Abstract
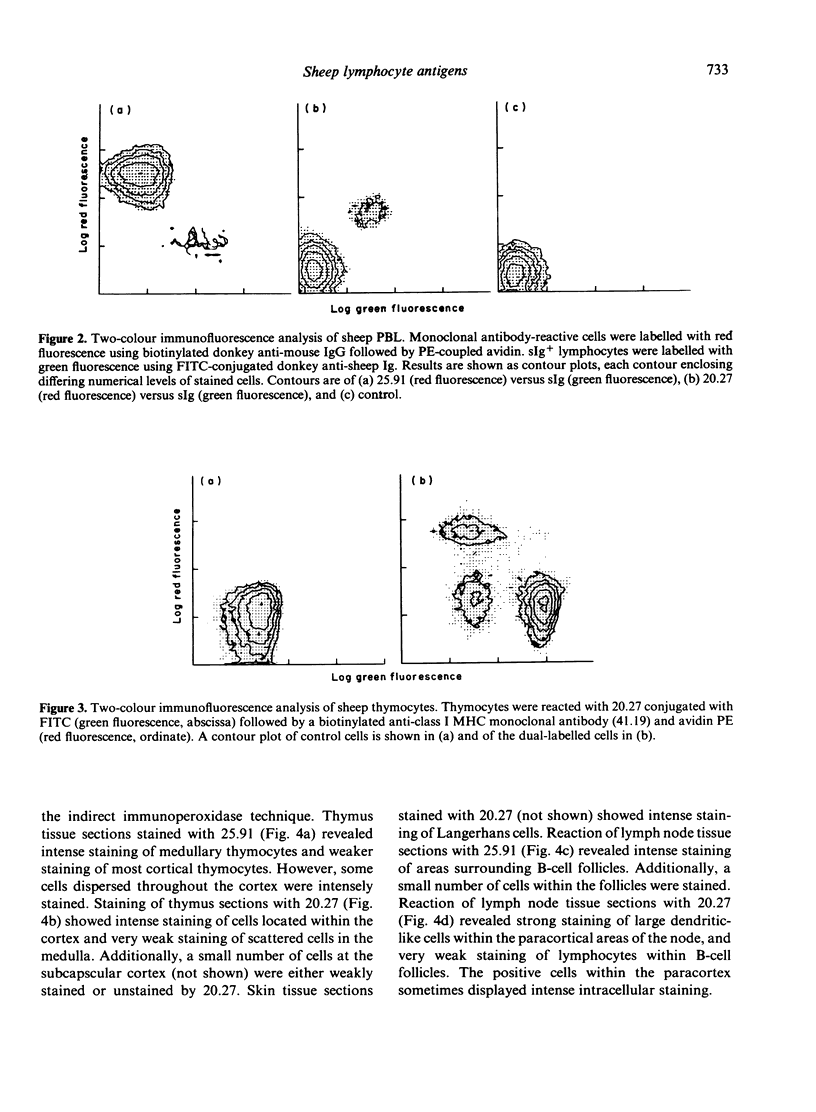
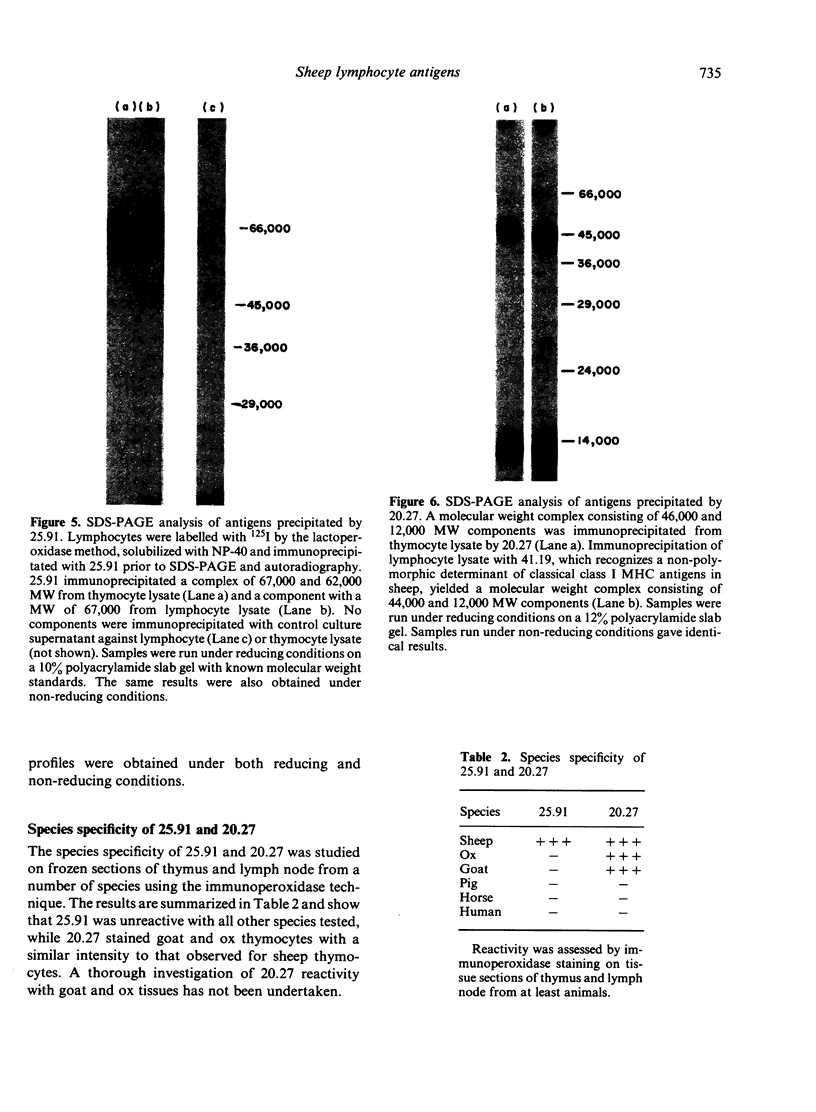
The monoclonal antibodies 25.91 and 20.27 define two lymphocyte cell surface antigens of sheep. 25.91 is reactive with 60-80% of lymphocytes and 98% of thymocytes, and only stains surface immunoglobulin-negative peripheral lymphocytes. 25.91 immuno-precipitates a 67,000 MW protein from lymphocyte lysates under both reducing and non-reducing conditions, whereas immunoprecipitation of thymocyte lysates reveals a 67,000, 62,000 MW complex. The tissue distribution and molecular weight analysis reported here for the antigen recognized by 25.91 indicate that this antigen is the sheep homologue of the human T1 and mouse Ly 1 antigens. The monoclonal antibody 20.27 is reactive with 80% of thymocytes and the majority of cell surface immunoglobulin-positive peripheral blood lymphocytes (B cells), but is unreactive with peripheral blood T cells. 20-27 also stains Langerhans cells in skin tissue sections and large dendritic-like cells in the paracortex sections and large dendritic-like cells in the paracortex of lymph node tissue sections. Immunoperoxidase staining of thymus tissue sections with 20.27 shows intense staining of cortical thymocytes and an absence of staining within the medulla. Molecular weight analysis of the 20.27 antigen reveals two major bands of 46,000 and 12,000 MW under both reducing and non-reducing conditions. The 20.27 antigen has properties resembling MHC class I-like antigens such as T6 in the human and TL in the mouse.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhan A. K., Reinherz E. L., Poppema S., McCluskey R. T., Schlossman S. F. Location of T cell and major histocompatibility complex antigens in the human thymus. J Exp Med. 1980 Oct 1;152(4):771–782. doi: 10.1084/jem.152.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman M. J., Thomas M. L., Green J. R. MRC OX-19: a monoclonal antibody that labels rat T lymphocytes and augments in vitro proliferative responses. Eur J Immunol. 1984 Mar;14(3):260–267. doi: 10.1002/eji.1830140311. [DOI] [PubMed] [Google Scholar]
