Abstract
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is a lipid phosphatase. PTEN inhibits the action of phosphatidylinositol-3-kinase and reduces the levels of phosphatidylinositol triphosphate, a crucial second messenger for cell proliferation and survival, as well as insulin signaling. In this study, we deleted Pten specifically in the insulin producing β cells during murine pancreatic development. Pten deletion leads to increased cell proliferation and decreased cell death, without significant alteration of β-cell differentiation. Consequently, the mutant pancreas generates more and larger islets, with a significant increase in total β-cell mass. PTEN loss also protects animals from developing streptozotocin-induced diabetes. Our data demonstrate that PTEN loss in β cells is not tumorigenic but beneficial. This suggests that modulating the PTEN-controlled signaling pathway is a potential approach for β-cell protection and regeneration therapies.
β cells are produced through neogenesis from precursor cells and/or replication of mature and differentiated β cells. During embryonic development, both neogenesis and replication contribute to the growth of the pancreas. The proliferation rate of β cells in the adult pancreas, however, is relatively low. The adult pancreas undergoes slow turnover, with only approximately 0.5% mitotic activity, primarily by replication of differentiated cells (16). Nutrient and growth factors, such as high glucose concentrations, insulin-like growth factor 1 (IGF-1), and growth hormone, can induce β-cell replication (6, 39). Adult β-cell proliferation has been observed among pregnant and obese individuals for whom the demand for insulin is increased (40). During β-cell or pancreatic injuries, mitotic activity of β cells is also increased (4, 6, 7, 20, 22, 40). It has been suggested that after injury, β-cell regeneration may be the result of progenitor cell proliferation and differentiation (5). However, recent findings by Dor et al. suggest that in murine islets, proliferation of preexisting β cells is the major mechanism for regeneration under both physiological and injury conditions (15). Together, these findings suggest that β cells in pancreatic islets have a significant potential for replication. However, little is known about the factors regulating β-cell mass during neogenesis and adult pancreas turnover.
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is an indispensable regulator for cell growth and survival (48, 51). PTEN functions as a lipid phosphatase to dephosphorylate phosphatidylinositol triphosphate, the product of phosphatidylinositol-3-kinase (PI3K) (35, 56). By antagonizing PI3K function, PTEN inhibits the signals of insulin, IGF-1, and platelet-derived growth factor, the major mitogenic and survival factors of β cells (13). As a consequence of PTEN loss, AKT serine/threonine kinase and its downstream effectors are hyperactivated (48, 51). AKT is critical for β-cell survival both in vitro and in transplantation models (1, 10, 12, 33, 34, 46, 54). Recent studies using genetic approaches have further demonstrated that a constitutively activated form of AKT enhances not only cell survival, but also cell proliferation and cell size, thereby increasing overall β-cell mass (3, 52). In these two studies, constitutive activation of AKT is produced by attaching myristylation signal to full-length or truncated AKT. This manipulation brings the AKT transgene to the cell membrane and results in its activation. Both studies showed increased islet mass and number as a result of constitutive AKT activation. The transgenic animals also demonstrated resistance to streptozotocin (STZ)-induced islet destruction. Furthermore, deletion of S6 kinase (S6K), a downstream kinase of the PI3K/AKT pathway, resulted in diminished β-cell size (41). The effect of PI3K/AKT activation is also demonstrated in insulin receptor substrate 2 (IRS2)-transgenic animals showing a dose-dependent response that increased islet mass and rescued animals from STZ-induced hyperglycemia (36). PTEN deficiency was directly evaluated with an experimental model that lacks IRS2. IRS2 mutant mice developed diabetes within the first 3 months of age (29). Heterozygous deletion of Pten in IRS2 mutants was able to improve insulin sensitivity and stimulate islet growth (29). This study provides direct evidence that Pten deficiency may stimulate islet regeneration, but peripheral Pten deletion and homeostasis-induced islet changes cannot be ruled out. Together, these findings suggest that the PI3K/AKT pathway is important for β-cell growth and survival. Therefore, we hypothesize that activation of this pathway could promote β-cell regeneration and thus lead to resistance to injury-induced diabetes. In this study, we generated a murine model of β-cell-specific Pten deletion to test whether a physiological gain of PI3 kinase activity leads to a similar enhancement in islet and β-cell mass. We showed that Pten deletion in β cells resulted in increased islet mass and demonstrated that activation of the PI3K/AKT pathway can rescue STZ-induced β-cell damage and subsequent diabetes development in vivo.
MATERIALS AND METHODS
Animals.
Targeted deletion of Pten in β cells was achieved by crossing PtenloxP/loxP mice (C57/129/J background) (30) with rat insulin promoter-Cre (RIP-Cre) mice (C57 background) (43). F1 generation compound heterozygous animals were backcrossed with PtenloxP/loxP mice to produce F2 generation experimental animals. Animals were genotyped by standard genomic PCR techniques (30). Animals were housed in a temperature-, humidity-, and light-controlled room (12-h light/dark cycle), allowing free access to food and water. All experiments were conducted according to the research guidelines of the UCLA Chancellor's Animal Research Committee.
Glucose, insulin, and lipid determinations.
Blood samples were collected from 3-month-old mice. After mice fasted overnight, blood samples were collected by orbital eye bleeding into lithium chloride-coated plasma collection tubes. Plasma was obtained by centrifugation. Plasma insulin levels were measured by enzyme-linked immunosorbent assay according to the manufacturer's protocols (Alpco, Windham, N.H.). Plasma samples were also analyzed for nonesterified fatty acid (Wako) and triglyceride (Thermo DNA) using the manufactured kits. Glucose levels were determined with a Freestyle glucometer (Therasense) with blood samples from tail vein punctures in mice that fasted for 16 h.
Glucose and insulin tolerance tests.
After overnight fasting, mice 3 months of age were injected intraperitoneally (i.p.) with 30% d-glucose (2 g/kg of body weight; Sigma, St. Louis, Mo.) or insulin (1 U/kg; Lilly) (50). Blood glucose concentrations were measured at indicated time points as previously described (50).
Western electrophoresis.
Protein lysates were collected from isolated control and mutant islets. Western blot analyses were performed with PTEN, phosphor-S6, cyclin D, and p27 antibodies. All membranes were also probed with antibodies for β-actin as a loading control.
Immunohistochemistry.
