Abstract
Objective
To compare the characteristics and outcomes of patients with intraabdominal infections enrolled in prospective randomized trials (PRTs) with those of a cohort of patients not enrolled in a trial.
Summary Background Data
Prospective randomized trials are the gold standard for the evaluation of new treatments. Patients are screened using rigorous eligibility criteria and sometimes are excluded from PRTs because of associated medical conditions or more severe illness. However, the effect that the exclusion of these patients has on the applicability of clinical trial outcomes has not been defined.
Methods
One hundred sixty-eight adults with intraabdominal infection were treated at a single institution during 7 years. Fifty-three patients were enrolled in four PRTs comparing various antibiotic regimens for treatment; 115 were not enrolled. Patient characteristics and outcomes of these two groups were compared.
Results
Patients with infections from appendicitis (n = 68) had a low severity of illness and similar outcomes in both groups. These patients and those for whom a concurrent PRT was unavailable were excluded from subsequent analysis. Eighty-eight patients (42 PRT, 46 not enrolled) with serious infection remained for analysis. Patients enrolled in PRTs were younger, had less severe illness, had a decreased length of stay, a lower incidence of antibiotic resistance, and less frequent extraabdominal infections than those not enrolled in a trial. Patients enrolled in PRTs were more likely to be cured and were less likely to die. Logistic regression analysis demonstrated that cure was associated with a lower initial severity of illness, absence of antibiotic resistance, and participation in a PRT.
Conclusions
Patients with intraabdominal infection enrolled in PRTs have an increased likelihood of cure and survival. This is due in part to a lower incidence of antibiotic resistance, which may reflect improved drug selection. Patients not enrolled in PRTs are at greater risk for treatment failure and death because of concomitant illness. Outcomes from PRTs may not be applicable to all patients with intraabdominal infections.
More than 250 years have passed since a British surgeon, James Lind, 1,2 randomly administered various unproven concoctions for the treatment of scurvy to six groups of 12 men on the deck of a sailing ship. Applying this method, Lind discovered the cure for scurvy and showed the early application of clinical trial methodology.
Prospective randomized trials (PRTs) have become the gold standard for evaluating the efficacy of new treatments. 2,3 They are considered the highest standard of evidence-based medicine 4 and are the method of choice for testing the efficacy of new antibiotics. 3 The goals of clinical trials are to show an advantageous or comparable treatment effect for a new agent and to identify a population of patients with a specific disease for which the treatment will be beneficial. 3,5
To ensure a carefully controlled study that protects the safety of patients and the integrity of analysis, homogeneous treatment groups are established by screening prospective enrollees through rigorous eligibility criteria before consent and randomization. 5 This process often excludes selected patients from participating in PRTs despite the fact that they may have the disease under evaluation in the study. These excluded patients and their risk characteristics are typically not recorded, followed, or evaluated in the clinical trial.
To determine the treatment impact of PRT enrollment, we compared the characteristics and outcomes of patients with intraabdominal infections enrolled in PRTs with a concurrent cohort of patients who were not enrolled in trials. We hypothesized that patients not enrolled in PRTs have a greater intensity of clinical illness or more severe intraabdominal infections, and that their clinical outcomes differ significantly from the cohort of patients evaluated in a PRT.
METHODS
This was a retrospective cohort study of 168 adults 18 years of age or older with the diagnosis of intraabdominal infection (defined as diffuse peritonitis or an intraabdominal abscess) who were treated at a single institution during 7 years. All charts were available and were reviewed for evidence of participation in a PRT specifically examining antibiotic efficacy for the treatment of intraabdominal infection. Four consecutive Food & Drug Administration-approved PRTs, all of which were multicenter trials examining the antimicrobial treatment of intraabdominal infections, were available for patient enrollment during this period. All studies were double-blinded and approved by the Institutional Review Board of MetroHealth Medical Center. Dedicated clinical research nurses screened all patients against preestablished criteria to determine their eligibility for enrollment.
Patients were eligible for enrollment and review for this study if they had a stomach or duodenal perforation for greater than 24 hours, small or large bowel perforation, perforated or gangrenous (complicated) appendicitis, postoperative peritonitis or intraabdominal abscess, traumatic perforation of the intestine with a greater-than-12-hour delay before surgery, or other conditions resulting from secondary bacterial peritonitis.
