Abstract
Formation of NADP+ from NAD+ is catalyzed by NAD kinase (NadK; EC 2.7.1.23). Evidence is presented that NadK is the only NAD kinase of Salmonella enterica and is essential for growth. NadK is inhibited allosterically by NADPH and NADH. Without effectors, NadK exists as an equilibrium mixture of dimers and tetramers (KD = 1.0 ± 0.8 mM) but is converted entirely to tetramers in the presence of the inhibitor NADPH. Comparison of NadK kinetic parameters with pool sizes of NADH and NADPH suggests that NadK is substantially inhibited during normal growth and, thus, can increase its activity greatly in response to temporary drops in the pools of inhibitory NADH and NADPH. The primary inhibitor is NADPH during aerobic growth and NADH during anaerobic growth. A model is proposed in which variation of NadK activity is central to the adjustment of pyridine nucleotide pools in response to changes in aeration, oxidative stress, and UV irradiation. It is suggested that each of these environmental factors causes a decrease in the level of reduced pyridine nucleotides, activates NadK, and increases production of NADP(H) at the expense of NAD(H). Activation of NadK may constitute a defensive response that resists loss of protective NADPH.
Keywords: NAD metabolism, pyridine nucleotides, metabolic control, NADP synthesis, sedimentation equilibrium
The pyridine nucleotide NADP+ is synthesized by 2′-phosphorylation of NAD+ (EC 2.7.1.23). The responsible enzyme, an NAD kinase (NadK), is expected to be essential because NADPH supports reductive biosynthetic reactions and defends cells against oxidative stress (1–3). NADH carries electrons from oxidative catabolic reactions and is toxic during oxidative stress, because it contributes electrons for iron reduction and formation of damaging hydroxyl radicals from H2O2 (4–6). In contrast, NADPH does not contribute to iron reduction but protects against stress by providing electrons for reductive repair and synthesis of deoxynucleotides. In other aspects of metabolism, NAD+ activates single-strand ends in DNA ligation (7, 8), provides ribose in the synthesis of B12 (9), donates ADP-ribose moieties in protein modification (10), and accepts acetyl moieties during protein deacetylation (11). Because NAD(H) and NADP(H) play a variety of distinct physiological roles, their levels are likely to be subject to control and NadK is likely to be a key regulation point.
Evidence for control is the change in NAD and NADP pools seen when growth conditions are varied (12–15). Aeration stimulates destruction and resynthesis of NAD, the pyridine nucleotide cycle (16, 17). After UV irradiation, pools of NAD are lost and pyridine compounds are ultimately dumped into the medium (18). Peroxide treatment of anaerobic cells causes a large increase in NADPH levels with the loss of NADH (19). Especially striking, limitation of de novo pyridine synthesis decreases NAD but not NADP pools (14). It is suggested that all of these phenomena reflect release of NadK from feedback inhibition.
Consistent with its essential role in metabolism, NAD kinase activity has been demonstrated in a variety of organisms (20) and genome sequences include at least one nadK homolog (21–23). (An exception is the genome of the intracellular parasite Chlamidia trachomatis, which has no homolog of any pyridine metabolic gene.) In view of the importance of NADP, it is surprising that null mutations in the single nadK homologue of Mycoplasma were found to be unessential (24) and yeast tolerates simultaneous elimination of all three of its nadK homologs (25–27).
The gene responsible for NADP synthesis was identified only recently because NadK is difficult to purify and no mutants were available. Kawai et al. (28) purified sufficient NAD kinase from Mycobacterium flavus to allow determination of a partial amino acid sequence. This data permitted unambiguous correlation of NAD kinase activity with a particular DNA base sequence (28). Using this information, nadK homologues have been cloned and the kinase characterized from S. cerevisiae (29), E. coli (30), B. subtilis (31), and humans (32).