We performed immunohistochemistry on Zn-formalin-fixed, paraffin-embedded sections, following antigen retrieval. Antibodies for PTEN (26H9) and phospho-AKT (Ser473) antibodies were obtained from Cell Signaling Technology, Beverly, Mass.). Glucose transporter 2 (GLUT2) antibody was purchased from Calbiochem, San Diego, Calif. Antibodies for insulin, glucagons, somatostatin, and pancreatic polypeptides were provided by Zymed. Sections were counterstained with hematoxylin.
Stereology measurement of islet area.
Insulin-stained pancreatic sections were analyzed using a MicroBrightField Stereology-assisted microscope mounted on a remote controlled platform. Islet area and number were determined with five cell clusters considered to be islets. Three slides per pancreas, 120 μm apart, were counted on a total of five animals that were 3 months old.
Determination of cell proliferation and apoptosis.
We evaluated cell proliferation on embryonic day 17.5 (E17.5) mice with bromodeoxyuridine (BrdU) pulse-labeling. Pregnant mothers at 17.5 days after plugging were injected with BrdU (100 μg/g of body weight) and sacrificed 45 min later. Embryos were retrieved, decapitated, and fixed in Zn-formalin. For adult animals, BrdU was given i.p. 30 to 45 min before euthanization. BrdU staining was done using a kit from Roche with a modified protocol for immunofluorescence. Sections were costained with insulin. Apoptosis was determined with P17- and STZ-treated mice with a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) kit from Roche with a modified protocol for immunofluorescence. The same sections were stained with insulin.
Islet culture and glucose stimulation.
Islets were isolated from 3-month-old male animals by collagenase digestion of the pancreas, followed by purification using a Ficoll gradient. Islets were handpicked twice and cultured in RPMI complete medium before stimulation with glucose. After overnight culture, islets (50) were stimulated with 3 mM (low) and 30 mM (high) glucose in RPMI 1640 for 30 min. The amount of insulin released to the culture medium was measured with an enzyme-linked immunosorbent assay kit from ALPCO. The islets were collected and lysed for DNA analysis. For STZ treatment, cultured islets were treated with 2 mM STZ in the culture medium for 30 min. Islets were then allowed to recover overnight. Insulin production in response to 30 mM glucose was then assayed.
Fluorescence-activated cell sorter (FACS) analysis for cell size.
Islet cells were dissociated with trypsin and passed through a 70 μm cell strainer (BD Falcon, Bedford, MA) to obtain a single-cell suspension. The trypsinized cells were run on a BD FACSCalibur (BD Immunocytometry Systems, San Jose, CA) to measure the cell size, based on forward scatter signal.
STZ-induced diabetes.
Eight-week-old male mice were injected i.p. with multiple subdiabetogenic doses of streptozotocin (31, 38) at a dose of 50 mg of streptozotocin/kg of body weight daily for 5 consecutive days (Sigma, St. Louis, Mo.) to produce a β-cell injury model. On day 8, a group of three animals were sacrificed following BrdU pulse-labeling. Pancreas sections were costained with antibodies against BrdU and insulin or for TUNEL and insulin. For long-term effect, the remaining six animals were evaluated weekly for the development of diabetes (defined as persistent random blood glucose levels of >300 mg/dl). Animals were sacrificed 2 months later, following BrdU pulse-labeling. For the high-dose STZ experiment, 200-mg/kg STZ was given as one dose, and animals were sacrificed 36 h later for tissue collection.
Statistical analysis.
All data are presented as means ± the standard error of the mean. Statistical calculations were performed with Microsoft Excel analysis tools. Differences between individual groups were analyzed by Student's t test, with two-tailed P values of <0.05 considered statistically significant.
RESULTS
β-cell-specific Pten deletion.
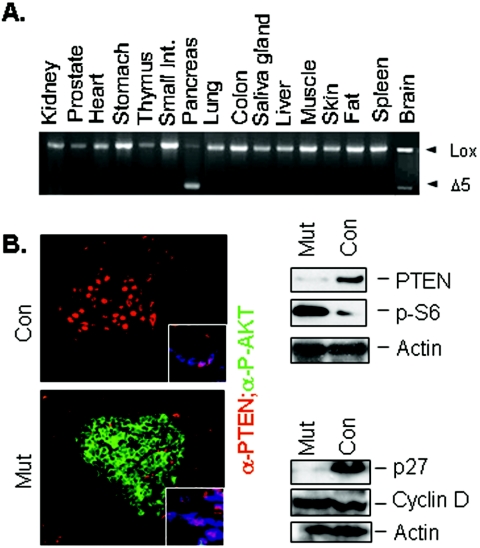
To establish a mouse model carrying Pten deletion in the insulin-producing cells, we crossed Pten conditional knockout mice (PtenloxP/loxP) (30) with the β-cell-specific Cre transgenic line driven by the rat insulin promoter (43). Of the offsprings generated, we used PtenloxP/loxP;Cre+ mice as mutants and PtenloxP/loxP; Cre− mice as controls for the experiment. PCR analysis of genomic DNA prepared from Ptenloxp/loxp; RIP-Cre+ (mutant) mice demonstrated that Cre-mediated Pten deletion was pancreatic specific, with little leakage in the brain (Fig. 1A), consistent with the previous report (17). Immunofluorescent analysis demonstrates that PTEN was localized in the ducts (Fig. 1B, top left, inset) and the nucleus of the murine islet cells (Fig. 1B, top left), similar to the subcellular localization observed in human islets (42). In the mutant pancreatic islets, PTEN was present in the ducts (Fig, 1B, bottom left, inset) but lost in the majority of the β cells, accompanied by increased phospho-AKT staining (Fig. 1B, bottom left). Loss of Pten and activation of AKT also led to the activation of S6K, as indicated by the increased phosphor-S6 protein levels in the mutant pancreatic islet protein lysate (Fig. 1B, top right). We further evaluated the expression of two cell cycle regulators, p27 and cyclin D; both are potential targets for regulation by the PI3K pathway. We found that the cell cycle inhibitor p27 was diminished significantly as a result of Pten deletion in the pancreas, whereas the level of cyclin D was minimally altered (Fig. 1B, bottom right).
FIG. 1.
Generation of mouse model carrying Pten deletion in insulin-producing cells. (A) Tissue PCR confirms deletion of Pten in pancreas. Minor leakage in the brain is observed. (B) Immunofluorescent staining shows PTEN staining (red) in the duct (inset) and nuclei of β cells in the control pancreas. In the mutant animals, loss of PTEN immunoreactivity is associated with increased membrane P-AKT staining (green). Images (original magnification, ×60) were overlaid by confocal microscopy. (Right) Western blot analysis of protein lysates isolated from control and mutant islets. Blots were probed with PTEN, p27, phosphor-S6, and β-actin (as a loading control).