Using a standardized data collection sheet, information including age, gender, race, Acute Physiologic and Chronic Health Evaluation (APACHE) II score, 6 the type and source of the infection, and the occurrence of extraabdominal infections was abstracted from the patient records. Initial culture results, evidence of antibiotic resistance, and days of intravenous antibiotic therapy were also recorded.
Patients who satisfied eligibility criteria were enrolled in a trial and randomized to a treatment arm. Clinical research nurses monitored and prospectively collected information about the clinical course of all enrolled patients for the duration of their hospital stay and for 4 to 6 weeks after discharge. Patients not enrolled in a PRT did not participate because of inability to obtain consent, patient refusal, exclusion criteria, lack of an available study, or failure to identify the patient for possible enrollment. Research nurses did not follow up patients excluded from PRTs.
Standard exclusion criteria for these studies included pregnancy or lactation; a history of immune suppression (≥30 mg/day prednisone or active chemotherapy); renal failure (serum creatinine ≥3.0 mg/dL); hepatic failure (aspartate aminotransferase [AST] or alanine aminotransferase [ALT] three times normal or more, or a history of hepatic disease); participation in another Food & Drug Administration-approved study within the last 30 days; or an allergy to penicillin or the antibiotics under study.
Primary outcome measures included treatment cure or failure. Patients were defined as cured if their initial surgical and antibiotic management resulted in discharge from the hospital without the need for reoperation, drainage of an intraabdominal abscess, or additional antimicrobial therapy to treat the intraabdominal infection. Treatment failure was defined as the need for additional antibiotic therapy, reoperation, drainage of an intraabdominal abscess, or death. Other outcome measures assessed included length of hospital stay (LOS) and length of intensive care unit stay (ICU LOS).
Continuous variables were compared using the Student t test or the Mann-Whitney test and discrete variables were assessed using chi-square analysis where appropriate. P < .05 was considered statistically significant. Odds ratios (OR) and their 95% confidence intervals (CI) were calculated from the data. All analysis was done with SPSS software (SPSS, Inc., Chicago, IL).
RESULTS
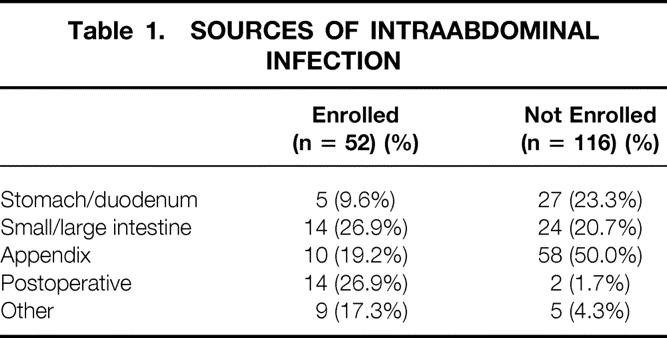
One hundred sixty-eight patients were reviewed. The source of intraabdominal infection is listed in Table 1. Complicated appendicitis and perforations of the small and large intestine were the most common sites of infection. Other sites of infection included gangrenous cholecystitis (n = 4), trauma (n = 2), psoas muscle abscess (n = 2), tuboovarian abscess (n = 2), Crohn’s disease (n = 1), and esophageal perforation (n = 1). A definitive source of infection could not be established in two patients. Fifty-two patients (31%) were enrolled in a PRT; 116 (69%) were not enrolled.
Table 1. SOURCES OF INTRAABDOMINAL INFECTION
One hundred sixteen patients (69%) were cured. Cure was more likely if patients were younger than 50 years of age, had an APACHE II score of 8 or less, had appendicitis as the cause of infection, had no evidence of antibiotic resistance, and did not have an extraabdominal infection (Table 2).
Table 2. FACTORS ASSOCIATED WITH TREATMENT CURE IN ALL PATIENTS WITH INTRAABDOMINAL INFECTION
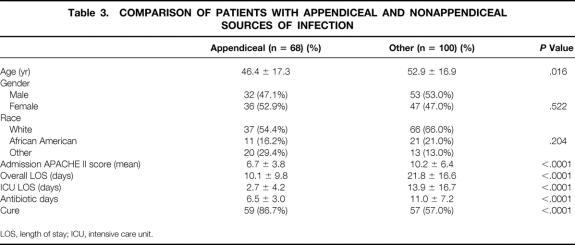
Sixty-eight patients (40%) had infections from complicated appendicitis. These patients were younger, had a lower APACHE II score at admission, a shorter hospital LOS, and a shorter ICU LOS, and required fewer days of intravenous antibiotic treatment than the patients with other sources of infection (Table 3). Patients with appendicitis had similar characteristics and a high rate of cure regardless of whether they were enrolled in a PRT (Table 4). Because other reports 7–12 also have shown superior rates of cure for appendicitis compared with other sources of intraabdominal infection, patients with appendicitis, as well as those for whom a PRT was not available at admission (n = 12), were excluded from further analysis.