Evidence is provided that the nadK gene encodes the only NAD kinase in Salmonella enterica and is essential for growth. The NadK enzyme of S. enterica was purified and shown to be similar to that of Escherichia coli (30) in that both show allosteric inhibition by NADPH and NADH. A change in subunit structure is shown to accompany and probably underlie feedback inhibition. This inhibition is central to a model proposed here to explain the changes in NAD and NADP pools that occur in response to aeration, peroxide exposure, UV irradiation, and blockage of pyridine synthesis.
Results
NadK Is Essential for Growth.
In the genomes of S. enterica and E. coli, the yfjB gene is the only close homologue of the initially identified NAD kinase gene from Mycobacterium tuberculosis (28). This homologue encodes the NAD kinase of E. coli (30) and S. enterica (see below) and will be referred to as nadK.
A nadK mutation was constructed in a strain that carries a tandem duplication of the nadK gene (see Materials and Methods). The mutant allele has a chloramphenicol-resistance determinant (CmR) in place of nadK-coding sequence. The constructed heterozygote (TT23196) also carries a normal nadK+ allele with intact control elements. At the duplication join point, this merodiploid strain carries a MudA element with an expressed lacZ+ gene (top of Fig. 1A).
Fig. 1.
NadK is essential. (A) Segregation of a duplication with both nadK+ and nadK:Cm alleles. (B and C) Colonies of duplication strain carrying nadK+ and a nadK:CmR allele (TT23464) growing on rich medium wiith X-Gal (B) or the same medium with added chloramphenicol (C).
To test whether the nadK gene is essential, single cells of the duplication strain were plated on rich medium containing X-Gal (to detect the join point lac+ allele). Duplication segregation (loss of the lac+ join point marker) can be visualized as white (Lac−) sectors in an otherwise blue (Lac+) colony (Fig. 1A). With no chloramphenicol, these haploid segregants, in principle, could retain either the nadK+ or nadK:Cm allele. However, if NadK is essential, only the NadK+, drug-sensitive segregants can grow. In the presence of chloramphenicol, any viable segregant must carry the nadK:Cm allele and would lack NadK activity; these segregants can appear only if NadK is nonessential. Thus, if NadK is essential, segregants (Lac− sectors) should not appear in the presence of chloramphenicol; if NadK is not essential, segregants will form with or without chloramphenicol. As can be seen in Fig. 1 B and C, Lac− segregants appeared only in the absence of chloramphenicol. All of these segregants had lost the mutant nadK:Cm allele (were sensitive to chloramphenicol). We conclude that the nadK+ gene is essential.
The nadK:CmR mutation could not be transduced into a WT haploid recipient but was efficiently transduced into a recipient having either a nadK+ duplication (TT23194) or a plasmid encoding either the tagged or the untagged form of the functional nadK+ gene. These plasmids allowed introduction of nadK:CmR into the chromosome, whether the plasmid nadK gene was induced by isopropyl β-d-thiogalactoside, suggesting that the basal expression level is sufficient for growth.
The essentiality of nadK in Salmonella contrasts with the finding that a nadK insertion mutation was not lethal in Mycoplasma (24). By PCR, the nadK insertion mutant was shown to be present in a cell population subjected to heavy insertional mutagenesis. It is possible that the nadK mutation detected by PCR arose in a Mycoplasma cell with a nadK+ duplication. Insertions in essential genes previously have been obtained in this way (33, 34).
Evidence for a Single NAD Kinase Activity in S. enterica.
Two chromatographically separable NAD kinase activities were reported in E. coli (21) and S. enterica (35). In the course of purifying NadK ≈2,000-fold from WT S. enterica (without overexpression), we have seen only a single activity (data not shown). However, only 10% of the initial activity was recovered, and we were unable to obtain N-terminal sequence. Other investigators have reported similar activity losses and low yields of NAD kinase protein (28, 36). In support of a single NAD kinase, only one band of NAD kinase activity was seen when crude extracts of S. enterica were run on Native-PAGE and stained for NAD kinase activity (Fig. 2). The mobility of this NAD kinase activity corresponded to that of purified NadK kinase activity run on the same gel. The previously reported, chromatographically separable NAD kinase activities may reflect different oligomerization states of the single NadK protein as described below.