Pten deletion leads to an increase in islet numbers and total islet mass.
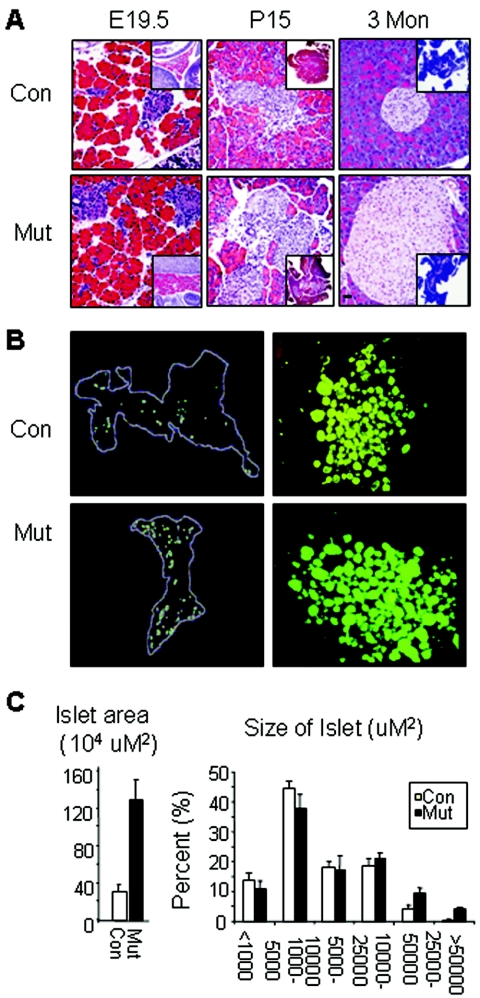
We examined the impact of Pten deletion on pancreatic islets and β cells. Development of the endocrine pancreas with insulin-expressing cells started as early as E12.5. Just prior to birth, endocrine cells migrate and aggregate to form mature islets with localized insulin secretion. At this stage, more islet clusters were seen in Pten mutant pancreas than in the control pancreas (Fig. 2A, left). Compared to islet mass in control animals, islet mass in postnatal animals was significantly increased in mutant mice as early as postnatal day 15 (P15) and persisted throughout adulthood (Fig. 2A, middle and right). This increase was likely due to increases in both islet number and islet size (Fig. 2B). We quantified islet number and islet size in 3-month-old male mice and demonstrated a 4.5-fold increase in total islet area (Fig. 2C, left). The increase in islet number was true for both small and large islets. Islets larger than 5,000 μM2 were only observed in the mutant pancreas but not in the control pancreas (Fig. 2C, right). The increase in islet area was also observed when islets were isolated (Fig. 2B, right) and scored. In this case, when islets from four mutant or control animals were pooled and counted, there was a 1.5-fold increase in the total number of islets recovered (200 versus 300 islets/sample pooled from four animals) and a 2-fold increase in islet size. The phenotype analysis of the mutant animals suggested that PTEN is a crucial player in regulating islet mass. This may happen during embryonic development when neogenesis dominates the islet mass, as well as during adulthood when most β cells come from self-regeneration.
FIG. 2.
Pten deletion in insulin-producing cells resulted in increased islet mass. (A) Increased islet mass is observed in the Pten mutant pancreas from late embryonic stage through adulthood. Insets show lower-magnification images with more islets in mutants than in control. Histology images are shown at ×40 magnification. Inserts are at ×10 magnification. Bar, 25 μm. (B) Increases in both islet mass and islet numbers are observed with Pten mutant mice. (Left) Stereology-assisted mapping of selected pancreas sections showed increased islets throughout the pancreas; images are representative of five animals, each 3 months of age. (Right) Islet culture isolated and pooled from four animals per genotype group. Larger and more islets were also observed in these organ cultures of isolated islets. (C) Quantification of islets in control and mutant animals. Islet number and area were averaged from five animals, with three sections from each animal, 120 μm apart. The right-hand panel shows the percentage of islets in each size category.
Pten deletion leads to increased cell proliferation and decreased cell death under physiological conditions.
During pancreatic development, cell proliferation peaks around late embryonic development and early postnatal life, followed by a wave of apoptosis at postnatal 2 to 3 weeks during pancreatic remodeling (16). Cell proliferation and cell death are limited in the adult endocrine pancreas (16). Deletion of Pten led to a significant increase in β-cell proliferation during embryogenesis, as measured by BrdU pulse-labeling at E17.5 (Fig. 3A). More than 8% of insulin-positive cells were BrdU+ in the mutant animals, compared to 3% double-positive cells in the controls. There was also significant proliferative activity adjacent to the insulin-positive cells in the mutant pancreas (Fig. 3A, arrows), which may represent increased progenitor cell activity. Deletion of Pten also led to a significant decrease in apoptotic rate during pancreas remodeling at an early postnatal stage. A twofold decrease in TUNEL-positive cells was observed in the mutant pancreas at P17 (Fig. 3B). Taken together, these data demonstrate that PTEN plays a critical role in regulating β-cell growth and survival.
FIG. 3.
Pten deletion led to increased proliferation and decreased apoptosis. (A) Increased BrdU-positive (green) cells were observed in mutant pancreas at E17.5. Double-positive cells for BrdU (green) and insulin (red) were quantified (bottom). Insets show higher-resolution images of BrdU and insulin staining. Arrows show proliferative activity adjacent to the insulin-positive cells in the mutant pancreas. (B) Apoptosis rate is measured in samples from P15 neonatal mice by TUNEL staining (green). TUNEL-positive cells were colocalized with insulin (red) and quantified (bottom). Inserts show higher-resolution images of TUNEL and insulin stainings. Immunofluorescent images were shown at ×40 magnification. Bar, 25 μm.
Pten-null pancreas retains normal islet functions.