Table 3. COMPARISON OF PATIENTS WITH APPENDICEAL AND NONAPPENDICEAL SOURCES OF INFECTION
LOS, length of stay; ICU, intensive care unit.
Table 4. COMPARISON OF PATIENTS WITH APPENDICITIS BY CLINICAL TRIAL ENROLLMENT STATUS
LOS, length of stay; ICU, intensive care unit.
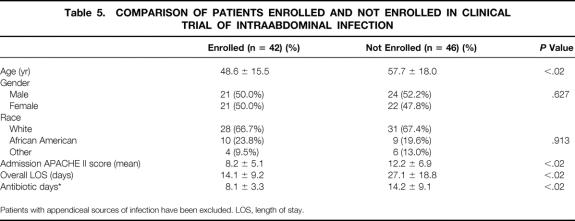
Of the remaining 88 patients, 42 (48%) were enrolled in a PRT and 46 (52%) were not enrolled (Fig. 1). Patients enrolled in PRTs were younger, had a lower admission APACHE II score and a lower incidence of antibiotic resistance, required fewer days of intravenous antibiotics, and had a shorter hospital LOS. Gender and race distribution was similar in these two groups (Table 5).

Figure 1. Outline for selection of patients with serious intraabdominal infections. PRT, prospective randomized trial.
Table 5. COMPARISON OF PATIENTS ENROLLED AND NOT ENROLLED IN CLINICAL TRIAL OF INTRAABDOMINAL INFECTION
Patients with appendiceal sources of infection have been excluded. LOS, length of stay.
Fifty-two (59%) of these 88 patients were cured. Twelve patients (13.6%) required reoperation, 5 (5.7%) failed to respond because of recurrent antibiotic resistance, and 19 (21.6%) died (Table 6). Patients who were cured were younger (50.3 ± 17.3 vs. 58.0 ± 16.8 years, P < .05), had a lower admission APACHE II score (8.1 ± 5.8 vs. 13.4 ± 5.9, P < .05), had a shorter hospital LOS (16.3 ± 14.0 vs. 27.8 ± 17.7 days, P < .05), and required fewer days of intravenous antibiotic treatment (8.8 ± 5.1 vs. 15.2 ± 9.1 days, P < .05) than patients who failed to respond to treatment. APACHE II scores and treatment failure were directly related in both the enrolled and not-enrolled groups (Table 7).
Table 6. OUTCOMES BASED ON ENROLLMENT STATUS

PRT, prospective randomized trial.
Table 7. IMPACT OF APACHE II SCORE ON OUTCOMES OF INTRAABDOMINAL INFECTION

Patients enrolled in PRTs were more likely to be cured (78.6% vs. 41.3%, OR 5.333, 95% CI 2.1–13.8, P < .0001) and were less likely to die (9.5% vs. 32.6%, OR 0.232, 95% CI 0.069–0.778, P < .013). Patients not enrolled in PRTs required more reoperations and percutaneous drainage procedures (n = 9) and had a higher incidence of failure resulting from antibiotic resistance (n = 3) and death (n = 15).
We performed a logistic regression analysis entering the independent variables of age, male gender, admission APACHE II score, evidence of antimicrobial resistance, and PRT enrollment as risk factors against the binary outcome of cure or failure. A low APACHE II score (P = .014), the absence of resistance (P = .036), and participation in a PRT (P = .034) were predictive of cure (R2 = 0.362, chi-square = 25.207, P < .0001).
Exclusion criteria for the 46 patients not enrolled in PRTs also were reviewed (Table 8). All patients who had evidence of an allergy to antibiotics were allergic to penicillins or the specific agents under study. Ten patients had no evidence of a specific exclusion criterion that would have prevented enrollment into a PRT.
Table 8. REASONS PATIENTS WERE NOT ENROLLED IN ANTIBIOTIC TRIALS

Total may not equal 100% due to rounding.