Fig. 2.
Evidence for a single NadK enzyme. Crude extracts were fractionated by native PAGE. Each gel was cut, and portions were stained separately for NadK activity or protein (see Materials and Methods). Lanes: 1, 50 mg of crude extract stained with the complete reaction mix; 2, 50 mg of crude extract stained with reaction mix lacking ATP; 3, 3 mg of purified Salmonella NadK-tagged protein stained with Coomassie blue. The assay mix included NAD+, ATP (to allow NADP production by kinase), and NADP-dependent G-6-PD to reduce kinase product to NADPH that, in turn, reduces phenazine methosulphate and iodonitrotetrazolium, producing a colored precipitate. The assay detects a few endogenous NAD-dependent reductases that produce NADH and can be detected even when ATP or G-6-PD is left out of the reaction mixture (data not shown). Only NAD kinase produces a ATP-dependent activity band (by providing NADP+ for included G-6-PD).
Enzyme Characterization.
NAD kinase was assayed in the presence of a variety of metal ions and several phosphate donors. In both E. coli (30) and S. enterica, purified enzyme and NadK activity in crude extracts used ATP and other nucleoside triphosphates as a phosphate donor, but did not use polyphosphate or glucose-6-phosphate (data not shown). In contrast, the M. tuberculosis enzyme (31% identical) uses either polyphosphate or ATP (28), and other bacterial NAD kinases have been reported to use glucose-6-phosphate (37). The Salmonella NadK enzyme uses Mn2+ (255%), Mg2+ (100%), or Zn2+ (117%) as metal ion, as well as Ca2+ (72.3%), Fe2+ (59.8%), and Co2+ (42.6%). Virtually no activity was detected with Cu2+ as the metal ion. In addition, NADH could not replace NAD+ as a substrate. The apparent Km values of the untagged styNadK for ATP and NAD+ (2.7 ± 0.20 mM for ATP and 2.1 ± 0.14 mM, respectively) were very similar to those reported by Kawai et al. (30) for E. coli NAD kinase, ecoNadK. The Km values of the native enzyme for ATP and NAD+ are in the range of in vivo concentrations of these nucleotides, 4.0 mM (38) and 0.9 mM (13), respectively, making the activity sensitive to slight variations in substrate pools. Enzyme with a C-terminal histidine tag showed slightly different Km values (appKm values 6.2 ± 0.1 mM and 4.0 ± 0.3 mM) than those of the untagged protein, consistent with recent evidence that the C-terminal domain of the M. tuberculosis NAD kinase is important for NAD+ recognition (39).
Inhibition of NAD Kinase by NADH and NADPH in Vitro.
A variety of NadK inhibitors, including NADH, NADPH, and quinolinic acid, have been reported for various organisms (31). The NAD kinase of S. enterica (styNadK) is strongly inhibited by NADPH (41% activity at 20 μM) and less strongly by NADH (40% activity at 40 μM). The feedback inhibitor NADPH shifts the NAD+ saturation curve from a typical Michaelis–Menten type to a sigmoidal shape characteristic of allosteric enzymes. The Hill coefficients were 1.5 in the presence of 10 μM NADPH and 2.0 in the presence of 20 μM NADPH. This data for Salmonella is presented as Fig. 4 and Supporting Text, which are published as supporting information on the PNAS web site. Similar kinetic parameters were reported for ecoNadK (30).
Because NadK is likely to be a central control point, other metabolites were tested for inhibitory effects. No inhibition was seen for any of the following (all tested at concentrations up to 0.5 mM): quinolinic acid, nicotinic acid, nicotinamide, nicotinamide mononucleotide, reduced nicotinamide mononucleotide, nicotinic acid mononucleotide, NADP+, AMP, ADP, and 2′5′ ADP.
The Effect of NADPH on the Oligomerization of NadK.