To determine whether Pten-null β cells are fully differentiated and functional, we first stained islets with anti-insulin antibody. Immunoreactivity to insulin was detected as early as E12.5 in both the control and mutant pancreases (data not shown). By E15.5, all pancreases analyzed contain insulin-positive cells (Fig. 4A, left). In the postnatal stage when endocrine cells form clustered islets, mutant islets showed immunoreactivity to insulin, although with less intensity than in the control islets in both P15 and 3-month-old mice (Fig. 4A). No significant changes in localization of the non-insulin-producing cells were observed (Fig. 4B), while the number of insulin-producing cells appeared to be increased in mutant islets (Fig. 4B). Insulin-producing cells were localized in the center of the islet, while non-insulin-producing cells were predominantly distributed near the periphery of the islets in both control and mutant pancreas. In some islets, non-insulin-producing cells were disbursed in the islets. This was observed for both control and mutant animals and was unlikely to be due to Pten deletion. The ability of Pten-null β cells to secrete insulin in response to glucose stimulation was further quantified using isolated islets. Pten-null islets were responsive to glucose stimulation and secreted insulin in a manner similar to islets from control animals (Fig. 4C, left). Consistent with this observation, no statistically significant changes in serum insulin levels could be observed in the mutant animals, compared to the controls (Fig. 4C, right).
FIG. 4.
Pten mutant islets exhibit normal hormone profiles and cell surface markers. (A) Insulin immunohistochemistry staining of pancreas from different age cohorts (original magnification, ×40). Inset, lower-magnification image showing more insulin stained islets in mutants than in controls. Original magnification of insets, ×10. Bar, 25 μm. (B) Control and mutant pancreas stained with insulin (red) and a cocktail of non-insulin islet antibodies (glucagons plus somatostatin plus pancreatic polypeptide, green). (C, left) Insulin secretion from islet culture (pooled from four animals) with low (3 mM, right two bars) and high (20 mM, left two bars) glucose levels, as well as calculated changes (n-fold). The figure shows a representative sample from three independent tests. (C, right) Plasma insulin levels in control and mutant animals after 16-h fast. (D) Cell size measurement. (Left) GLUT2 staining of control and mutant islets. (Right) FACS analysis of control and mutant β cells. Original magnification, ×100. Bar, 25 μm.
The mutant islets also displayed normal staining patterns of GLUT2 (Fig. 4D, left). With high-power microscopy (magnification, ×100), Pten-null β cells did appear slightly enlarged, compared to controls (Fig. 4D, left). To determine whether Pten deletion leads to changes in β-cell size, we subjected dissociated islet cells to FACS analysis. Forward scatter signal showed no significant difference in cell size between the control and mutant β cells (Fig. 4D, right).
Pten deletion in pancreatic β cells did not significantly alter the glucose homeostasis of mutant animals.
Similar to our previous studies with hepatocyte- and adipose tissue-specific Pten deletion (28, 50), PTEN loss in β cells led to hypoglycemia (Fig. 5A). This lower serum glucose level may be one explanation for the decreased insulin-staining density of the mutant islets. Nevertheless, mutant and control animals responded similarly to a glucose challenge (Fig. 5B). An insulin tolerance test was also performed (Fig. 5C). Although insulin injection led to a similar fold decrease in plasma glucose levels in control and mutant animals, most of the mutant animals could not complete the test, due to their significantly lower fasting glucose levels, and had to be rescued with glucose injection. No significant changes in serum lipid indexes in control and mutant animals were observed (Fig. 5D).
FIG. 5.
Pten deletion resulted in fasting hyperglycemia of mutant animals. (A) Fasting plasma glucose levels. Animals fasted for 16 h, and plasma glucose levels were measured (10 animals). (B) Glucose tolerance test. Animals fasted overnight (16 h) before glucose tolerance test. Glucose (2 g/kg of body weight) was injected at time zero, and glucose levels were measured before injection and at 5, 15, 30, 60, and 120 min thereafter (10 animals). (C) Insulin tolerance test. Animals fasted overnight (16 h) before insulin tolerance test. Insulin (1 U/kg of body weight) was injected at time zero; glucose measurements were obtained before injection and at 5, 15, 30, 60, and 90 min thereafter (eight animals). Arrows indicate times at which most mutant animals needed to be rescued, due to hypoglycemic seizures. (D) Fasting plasma triglyceride and nonesterified fatty acid levels in samples from five animals. Data are presented as means ± standard error of the mean. *, statistical significance at P values of ≤0.05.
Pten deletion protects animals from STZ-induced β-cell injury.
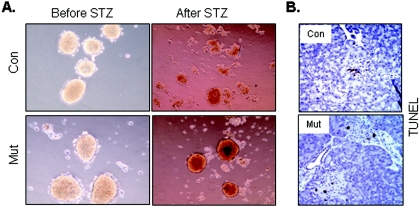
To determine the pathophysiological significance of Pten deletion in insulin-producing cells, we subjected control and mutant animals to low-dose STZ treatment. One week after initial STZ treatment, islets from the control pancreas started to atrophy, a sign of injury, while no obvious phenotype was observed on mutant islets (Fig. 6C, D, I, and J). Two months following initial STZ treatment, no visible islets could be found in the control pancreas under low-power microscopy, while isolated, irregular, atrophied islets could be found under high-power magnification (Fig. 6E and K). In contrast, a significant number of healthy islets could be found in the mutant pancreas (Fig. 6F and L). These morphologically healthy islets remained insulin positive (Fig. 6P and R). Lymphocyte infiltration was clearly visible in both control and mutant pancreases (Fig. 6E, F, I, J, K, and M). Two months after STZ treatment, small β-cell clusters with little or no lymphocyte infiltration could be observed in the mutant but not control pancreas (Fig. 6L, inset). These clusters may be islets newly formed after injury. Immunostaining to insulin was diminished in all animals treated with STZ but appeared to be retained more in the mutants (Fig. 6R).
FIG. 6.
Pten deletion protected mutant animals from STZ-induced β-cell loss. Histology analysis of islets treated with STZ. Untreated pancreas (left); pancreas 7 days after STZ treatment (middle); pancreas 2 months after STZ treatment (right). (A to L) Hematoxylin and eosin (H&E)-stained sections, showing islet morphology. Magnification, ×20 (A to F); ×100 (G to L). Bar, 50 μm (A to F) or 25 μm (G to L). Arrows indicate lymphocyte infiltration. (M to R) Immunohistochemical staining of insulin-positive cells. Bar, 25 μm.
Mutant pancreas exhibited increased BrdU-positive cells (5%) in comparison to control pancreas (1.5%) 7 days after STZ treatment (Fig. 7A). In control animals, BrdU-positive cells had weaker nuclear PTEN staining than the BrdU-negative cells (Fig. 7B, top, inset). Similar to the proliferative activity seen during the pancreatic development (Fig. 3), significantly increased proliferative activity peripheral to the islets was also observed in the mutant pancreas (Fig. 7A, bottom left). The Majority of these BrdU-positive/insulin-negative-staining cells stained negative for PTEN (Fig. 7B, bottom, inset). Cell death could be detected in the control pancreas in response to STZ treatment, while apoptotic cells were rare in mutant pancreas (Fig. 7C).