Patients in whom treatment failed had a higher incidence of antibiotic resistance (78.6% vs. 21.4%, P < .002), were more likely to have generalized peritonitis rather than an intraabdominal abscess (69.4% vs. 44.0%, P < .019), and had a greater incidence of extraabdominal infections (62.8% vs. 37.2%, P < .0001). Five patients (15.9%) who showed evidence of antimicrobial resistance died (three not enrolled in PRTs and two enrolled in PRTs).
Initial culture results were available for 89% of the patients. Patients not enrolled in PRTs had a higher incidence of yeast recovered on culture, but this was not statistically significant. There was no significant correlation between species recovered from initial culture and cure. Polymicrobial infections were more common among patients enrolled in PRTs (81.6% vs. 55.2%).
Fourteen patients (15.9%) had antibiotic-resistant organisms grown from the initial cultures, and these patients were more likely to fail to respond to treatment (78.6% vs. 36.1%, P < .003). Patients not enrolled in PRTs had a higher incidence of resistance to antibiotics (21.7% vs. 9.5%, P < .05). These patients required more days of intravenous antibiotic therapy (15.4 vs. 10.3 days, P < .12), and had a greater hospital LOS (28.9 vs. 19.9 days, P < .07).
Forty-seven patients (53.4%) had generalized peritonitis and 41 (46.6%) had an intraabdominal abscess. Patients with an abscess were more likely to be cured than those with generalized peritonitis (69.2% vs. 46.8%, P < .05) and were more likely to be enrolled in PRTs (64.3% vs. 30.4%, P < .05).
Sixty-eight episodes of extraabdominal infections occurred in 44 patients. These included urinary tract infections (n = 17), pneumonias (n = 12), wound infections (n = 11), and bloodstream infections (n = 10). Patients not enrolled in PRTs were more likely to have extraabdominal infections (65.9% vs. 34.1%, P < .01). The development of an extraabdominal infection was associated with a lower rate of cure (37.2% vs. 62.8%, P < .001); this was a consistent finding regardless of enrollment status.
The ICU LOS was assessed in both groups. Patients not enrolled in PRTs had a trend toward a longer ICU stay (14.7 vs. 6.4, P = .304). Thirty-five (76%) of the not-enrolled patients and five (12%) of the patients enrolled in PRTs required ICU care. Patients requiring ICU care were less likely to be cured (37.5% vs. 76.1%, OR 0.189, 95% CI 0.074–0.479, P < .0001).
All patients enrolled in PRTs who died (n = 4) did so as a consequence of infection. Of the patients not enrolled who died (n = 15), death was due to infection in eight patients, cardiac disease in five, and intestinal ischemia and acute leukemia in one each. Four patients not enrolled in PRTs who died had hepatic and/or renal failure, and three had pneumonia or acute respiratory distress syndrome.
DISCUSSION
At our institution, patients with intraabdominal infections who were enrolled in a PRT had better outcomes than those who were excluded or not entered into a clinical trial. Excluded patients as a group were older, had higher mean admission APACHE II scores, stayed in the hospital longer, and required more days of intravenous antibiotics than their PRT counterparts.
Increasing age 7,8,10,13–21 and higher APACHE II scores 7,10,14,17,19–21 have previously been shown to be independent predictors of treatment failure and death for patients with intraabdominal infections. In the current study, patients older than 50 years and those with APACHE II scores greater than eight had significantly higher rates of treatment failure, a finding observed in both the PRT and not-enrolled groups.
Concomitant illness has been reported to be a significant risk factor for treatment failure in other studies evaluating intraabdominal infection. 8,9,13,17,19 Patients not enrolled in PRTs had a higher incidence of significant medical problems (cardiac disease, renal failure, hepatic failure, steroid or chemotherapy use) than those enrolled in PRTs.
Trial eligibility criteria restricted the participation of patients with comorbid diseases that have been reported to increase the death rate of patients with intraabdominal infections. 13,18,19
Excluding sicker patients from trial participation based on rigid eligibility criteria may have had the unintended effect of creating two heterogeneous groups of patients. The group not enrolled in PRTs may have been less likely to be cured because of factors imposed by concomitant illnesses that limited their physiologic reserve. In contrast, patients who meet eligibility criteria and therefore can be enrolled in PRTs are healthier overall, have fewer comorbid diseases, and are more likely to be cured of infection.