- Fithian E., Kung P., Goldstein G., Rubenfeld M., Fenoglio C., Edelson R. Reactivity of Langerhans cells with hybridoma antibody. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2541–2544. doi: 10.1073/pnas.78.4.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Structural studies of murine lymphocyte surface IgD. J Immunol. 1980 May;124(5):2082–2088. [PubMed] [Google Scholar]
- HALL J. G., MORRIS B. The output of cells in lymph from the popliteal node of sheep. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:360–369. doi: 10.1113/expphysiol.1962.sp001620. [DOI] [PubMed] [Google Scholar]
- Knowles R. W., Bodmer W. F. A monoclonal antibody recognizing a human thymus leukemia-like antigen associated with beta 2-microglobulin. Eur J Immunol. 1982 Aug;12(8):676–681. doi: 10.1002/eji.1830120810. [DOI] [PubMed] [Google Scholar]
- LASCELLES A. K., MORRIS B. Surgical techniques for the collection of lymph from unanaesthetized sheep. Q J Exp Physiol Cogn Med Sci. 1961 Jul;46:199–205. doi: 10.1113/expphysiol.1961.sp001536. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. J., Hansen J. A., Nowinski R. C., Brown M. A. A new human T-cell differentiation antigen: unexpected expression on chronic lymphocytic leukemia cells. Immunogenetics. 1980;11(5):429–439. doi: 10.1007/BF01567812. [DOI] [PubMed] [Google Scholar]
- Miyasaka M., Heron I., Dudler L., Cahill R. N., Forni L., Knaak T., Trnka Z. Studies on the differentiation of T lymphocytes in sheep. I. Recognition of a sheep T-lymphocyte differentiation antigen by a monoclonal antibody T-80. Immunology. 1983 Jul;49(3):545–553. [PMC free article] [PubMed] [Google Scholar]