FIG. 7.
Pten deletion increases β-cell proliferation, decreases apoptosis, and prevents hyperglycemia in STZ-treated animals. (A) BrdU pulse-labeling of control and mutant islets 7 days after initial STZ treatment (magnifications, ×40 [left] and ×100 [middle]). Red, insulin; green, BrdU. Quantification is shown in the right-hand panel. (B) Colocalization of PTEN and BrdU+ proliferating cells (confocal image magnification, ×60). Red, PTEN; green, BrdU. Insets, higher magnification showing colocolization of BrdU and PTEN in control but not in mutant pancreas. (C) TUNEL staining of control and mutant islets 7 days after initial STZ treatment (magnification, ×100). Red, insulin; green, TUNEL. Quantification is shown in the righthand panel. (D) STZ-induced hyperglycemia. (Left) Glucose levels of six STZ-treated animals. (Right) Percentage of animals free of diabetes after STZ treatment.
As a result of β-cell damage, blood glucose levels increased significantly in the control animals from approximately 120 mg/dl to 400 mg/dl, while the blood glucose levels of mutant animals did not change significantly (from 106 mg/dl to 120 mg/dl) (Fig. 7D, left). Three weeks after STZ treatment, 80% of the control animals developed hyperglycemia (random glucose level, >300 mg/dl). No mutant animals (n = 6) developed hyperglycemia during the entire experimental period of 2 months (Fig. 7D, right). Furthermore, the glucose levels of mutant animals remained at 120 mg/dl for the remainder of the 2 months of the experimental period. This normal glucose level in Pten mutant mice was consistent with the healthy islet morphology observed in the mutant pancreas and suggests that PTEN loss could efficiently protect β cells from STZ-induced injury and maintain glucose homeostasis.
Pten deletion in the pancreas protected islets from STZ-induced oxidative stress.
To dissect the mechanism of Pten deletion-induced cytoprotection of β cells, we subjected isolated control and mutant islets to STZ treatment in vitro to avoid the complication of in vivo immune response. Immediately after a 30-min treatment with STZ (2 mM), both control and mutant islets lost responsiveness to glucose stimulation (data not shown). After overnight recovery was permitted, control islets were mostly destroyed, while mutant islets still retained their islet morphology (Fig. 8A), suggesting that PTEN loss protects islets from oxidative stress induced by STZ. To further test whether Pten deletion will protect islets from high-dose STZ (200 mg/kg) treatment in vivo, we examined the mutant pancreas 36 h after STZ treatment, when control animals started to show significant damage (Fig. 8B, top). Deletion of Pten did not protect the animals from the high-dose STZ treatment, as indicated by TUNEL-positive staining (Fig. 8B, bottom). Both control and mutant islets had diminished insulin staining after STZ treatment (data not shown). Thus, although Pten deletion can effectively protect the islet from low-dose STZ treatment, it cannot prevent cell death caused by higher doses of oxidative stress.
FIG. 8.
Pten deletion protects mutant animals from oxidative stress. (A) Isolated islets from control and mutant animals were subjected to 2 mM STZ treatment for 30 min. Islets were allowed to recover overnight. Images were taken the next morning. Images are representative of three experiments. (B) TUNEL staining of pancreatic sections from animals treated with a single dose of 200 mg STZ/kg for 36 h. Experiment used two animals per genotype group.
DISCUSSION
Replication of differentiated β cells and neogenesis of progenitor cells are two possible processes that control β-cell production and β-cell mass. However, little is known about the molecular mechanisms governing these two processes. We demonstrate here that PTEN phosphatase plays a critical role in modulating β-cell growth both during pancreatic development and in adulthood. Pten deletion in β cells results in increased cell proliferation and decreased apoptosis during embryonic development and neonatal remodeling when neogenesis plays a predominant role in regulating pancreatic β-cell production and mass. In the adult pancreas, when replication of differentiated β cells is the major mechanism for β-cell regeneration, PTEN also plays an important role in modulating injury response and postinjury recovery of the β cells. Our study provides confirmation of earlier studies suggesting that increase of endogenous PI3K and AKT activity leads to increased β-cell mass and protects β cells from STZ-induced diabetes (3, 36, 52). The results from this study imply that PTEN and PTEN-controlled signaling pathways are potential therapeutic targets for β-cell protection and regeneration.
The PI3K/AKT pathway is a common pathway used by a variety of growth factors to stimulate cell growth and survival. Growth factors, such as platelet-derived growth factor, IGF, and insulin, have been shown to play roles in β-cell neogenesis, as well as in regeneration (4). Deletion of igf-1 and igf-2 and their receptors leads to impaired β-cell growth and glucose-stimulated insulin secretion (26). In vitro stimulation of insulin, IGF, and glucagon-like peptide 1 leads to activation of PI3K/AKT pathways in cultured β cells (8, 32). In animal models, overexpression of IRS2 and the constitutive active form of AKT-1, one of the AKT isoforms, in the β cells results in increased islet size (3, 36, 52), while deletion of AKT-2 causes insulin resistance and diabetes (11). Expression of a dominant negative form of AKT, which may inhibit all AKT activities, attenuates insulin secretion and sensitizes animals to experimental diabetes (2). Together, these studies suggest that PI3K/AKT signaling pathway is important in mediating both pancreatic β-cell proliferation and function.
Compared to the aforementioned AKT models, our Pten β-cell deletion model differs in two respects. First, we did not observe significant changes in β-cell size; second, the mutant β cells appeared to secrete insulin in a manner similar to that of the control islets. The differences may lie in the existence of the three AKT isoforms, which are known to distribute differently in various tissues and which may play overlapping and distinct biological functions. PTEN lies upstream of AKT, and PTEN loss can lead to activation of all three AKT isoforms, as well as other PI3K downstream effectors, which may explain the phenotypic differences observed between Pten knockout mice and those models with a specific AKT isoform activated. Another reason for the apparent cell size differences may be the way these animals are generated. The transgenic animals overexpressing AKT are generated with membrane myristylation-tagged AKT constructs. Our mutant animals test the loss of function effect of PTEN efficiency induced AKT activation, thereby, testing the physiological activation of the PI3K/AKT pathway. Although subtle increases of cell size are observed under high-power microscopy, the single-cell-based FACS assay, which more accurately reflects cell size without interference from surrounding cells, suggests no significant changes in cell size after PTEN loss. Finally, it is now well accepted that activation of the PI3K pathway (at least in epithelial cells) leads to enlarged cell size (23, 49). However, several transgenic lines targeted to the β cells did not result in the same phenotype (14, 36). The mechanism for β-cell size control may not entirely rely on PI3 kinase activation. Unlike many of the topical epithelial cells, β cells contain secretory vesicles that may alter cell size. The enlarged cell size in one of the AKT transgenic lines showing increased insulin secretory potential may be due to increased secretory vesicle storage in the cells (3). The exact mechanism for β-cell size control remains to be determined.