The source of intraabdominal infection has been shown to be another important determinant of outcomes. 8,11,17 Patients not enrolled in PRTs had a higher frequency of appendicitis and gastroduodenal perforation and fewer episodes of postoperative infection than those enrolled, all of which would have been expected to result in better outcomes. Patients with complicated appendicitis have repeatedly been shown to have a lower death rate and fewer complications than patients with other causes for intraabdominal infection. 7–12 The incidence of complicated appendicitis in the PRT group was considerably less than in other clinical trials of intraabdominal infections. 11,14,20 Analysis of outcomes after excluding these patients allowed this study to concentrate on patients with more serious infection who were at greater risk for treatment failure and death.
Postoperative infections have been associated with higher death rates than many other sources of intraabdominal infection. 8,12 Eighty-eight percent of patients who had a postoperative infection were in the PRT group; however, treatment failure was still lower in this group of patients. Although there are multiple factors that affect cure and survival in intraabdominal infections, the importance of risk stratification, especially by source of infection, cannot be overemphasized. 11
Intraabdominal abscesses were more common among patients with postoperative infection. Previous reports have shown that patients with a localized infection have better outcomes than those with generalized peritonitis. 7,10,16,19 This was true in our study as well, and overall more patients with intraabdominal abscess were enrolled in PRTs.
The beneficial effect that enrollment in a clinical trial can have on outcomes is not new. One theory suggests that patients enrolled in clinical trials receive better medical care. 22,23 Research nurses carefully followed up the patients enrolled in these clinical trials. This increased surveillance, and the fact that all caregivers are aware of the patient’s participation, may have had the unintended effect of ensuring that enrolled patients receive better or more consistent care than those not enrolled.
Patient motivation has been speculated to improve outcomes in cancer chemotherapy trials. 24 These studies are more likely to enroll patients who are healthier, more motivated, and have a stronger “will to live,” which may result in a bias toward improved survival. 24 This factor is probably less important in clinical trials of intraabdominal infection because this disease carries a lower death rate than many malignancies. In our experience, the overall desire of patients to participate in PRTs is high, which should reduce the potential for this type of bias.
Microbial resistance to the selected antibiotic treatment has been shown to increase the risk of failure for patients with intraabdominal infections. 12,21,25 In the current study, patients who had evidence of drug resistance were less likely to be cured regardless of enrollment status. Patients in PRTs had a lower incidence of resistance, which may be due to better antibiotic selection or a less severe disease process.
Enrollment in a clinical trial regulates drug selection and may ensure that more effective antibiotics are prescribed initially. Ineffective empirical antimicrobial therapy has been associated with a greater likelihood of treatment failure in patients with peritonitis 25 and other infections. 26 The rigorous drug selection and dosing criteria imposed by clinical trials may result in a lower incidence of antimicrobial resistance. This factor, combined with the increased monitoring of care by clinical investigators and research nurses, may account for the better outcomes of patients enrolled in PRTs.
Lead-time bias is a well-described phenomenon in screening studies. 27 This also may affect the eligibility of patients for enrollment in clinical trials and ultimately influence their results. Protocols generally require immediate screening of patients with the index disease for enrollment into a trial. Delays in patient presentation and disease recognition may ultimately lead to the exclusion of a patient from trial participation. For example, a patient may enter the hospital with normal renal function, and subsequently peritonitis may develop. A delay in recognition of the infection causes renal function to worsen. If this patient were not immediately screened for enrollment, the subsequent impairment of renal function would lead to exclusion from participation in a trial.
A primary goal of a clinical trial is to select similar groups of patients with a relatively uniform disease process for each treatment arm. 5 This process ensures that the results of the study are applicable to a broader group of patients with the same disease. The development of homogeneous treatment groups generally is accomplished through the process of randomization, 28 which reduces systematic bias and equally distributes patients with known and unknown risk characteristics into the various treatment arms. Randomization takes place, however, only after patients have been screened for all potential exclusion criteria; this is an important point that often is not considered. Thus, the treatment arms are balanced with similar groups of patients derived from a homogeneous patient population with a lower severity of disease. The risk characteristics of patients excluded before randomization are not routinely recorded or analyzed, and the treatment results of these patients have not been reported.