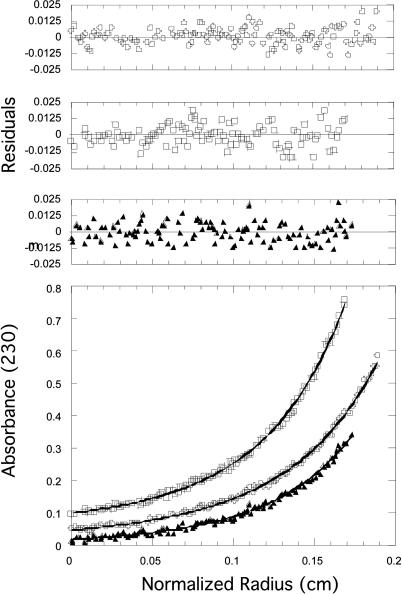
Sedimentation equilibrium analysis was used to assess the subunit structure of purified styNadK (Fig. 3) and ecoNadK (data not shown). In the absence of inhibitors, both proteins were best described by a dimer-tetramer equilibrium, with a KD of 1.0 μM ± 0.9 μM and 0.73 ± 0.5 μM, respectively.
Fig. 3.
Sedimentation equilibrium analysis of styNadK protein. (Lower) Data for three protein concentrations (0.26, 0.13, and 0.065 mg/ml) with the corresponding calculated curve fit (solid line). The data fit a dimer-tetramer equilibrium with a KD = 1.0 ± 0.9 μM. (Upper) The closeness of the fit is evident by the small random residuals.
In the presence of its inhibitor NADPH, styNadK existed entirely as a tetramer as judged by equilibrium sedimentation (molecular mass 145,727 ± 1,000 Da). Simultaneous absorbance and interference data were taken with and without NADPH; however, only the interference data could be used in the presence of this inhibitor because of the high absorbance of NADPH (see Materials and Methods). Without an inhibitor, both absorbance and interference data suggested a dimer-tetramer equilibrium model, with a KD of 0.22 ± 0.06 μM. Thus, feedback inhibition of NadK by NADPH favors the tetrameric state and is likely to be caused by this oligomerization.
Although both styNadK and ecoNadK oligomerize reversibly in the absence of inhibitors (see above), they both appear as a single species when analyzed by gel filtration or native gel electrophoresis, presumably due to the high local protein concentration (data not shown). Our early gel filtration results suggested a tetrameric structure for styNadK, whereas a hexameric structure was reported for the 96% identical E. coli protein (30). The conclusions are likely to reflect different standards used for the interpolation, and the conflict is resolved in favor of tetramers by the equilibrium sedimentation assays (for both ecoNadK and styNadK).
Estimates of in Vivo NAD Kinase Activity.
To estimate the activity of NadK in vivo, purified, untagged styNadK was assayed at the concentrations of substrates and inhibitors found within cells. Because published values for pool sizes vary significantly, these pools were redetermined by the extraction procedure of Lundquist et al. (13), which does not allow oxidation, reduction, or breakdown of nucleotides during sample preparation and is not sensitive to variation in the concentration of cells used. The pool sizes found for S. enterica during aerobic growth (Table 1) agree closely with those determined previously for E. coli by using the same extraction method (13). During aerobic growth on glucose, the NadK substrate NAD+ was present at 0.90 mM, and the inhibitory species were present at 0.16 mM (NADH) and 0.18 mM (NADPH). Published values for ATP levels are 4 mM (38).
Table 1.
Internal pyridine nucleotide pools of S. enterica
| Pyridine nucleotide | Anaerobic* | Aerobic* |
|---|---|---|
| NAD+ | 0.18 ± 0.08 mM | 0.90 ± 0.10 mM |
| NADH | 0.43 ± 0.08 mM (71%)† | 0.16 ± 0.04 mM (14%)† |
| NADP+ | 0.012 ± 0.010 mM | 0.54 ± 0.10 mM |
| NADPH | 0.036 ± 0.005 mM (75%)† | 0.18 ± 0.07 mM (25%)† |
| 0.08 | 0.68 | |
| 2.6 | 0.38 |
*Determined as described in Materials and Methods.