The increased islet mass phenotype is observed during embryo development, as well as in the adult pancreas. During embryonic development, neogenesis leads to increased β-cell numbers, resulting in islet formation. PTEN/AKT may regulate this neogenesis process by modulating the subcellular localization of Foxa-2, a critical transcriptional factor for pancreas development (53). Alternatively, PTEN may modulate pancreatic stem/progenitor cell self-renewal, proliferation, and survival, similar to its function in regulating neural stem cells (21). The identity of pancreatic or islet stem/progenitor cells in the adult stage is currently unknown. However, we did observe that mice with Pten β-cell deletion have increased cell proliferation in both insulin-producing and adjacent non-insulin-producing cells during both pancreatic development and post-STZ treatment. Whether these are the bona fide islet progenitor/stem cells or some β cells that have undergone dedifferentiation remains to be resolved. It is unlikely that phenotypes described in this study are due to leakage in the embryonic pancreatic progenitors, since Pten deletion in pancreatic-duodenal homeobox gene 1-positive embryonic progenitors resulted in the pancreatic adenocarcinoma phenotype, in conjunction with increased islet mass (47). Therefore, the RIP-Cre-Pten model most likely targeted to a different cell population than the pancreatic-duodenal homeobox gene 1 model we reported earlier.
While enhanced β-cell proliferation most likely contributes to increased islet mass, cells peripheral to the islets may also proliferate and differentiate to insulin-expressing cells and contribute to overall islet mass. The low-dose STZ treatment induces an inflammatory response that may contribute both cells and stimulatory factors to the injury sites. Mobilized bone marrow cells may be such cells. At these sites, they may differentiate or fuse with existing β cells to increase the β-cell pool (24, 25, 27). Furthermore, the presence of these immune cells may induce an epithelial-mesenchymal transition through a transforming growth factor β-mediated pathway (37). Recent data suggests that human β cells have the capacity to dedifferentiate through epithelial-mesenchymal transition to mesenchyma-like cells with no insulin production (19). These mesenchyma-like cells retain the ability to differentiate to insulin-producing cells (9, 19). It will be interesting to study whether Pten deletion can protect β cells from immunity-induced cell death and thus promote the survivor cells to become mesenchymal cells in response to transforming growth factor β stimulation.
Self-renewal of β cells is proposed to be the mechanism for β-cell regeneration in the adult stage. This replication of β cells is subjected to cell cycle regulation. When cyclin D2 is deleted, β-cell replication is significantly hindered in the postnatal pancreas (18). As a result, cyclin D2-deficient mice had fourfold-less islet mass than the control mice. However, deletion of cyclin D2 in other tissues, especially those insulin response organs, complicated the interpretation of data in this case. PTEN and PTEN-regulated pathways regulate a number of cell cycle regulators, including the D-type cyclins, p27, and p21 (44, 48, 51, 55). We showed that expression of p27, a G1-S cell cycle inhibitor, is significantly decreased upon Pten deletion. Together with enhanced S6K activity, this downregulation of p27 may enhance cell cycle progression, induce β cells to proliferation, and increase the β-cell self-renewal rate, especially in response to injury. Increased β-cell proliferation in adults is, at least in part, responsible for the enlarged islet size observed in the mutant pancreas. These enlarged islets might go through a fission process to generate more islets (45).
Of note, as a result of Pten deletion, the mutant mice were significantly smaller (see Fig. S1 in the supplemental material) and had shortened life spans compared to those of PtenloxP/loxP;RIP-Cre− mice. This phenotype is most likely due to RIP-Cre expression (therefore, Pten deletion) in the hypothalamus of the embryonic brain (17). Compared to green fluorescent protein and saline controls, deletion of Pten in the adult hypothalamus through stereotactic injection of Cre-expressing adenovirus did not cause dramatic changes in body weight, food intake, or glucose and lipid indexes (data not shown; see Fig. S2 in the supplemental material). Contrarily, these animals showed slightly increased food intake and body weight, compared to the controls (see Fig. S2 in the supplemental material). β-cell Pten deletion did not seem to affect food intake (data not shown). Furthermore, inducing Pten deletion in the adult pancreas, using RIP-CreER transgenic mice, which express Cre recombinase only when infected with tamoxifen, did not alter the body weight of the animals either (data not shown). The RIP-Cre Pten deletion model did not develop insulinomas in the limited number of older (12- to 15-month-old) animals (see Fig. S3 in the supplemental material; three mice) we have observed. Therefore, insulinoma is a later event, if it does occur.
In our study, we showed that PTEN loss in the developing pancreas leads to increased islet number and size, as well as overall β-cell mass. PTEN loss does not appear to change the normal course of cellular differentiation, as evident by the expression of β-cell-specific markers such as insulin, glucagon, somatostatin, pancreatic polypeptide, and GLUT2. The morphology of the mutant islets is not altered, and their response to glucose stimulation is indistinguishable from that of the controls. PTEN loss enhances survival and replication of β cells following chemically induced β-cell injury. It is not clear whether this effect is solely due to PTEN loss in the β cells or if as-yet-unidentified pancreatic progenitor cells are also involved. Furthermore, whether adult-onset Pten deletion can also protect β cells from injury and induce their proliferation remains to be tested. Nevertheless, our results imply strongly that PTEN and the PTEN-controlled signaling pathway play important roles in β-cell development, function, survival, and regeneration.
Supplementary Material
Acknowledgments
We thank Eric Kremer from the Institut de Genetique Moleculaire de Montpellier, France, for providing the Cre adenovirus. We thank Michael Mendoza, Yuval Dor, Ben Stanger, and colleagues in our laboratory for helpful suggestions and discussion in preparing the manuscript. We also thank Bryan Stiles for his help in proofreading the paper.