Clinical trial investigators attempt to ensure a relatively uniform patient population by carefully controlling enrollment through the establishment of restrictive inclusion and exclusion criteria. 5 There are two approaches to the development of eligibility criteria for clinical trials, both of which have obvious advantages and disadvantages. One approach is to establish rigid criteria; this leads to a more homogeneous study population and ensures that the observed treatment effect is robust and incontrovertible. Alternatively, the use of less restrictive eligibility criteria selects a more heterogeneous patient population. This approach requires a more sophisticated stratification system based on patient risk factors to analyze the results properly.
The expense and time involved in conducting a carefully designed clinical trial can be enormous, and therefore the need to find a clear treatment effect favors enrollment of a homogeneous patient population. Under these circumstances, there is a risk of limiting the applicability of the results to other patients who have a similar disease but who were excluded from the study. Better record keeping and the more extensive collection of additional clinical data allows more accurate risk adjustment in a larger, more heterogeneous patient population. This approach excludes fewer patients and favors the applicability of study findings to a broader group of patients.
The results of the current study show that the degree of illness as measured by admission APACHE II score, the presence of antimicrobial resistance on initial culture, and enrollment into a PRT were significant determinants of cure and ultimate survival for patients with serious intraabdominal infections. As surgeons, we can exercise some control over each of these parameters. Although the severity of illness is often determined at the time the patient is initially seen, avoiding delays in treatment as well as prompt resuscitation and support of organ systems may reduce treatment failure by preventing further physiologic derangements.
The proper selection of antimicrobial agents for the treatment of intraabdominal infections should minimize the occurrence of antibiotic resistance. The advantages of being enrolled in a PRT may relate to the mandated selection of empirical antibiotic dose and therapy, or to improved delivery of care. Although the exact cause cannot be determined at present, these observations nevertheless show a benefit to study participation, which is a key element of informed consent. 29 If this benefit is due to regulation of drug choice, then appropriate selection of antibiotics must be made for all patients with these infections. We hope that these observations will stimulate further investigations of this and other possible benefits of PRT participation.
Some patients are excluded from study participation because of associated organ dysfunction, such as severe renal or hepatic failure. These diseases often require dosage adjustment of antimicrobial agents, which may lead to subtherapeutic drug concentrations and thus reduce their effectiveness. Other exclusion criteria prohibit the enrollment of patients with compromised immune function, because these patients have a greater risk of treatment failure. It may be more relevant to avoid the routine exclusion of more seriously ill patients, instead using more rigorous methods to stratify risk characteristics. This would provide a more realistic assessment of the expected outcomes for more seriously ill patients and would improve the external validity and applicability of the results of clinical trials.
Discussion
DR. HIRAM C. POLK, JR. (Louisville, Kentucky): Dr. Aust, Dr. Townsend, ladies and gentlemen. Mark is to be congratulated for taking up a tough subject. This has been in the background of many clinical trials for a long time. In fact, it was about 20 years ago before this Association when Dr. Stone, perhaps with Dr. Fabian’s help, described that patients in trials did better. He assumed at the time and discussed briefly that this was because they got better care, the study coordinator was looking after them, the professor was interested, and all the other factors Mark has described.
Mark took this a very important further step today, because he says it is not just that. When I looked at the manuscript, it says these patients are younger, they are less sick, and that those two factors directly lead to shorter stay, less antibiotic resistance and less frequency of a whole variety of interventions. They are more likely to live, and they are more likely to be cured.
The lead-time bias issue has been mentioned, and it is part of many of these trials. But I’d like to ask Mark the hard question: To what extent are these biases the products of the demands of the sponsoring pharmaceutical companies? Have they always known these answers, and have they always wanted them to be the outcome? I think that the issue of ethics of dealing with pharmaceuticals in these areas, with billions and billions of dollars at stake, is really important, and I would be interested in his thoughts about how much is investigator-generated and how much is sponsor-generated.
Thank you very much.
DR. TIMOTHY C. FABIAN (Memphis, Tennessee): Dr. Aust, Dr. Townsend, members, and guests. I’d like to thank the authors for asking me to discuss this nicely executed study. The manuscript is very well written, a lot of important material dealing with the outcomes and clinical trials is referenced, which I found quite educational personally.
They document an important fact concerning antibiotic clinical trials of intraabdominal infection that many investigators in the area are aware of, but these authors are the first, to my knowledge, to well document and define this problem.