- Pescovitz M. D., Lunney J. K., Sachs D. H. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984 Jul;133(1):368–375. [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., McCluskey R. T., Schlossman S. F. Distribution of T cell subsets in human lymph nodes. J Exp Med. 1981 Jan 1;153(1):30–41. doi: 10.1084/jem.153.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg-Sinakin I., Thorbecke G. J., Baer R. L., Rosenthal S. A., Berezowsky V. Antigen-bearing langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell Immunol. 1976 Aug;25(2):137–151. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- Terhorst C., van Agthoven A., LeClair K., Snow P., Reinherz E., Schlossman S. Biochemical studies of the human thymocyte cell-surface antigens T6, T9 and T10. Cell. 1981 Mar;23(3):771–780. doi: 10.1016/0092-8674(81)90441-4. [DOI] [PubMed] [Google Scholar]
- Vitetta E., Uhr J. W., Boyse E. A. Isolation and characterization of H-2 and TL alloantigens from the surface of mouse lymphocytes. Cell Immunol. 1972 Jun;4(2):187–191. doi: 10.1016/0008-8749(72)90019-6. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Good R. A., Ammirati P., Dymbort G., Evans R. L. Identification of a p69,71 complex expressed on human T cells sharing determinants with B-type chronic lymphatic leukemic cells. J Exp Med. 1980 Jun 1;151(6):1539–1544. doi: 10.1084/jem.151.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]
- van Ewijk W., van Soest P. L., van den Engh G. J. Fluorescence analysis and anatomic distribution of mouse T lymphocyte subsets defined by monoclonal antibodies to the antigens Thy-1, Lyt-1, Lyt-2, and T-200. J Immunol. 1981 Dec;127(6):2594–2604. [PubMed] [Google Scholar]
- van de Rijn M., Lerch P. G., Knowles R. W., Terhorst C. The thymic differentiation markers T6 and M241 are two unusual MHC class I antigens. J Immunol. 1983 Aug;131(2):851–855. [PubMed] [Google Scholar]