B.L.S. acknowledges support from the Department of Defense; this work is also partially supported by grants from NIH: UO1 CA84128-06 and RO1 CA107166 to H.W.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aikin, R., L. Rosenberg, and D. Maysinger. 2000. Phosphatidylinositol 3-kinase signaling to Akt mediates survival in isolated canine islets of Langerhans. Biochem. Biophys. Res. Commun. 277:455-461. [DOI] [PubMed] [Google Scholar]
- 2.Bernal-Mizrachi, E., S. Fatrai, J. D. Johnson, M. Ohsugi, K. Otani, Z. Han, K. S. Polonsky, and M. A. Permutt. 2004. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J. Clin. Investig. 114:928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernal-Mizrachi, E., W. Wen, S. Stahlhut, C. M. Welling, and M. A. Permutt. 2001. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Investig. 108:1631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard-Kargar, C., and A. Ktorza. 2001. Endocrine pancreas plasticity under physiological and pathological conditions. Diabetes 50(Suppl. 1):S30-S35. [DOI] [PubMed] [Google Scholar]
- 5.Bonner-Weir, S., L. A. Baxter, G. T. Schuppin, and F. E. Smith. 1993. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes 42:1715-1720. [DOI] [PubMed] [Google Scholar]
- 6.Bonner-Weir, S., D. Deery, J. L. Leahy, and G. C. Weir. 1989. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes 38:49-53. [DOI] [PubMed] [Google Scholar]
- 7.Brockenbrough, J. S., G. C. Weir, and S. Bonner-Weir. 1988. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes 37:232-236. [DOI] [PubMed] [Google Scholar]
- 8.Buteau, J., R. Roduit, S. Susini, and M. Prentki. 1999. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia 42:856-864. [DOI] [PubMed] [Google Scholar]
- 9.Chen, L. B., X. B. Jiang, and L. Yang. 2004. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J. Gastroenterol. 10:3016-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, W., K. V. Salojin, Q. S. Mi, M. Grattan, T. C. Meagher, P. Zucker, and T. L. Delovitch. 2004. Insulin-like growth factor (IGF)-I/IGF-binding protein-3 complex: therapeutic efficacy and mechanism of protection against type 1 diabetes. Endocrinology 145:627-638. [DOI] [PubMed] [Google Scholar]
- 11.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 12.Contreras, J. L., C. A. Smyth, G. Bilbao, C. J. Young, J. A. Thompson, and D. E. Eckhoff. 2002. Simvastatin induces activation of the serine-threonine protein kinase AKT and increases survival of isolated human pancreatic islets. Transplantation 74:1063-1069. [DOI] [PubMed] [Google Scholar]
- 13.da Silva Xavier, G., A. Varadi, E. K. Ainscow, and G. A. Rutter. 2000. Regulation of gene expression by glucose in pancreatic beta-cells (MIN6) via insulin secretion and activation of phosphatidylinositol 3′-kinase. J. Biol. Chem. 275:36269-36277. [DOI] [PubMed] [Google Scholar]
- 14.Devedjian, J. C., M. George, A. Casellas, A. Pujol, J. Visa, M. Pelegrin, L. Gros, and F. Bosch. 2000. Transgenic mice overexpressing insulin-like growth factor-II in beta cells develop type 2 diabetes. J. Clin. Investig. 105:731-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dor, Y., J. Brown, O. I. Martinez, and D. A. Melton. 2004. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41-46. [DOI] [PubMed] [Google Scholar]
- 16.Finegood, D. T., L. Scaglia, and S. Bonner-Weir. 1995. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44:249-256. [DOI] [PubMed] [Google Scholar]
- 17.Gannon, M., C. Shiota, C. Postic, C. V. Wright, and M. Magnuson. 2000. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis 26:139-142. [DOI] [PubMed] [Google Scholar]
- 18.Georgia, S., and A. Bhushan. 2004. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J. Clin. Investig. 114:963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gershengorn, M. C., A. A. Hardikar, C. Wei, E. Geras-Raaka, B. Marcus-Samuels, and B. M. Raaka. 2004. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science 306:2261-2264. [DOI] [PubMed] [Google Scholar]
- 20.Green, I. C., S. El Seifi, D. Perrin, and S. L. Howell. 1981. Cell replication in the islets of langerhans of adult rats: effects of pregnancy, ovariectomy and treatment with steroid hormones. J. Endocrinol. 88:219-224. [DOI] [PubMed] [Google Scholar]
- 21.Groszer, M., R. Erickson, D. D. Scripture-Adams, R. Lesche, A. Trumpp, J. A. Zack, H. I. Kornblum, X. Liu, and H. Wu. 2001. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294:2186-2189. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann, K., W. Besch, and H. Zuhlke. 1989. Spontaneous recovery of streptozotocin diabetes in mice. Exp. Clin. Endocrinol. 93:225-230. [DOI] [PubMed] [Google Scholar]
- 23.Hay, N. 2005. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8:179-183. [DOI] [PubMed] [Google Scholar]
- 24.Hess, D., L. Li, M. Martin, S. Sakano, D. Hill, B. Strutt, S. Thyssen, D. A. Gray, and M. Bhatia. 2003. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat. Biotechnol. 21:763-770. [DOI] [PubMed] [Google Scholar]
- 25.Ianus, A., G. G. Holz, N. D. Theise, and M. A. Hussain. 2003. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J. Clin. Investig. 111:843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kido, Y., J. Nakae, M. L. Hribal, S. Xuan, A. Efstratiadis, and D. Accili. 2002. Effects of mutations in the insulin-like growth factor signaling system on embryonic pancreas development and beta-cell compensation to insulin resistance. J. Biol. Chem. 277:36740-36747. [DOI] [PubMed] [Google Scholar]
- 27.Kodama, S., W. Kuhtreiber, S. Fujimura, E. A. Dale, and D. L. Faustman. 2003. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science 302:1223-1227. [DOI] [PubMed] [Google Scholar]
- 28.Kurlawalla-Martinez, C., B. Stiles, Y. Wang, S. U. Devaskar, B. B. Kahn, and H. Wu. 2005. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol. Cell. Biol. 25:2498-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kushner, J. A., L. Simpson, L. M. Wartschow, S. Guo, M. M. Rankin, R. Parsons, and M. F. White. 2005. Phosphatase and tensin homolog regulation of islet growth and glucose homeostasis. J. Biol. Chem. 280:39388-39393. [DOI] [PubMed] [Google Scholar]