†Percentage of this total nucleotide present in reduced form.
When NadK was assayed in vitro by using substrate concentrations that prevail in vivo (but without inhibitory effectors), purified untagged protein produced 1.6 μmol·min−1·mg−1 NADP+. However, activity dropped 100-fold when inhibitory nucleotides were added at concentrations that prevail during aerobic growth. This drop suggests that NadK is heavily inhibited during normal aerobic growth and, therefore, can increase its activity substantially in response to occasional increases in the level of substrate (NAD+) or decreases in the pool of inhibitory reduced nucleotides (NADH and NADPH).
Evidence for inhibition of NadK in Vivo.
When the NadK protein was overexpressed 1,000-fold from a plasmid, no adverse effects on growth were seen, consistent with the idea that excess NadK enzyme was strongly inhibited. The effect of excess NadK on NAD+ level then was tested more directly by using a nadB:lac fusion, which is known to be repressed by NAD+ (40, 41). Plasmids encoding NadK were introduced into a duplication-bearing strain with a WT nadB+ allele and a nadB:lac fusion allele. Transcription of nadB was measured by assaying β-gal activity produced by the fusion allele. Overexpression of NadK caused no change in nadB transcription (β-gal level) in this strain (data not shown). It seems likely that slight increases in NADP+ (and secondarily in NADPH) inhibit the excess NAD kinase activity and prevent depletion of NAD+ pools or overproduction of NADP+.
Additional evidence for in vivo inhibition of NadK is the surprising observation by Lundquist and Olivera (14), who placed a pyridine-requiring (nadB) mutant of E. coli into unsupplemented minimal medium. With time, the NAD pool dropped, whereas the NADP pool remained almost constant; the NAD:NADP ratio dropped from 2.00 to 0.3. Maintenance of the NADP pool in the face of reduced total pyridine is consistent with the feedback sensitivity of NadK reported here. That is, any marginal decrease in the NADPH level would release NadK from inhibition and maintain NADP(H) levels at the expense of the NAD pool. This response would allow biosynthetic processes to continue in the face of pyridine starvation.
Levels of the Four Pyridine Nucleotides During Anaerobic and Aerobic Growth.
Pool sizes were determined as described in Materials and Methods and are presented in Table 1. During anaerobic growth, the total NADP(H) pool is low compared with those of NAD(H). This ratio is higher during growth in the presence of oxygen, as expected if NadK activity increases (next-to-last line in Table 1). Furthermore, the level of NadK activity would be expected to reflect the ratio of the summed inhibitory pools to the pool of the substrate (NAD+); this ratio is much lower during anaerobic growth than aerobic, suggesting that aerobiosis is accompanied by activation of NadK. Possible biological significance of these changes and the response of these pools to stress are discussed below.
Discussion
A Rationale for the Existence of Two Biochemically Similar Electron Carriers.
It seems likely that living things employ two structurally and biochemically similar electron carriers (NAD and NADP) to control cellular processes. The large NAD+ pool (0.9 mM; 86% of total NAD) seen under aerobic conditions may serve to drive oxidative catabolic reactions that support respiration. The high level of NADH (0.41 mM; 71% of total NAD) seen under anaerobic conditions may support growth when the rate-limiting step in providing energy is reoxidation and recycling of NADH by using electron acceptors that are less avid than oxygen. The NADPH level is lower (0.036 mM) during anaerobic growth when growth is slow and higher (0.18 mM) during more rapid aerobic growth, consistent with the idea that the NADPH level increases to meet the higher demand of reductive biosynthetic reactions when growth is rapid. Thus, one can at least rationalize the observed pool changes in terms of cellular metabolism. Later in this section, we discuss the changes in pyridine nucleotide pools during periods of oxidative stress.
Proposal that NadK Inhibition Controls Pyridine Nucleotide Pool Sizes.