The mortality rates in most prospective randomized trials for antibiotics in intraabdominal sepsis approximate the 10% mortality in the authors’ trials, while historical mortality rates for intraabdominal sepsis are generally quoted as 30% to 40%, similar to the authors’ experience of 33%. Parenthetically, it is interesting to note that those mortality rates have not substantially changed over the last two to three decades. I have the following questions:
How did you identify the cohort not entered in the prospective randomized trials? What were the antibiotic regimens? Were the nonenrolled patients on generally similar regimens (e.g., triple therapy)? And is it possible that those regimens were in fact less effective? Did you note differences in flora between groups? Importantly, could differences in culture techniques affect resistance patterns (i.e., better microbiology techniques under study circumstances)? I believe what you alluded to with your comments on the Hawthorne effect already.
Finally, a key point deals with attributable mortality. While the crude mortality rates were significantly lower for the prospective randomized trials, 9.5% and 32%, the infection-related death rates were 9.5% and 17.4%, not statistically significantly different.
As you well demonstrated in this presentation, exclusion criteria in randomized trials eliminate patients with significant underlying disease, thus producing significantly younger study populations. To eliminate the “noise” of noninfection-related death, perhaps the exclusion criteria generally used are justified. As you have given this considerable study and thought, how do you weigh in on this issue for further studies and prospective randomized trials?
Thank you very much.
DR. MARK A. MALANGONI (Cleveland, Ohio): I’d like to thank both Dr. Polk and Dr. Fabian for their insightful comments on this. Dr. Polk asked about bias. And, clearly, the design of a prospective randomized trial is to try to enroll a group of patients who are relatively uniform with a single disease. They also try to show either an advantageous or comparable treatment effect.
We would suggest that on the basis of our results is that what we need to do is change from this homogenous group of patients to a more heterogenous group of patients and then apply better statistical analysis to our data collection in order to make certain that these patients are evenly distributed in outcome in both treatment arms. And this would lead to a more realistic appraisal of the effects of these treatments.
Dr. Fabian asked a number of questions. Tim, we relied on our screening logs to identify patients not enrolled in the trial. There were 46 in that group, and the remaining patients were found through review of our patient records.
The antibiotics given to patients not enrolled in trials were various, and we did not pass judgment on their effectiveness. The microbiology was not different between the two groups; however, on recovery of cultures, polymicrobial infections were more common in the enrolled patient group which, I think, reflects exactly what you stated, and that is we try harder, if you will, in the microbiology laboratory to identify the culprits in this case.
Lastly, the most important question that Dr. Fabian asked was about the difference in attributable mortality, and I think this is going to require a larger data set in order for us to make any firm conclusions about whether that is going to hold true or not.
We saw this as an initial study to bring home an important point, and we hope that it will stimulate others to examine this and other problems with prospective randomized trials further.
Once again, I’d like to thank the Association for the privilege of the floor.
Footnotes
Presented at the 112th Annual Meeting of the Southern Surgical Association, December 4–6, 2000, Palm Beach, Florida.
Correspondence: Mark A. Malangoni, MD, Chairperson, Department of Surgery, MetroHealth Medical Center, 2500 MetroHealth Dr., Cleveland, OH 44109.
E-mail: mmalangoni@metrohealth.org
Accepted for publication December 2000.
References
- 1.Bull JP. The historical development of clinical therapeutic trials. J Chron Dis 1959; 10: 218–248. [DOI] [PubMed] [Google Scholar]
- 2.Friedman LM, Furberg CD, DeMets DL. Fundamentals of clinical trials, 3d ed. New York: Springer-Verlag; 1998.
- 3.Leber P. The future of controlled clinical trials: is there an alternative to the randomized controlled trial? Psychol Bull 1991; 27: 3–8. [PubMed] [Google Scholar]
- 4.Preventative Services Task Force. Guide to clinical preventative services: Report of the U.S. Preventative Services Task Force, 2d ed. Baltimore: Williams & Wilkins; 1996.
- 5.Piantadosi S. Clinical trials: a methodologic perspective. New York: John Wiley & Sons; 1997.