- 30.Lesche, R., M. Groszer, J. Gao, Y. Wang, A. Messing, H. Sun, X. Liu, and H. Wu. 2002. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32:148-149. [DOI] [PubMed] [Google Scholar]
- 31.Like, A. A., and A. A. Rossini. 1976. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 193:415-417. [DOI] [PubMed] [Google Scholar]
- 32.Lingohr, M. K., R. Buettner, and C. J. Rhodes. 2002. Pancreatic beta-cell growth and survival—a role in obesity-linked type 2 diabetes? Trends Mol. Med. 8:375-384. [DOI] [PubMed] [Google Scholar]
- 33.Liu, W., C. Chin-Chance, E. J. Lee, and W. L. Lowe, Jr. 2002. Activation of phosphatidylinositol 3-kinase contributes to insulin-like growth factor I-mediated inhibition of pancreatic beta-cell death. Endocrinology 143:3802-3812. [DOI] [PubMed] [Google Scholar]
- 34.Maeda, H., K. G. Rajesh, R. Suzuki, and S. Sasaguri. 2004. Epidermal growth factor and insulin inhibit cell death in pancreatic beta cells by activation of PI3-kinase/AKT signaling pathway under oxidative stress. Transplant. Proc. 36:1163-1165. [DOI] [PubMed] [Google Scholar]
- 35.Maehama, T., and J. E. Dixon. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273:13375-13378. [DOI] [PubMed] [Google Scholar]
- 36.Mohanty, S., G. A. Spinas, K. Maedler, R. A. Zuellig, R. Lehmann, M. Y. Donath, T. Trub, and M. Niessen. 2005. Overexpression of IRS2 in isolated pancreatic islets causes proliferation and protects human beta-cells from hyperglycemia-induced apoptosis. Exp. Cell Res. 303:68-78. [DOI] [PubMed] [Google Scholar]
- 37.Moustakas, A., K. Pardali, A. Gaal, and C. H. Heldin. 2002. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol. Lett. 82:85-91. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien, B. A., B. V. Harmon, D. P. Cameron, and D. J. Allan. 1996. Beta-cell apoptosis is responsible for the development of IDDM in the multiple low-dose streptozotocin model. J. Pathol. 178:176-181. [DOI] [PubMed] [Google Scholar]
- 39.Parsons, J. A., A. Bartke, and R. L. Sorenson. 1995. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology 136:2013-2021. [DOI] [PubMed] [Google Scholar]
- 40.Parsons, J. A., T. C. Brelje, and R. L. Sorenson. 1992. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 130:1459-1466. [DOI] [PubMed] [Google Scholar]
- 41.Pende, M., S. C. Kozma, M. Jaquet, V. Oorschot, R. Burcelin, Y. Le Marchand-Brustel, J. Klumperman, B. Thorens, and G. Thomas. 2000. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature 408:994-997. [DOI] [PubMed] [Google Scholar]
- 42.Perren, A., P. Komminoth, P. Saremaslani, C. Matter, S. Feurer, J. A. Lees, P. U. Heitz, and C. Eng. 2000. Mutation and expression analyses reveal differential subcellular compartmentalization of PTEN in endocrine pancreatic tumors compared to normal islet cells. Am. J. Pathol. 157:1097-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Postic, C., M. Shiota, and M. A. Magnuson. 2001. Cell-specific roles of glucokinase in glucose homeostasis. Recent Prog. Horm. Res. 56:195-217. [DOI] [PubMed] [Google Scholar]
- 44.Radu, A., V. Neubauer, T. Akagi, H. Hanafusa, and M. M. Georgescu. 2003. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol. Cell. Biol. 23:6139-6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seymour, P. A., W. R. Bennett, and J. M. Slack. 2004. Fission of pancreatic islets during postnatal growth of the mouse. J. Anat. 204:103-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srinivasan, S., E. Bernal-Mizrachi, M. Ohsugi, and M. A. Permutt. 2002. Glucose promotes pancreatic islet beta-cell survival through a PI 3-kinase/Akt-signaling pathway. Am. J. Physiol. Endocrinol. Metab. 283:E784-E793. [DOI] [PubMed] [Google Scholar]
- 47.Stanger, B. Z., B. Stiles, G. Y. Lauwers, N. Bardeesy, M. Mendoza, Y. Wang, A. Greenwood, K. H. Cheng, M. McLaughlin, D. Brown, R. A. Depinho, H. Wu, D. A. Melton, and Y. Dor. 2005. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell 8:185-195. [DOI] [PubMed] [Google Scholar]
- 48.Stiles, B., V. Gilman, N. Khanzenzon, R. Lesche, A. Li, R. Qiao, X. Liu, and H. Wu. 2001. The essential role of AKT-1/protein kinase Bα in PTEN-controlled tumorigenesis. Mol. Cell. Biol. 22:3842-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stiles, B., M. Groszer, S. Wang, J. Jiao, and H. Wu. 2004. PTENless means more. Dev. Biol. 273:175-184. [DOI] [PubMed] [Google Scholar]
- 50.Stiles, B., Y. Wang, A. Stahl, S. Bassilian, W. P. Lee, Y. J. Kim, R. Sherwin, S. Devaskar, R. Lesche, M. A. Magnuson, and H. Wu. 2004. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity. Proc. Natl. Acad. Sci. USA 101:2082-2087. (Erratum, 101:5180.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun, H., R. Lesche, D. M. Li, J. Liliental, H. Zhang, J. Gao, N. Gavrilova, B. Mueller, X. Liu, and H. Wu. 1999. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc. Natl. Acad. Sci. USA 96:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tuttle, R. L., N. S. Gill, W. Pugh, J. P. Lee, B. Koeberlein, E. E. Furth, K. S. Polonsky, A. Naji, and M. J. Birnbaum. 2001. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 7:1133-1137. [DOI] [PubMed] [Google Scholar]
- 53.Wolfrum, C., D. Besser, E. Luca, and M. Stoffel. 2003. Insulin regulates the activity of forkhead transcription factor Hnf-3β/Foxa-2 by Akt-mediated phosphorylation and nuclear/cytosolic localization. Proc. Natl. Acad. Sci. USA 100:11624-11629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wrede, C. E., L. M. Dickson, M. K. Lingohr, I. Briaud, and C. J. Rhodes. 2002. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1). J. Biol. Chem. 277:49676-49684. [DOI] [PubMed] [Google Scholar]
- 55.Wu, R. C., X. Li, and A. H. Schonthal. 2000. Transcriptional activation of p21WAF1 by PTEN/MMAC1 tumor suppressor. Mol. Cell Biochem. 203:59-71. [DOI] [PubMed] [Google Scholar]
- 56.Wu, X., K. Senechal, M. S. Neshat, Y. E. Whang, and C. L. Sawyers. 1998. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 95:15587-15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.