- 6.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 7.Dellinger EP, Wertz MJ, Meakins JL, et al. Surgical infection stratification system for intra-abdominal infection. Arch Surg 1985; 120: 21–29. [DOI] [PubMed] [Google Scholar]
- 8.Bohnen JMA, Boulanger M, Meakins JL, et al. Prognosis in generalized peritonitis. Arch Surg 1983; 118: 285–290. [DOI] [PubMed] [Google Scholar]
- 9.Nystrom PO, Bax R, Dellinger PE, et al. Proposed definitions for diagnosis, severity scoring, stratification, and outcome for trials on intraabdominal infection. World J Surg 1990; 14: 148–158. [DOI] [PubMed] [Google Scholar]
- 10.Christou NV, Barie PS, Dellinger EP, et al. Surgical Infection Society Intra-abdominal Infection Study: prospective evaluation of management techniques and outcome. Arch Surg 1993; 128: 193–199. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SE, Faulkner K. Impact of anatomical site on bacteriological and clinical outcome in the management of intra-abdominal infections. Am Surg 1998; 64: 402–407. [PubMed] [Google Scholar]
- 12.Bohnen JMA, Solomkin JS, Dellinger EP, et al. Guidelines for clinical care: anti-infective agents for intra-abdominal infection: a Surgical Infection Society policy statement. Arch Surg 1992; 127: 83–89. [DOI] [PubMed] [Google Scholar]
- 13.Pine RW, Wertz MJ, Lennard ES, et al. Determinants of organ malfunction and death in patients With intra-abdominal sepsis. Arch Surg 1983; 118: 242–249. [DOI] [PubMed] [Google Scholar]
- 14.Solomkin JS, Dellinger EP, Christou NV, et al. Results of a multicenter trial comparing imipenem/cilastatin to tobramycin/clindamycin for intra-abdominal infections. Ann Surg 1990; 212: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomkin JS, Meakins JL, Allo MD, et al. Antibiotic trials in intra-abdominal infections: a critical evaluation of study design and outcome reporting. Ann Surg 1984; 200: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohnen JMA, Mustard RA, Oxholm SE, et al. APACHE II score, abdominal sepsis. A prospective study. Arch Surg 1988; 123: 225–229. [DOI] [PubMed] [Google Scholar]
- 17.Pacelli F, Doglietto GB, Alfieri S, et al. Prognosis in intra-abdominal infections: multivariate analysis on 604 patients. Arch Surg 1996; 131: 641–645. [DOI] [PubMed] [Google Scholar]
- 18.Milligan SL, Luft FC, McMurray SD, et al. Intra-abdominal infection and acute renal failure. Arch Surg 1978; 113: 467–472. [DOI] [PubMed] [Google Scholar]
- 19.Koperna T, Schulz F. Prognosis and treatment of peritonitis: do se need new scoring systems? Arch Surg 1996; 131: 180–186. [DOI] [PubMed] [Google Scholar]
- 20.Barie PS, Vogel SB, Dellinger EP, et al. A randomized double-blind clinical trial comparing cefepime plus metronidazole with imipenem-cilastatin in the treatment of complicated intra-abdominal infections. Arch Surg 1997; 132: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 21.Malangoni MA, Condon RE, Spiegel CA. Treatment of intra-abdominal infections is appropriate with single-agent or combination antibiotic therapy. Surgery 1985; 98: 648–655. [PubMed] [Google Scholar]
- 22.Wilhelmsen L. Ethics of clinical trials: the use of placebo. Eur J Clin Pharmacol 1980; 17: 1–4. [DOI] [PubMed] [Google Scholar]
- 23.Brown BW. The use of controls in the clinical evaluation of cancer therapies. In: Proceedings of a Symposium on Statistical Aspects of Protocol Design. National Cancer Institute, Bethesda, MD, Dec. 10, 1970:161–179.
- 24.Davis S, Wright PW, Schulman SF, et al. Participants in prospective, randomized clinical trials for resected non-small lung cancer have improved survival compared with nonparticipants in such trials. Cancer 1985; 56: 1710–1718. [DOI] [PubMed] [Google Scholar]
- 25.Mosdell DM, Morris DM, Voltura A, et al. Antibiotic treatment for surgical peritonitis. Ann Surg 1991; 214: 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kollef MH, Sherman G, Waro S, et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 1999; 115: 462–474. [DOI] [PubMed] [Google Scholar]
- 27.Gordis L. Epidemiology. New York: Saunders; 1996.
- 28.Zeller R, Good M, Anderson GC, Zeller DL. Strengthening experimental design by balancing potentially confounding variable across treatment groups. Nurs Res 1997; 46: 345–349. [DOI] [PubMed] [Google Scholar]
- 29.Federal Food, Drug, Cosmetic Act, Code of Federal Regulations, Chapter I, Title 21, Section 50.25(3).