Comparing NadK enzymatic kinetic parameters with in vivo pool sizes suggests that NADH is the primary inhibitor of NadK during anaerobic growth, and NADPH takes over under aerobic conditions. A drop in NADH caused by more rapid aerobic NAD oxidation would release NadK from inhibition (by decreasing NADH level) and provide more NadK substrate (by increasing NAD+). These two changes would combine to increase the NadK-dependent production of NADP. The increase in NADP would drive reactions that reduce NADP and cause a secondary increase in the rate of NADPH production. We suggest that this increased flux allows the cell to keep pace with the demands of reductive biosynthesis and protects against reactive oxygen species. Several lines of evidence support this rationalization.
As expected if NadK is activated during aerobic growth, the ratio of total NADP(H) to total NAD(H) was 8-fold higher under aerobic conditions (next-to-last line in Table 1). Also consistent with aerobic NadK activation, the combined level of inhibitors (NADH plus NADPH) was lower and the level of NAD+ (NadK substrate) was higher during aerobic growth; the ratio of total inhibitors to substrate was 2.6 during anaerobic growth and 0.38 under heavily aerobic conditions (last line in Table 1). These results are consistent with a higher level of NadK activity during aerobic growth.
A Model for Control of Pyridine Pools in Response to Oxidative Stress.
Just as NAD and NADP play different roles in normal cellular metabolism (degradative vs. synthetic reactions), they also play opposite roles during oxidative stress (19). During oxidative stress, NADH is thought to be dangerous because it contributes to reduction of Fe3+ and, hence, to the formation of damaging hydroxy radicals through the Fenton reaction (4–6). In contrast, NADPH does not contribute to iron reduction but defends cells against oxidative stress by supporting reductive repair reactions (1–3). Cells with impaired ability to reduce NADP+ (as in G6PDH mutants) are sensitive to oxidative stress (2, 3, 42, 43).
Oxidative stress is expected to impose a drain on NADH and NADPH because it activates a variety of repair processes that consume reduced pyridine nucleotides [i.e., glutathione reductase (44), alkyl hydroperoxide reductase (45), thioredoxin reductase (46), methionine sulfoxide reductase (47), and NADH-dehydrogenase (3)]. During periods of stress, many enzymes are induced that reduce NADP+ to NADPH (19, 48, 49), so an increase in NADP levels would support the formation of NADPH and compensate for the demands placed on this protective molecule.
The regulation of NadK activity by reduced nucleotides NADH and NADPH allows this enzyme to sense oxidative stress; reduced NADPH has been suggested as an indicator of oxidative stress (50). In response to this signal, NadK would convert NAD+ to NADP+ and, thereby, achieve two useful effects. It would reduce the level of toxic NADH and stimulate maintenance or increase in the pool of protective NADPH. This regulatory response could be initiated by any stress that reduces the pool of NADPH, including exposure to reactive oxygen species, UV irradiation, and aldehyde accumulation. A drop in NADPH after UV exposure seems likely in view of the recent demonstration (in yeast) that DNA damage stimulates ribonucleotide reductase and greatly increases deoxynucleotide production, which uses NADPH as a reducing agent (51). A drop in NADPH in response to aldehyde exposure is predicted based on the requirement for glutathione to permit growth on ethanolamine, which is associated with release of acetaldehyde (52–54).
A Test of the Oxidative Stress Model.
A test of the model is inherent in previous work by Brumaghim et al. (19), who treated anaerobic cells with hydrogen peroxide, imposing a severe oxidative stress. They observed a 90-fold increase in the NADPH:NADH ratio within 15 min; the total NADP(H) level increased 2.5-fold. They saw no increase in NAD kinase activity (assayed under standardized conditions in vitro), suggesting that the nadK gene did not derepress on this time scale. We suggest that the observed increase in NADP was due to the release of NAD kinase from feedback inhibition after marginal decreases in the levels of NADH and NADPH.
Two Additional Stress-Induced Changes in Pyridine Nucleotide Pools.
Feedback inhibition of NadK is likely to be involved in the oxygen-stimulated pyridine nucleotide cycle and the UV-induced loss of NAD pools. Olivera et al. (12, 13, 17) demonstrated that pyridine nucleotides are continuously broken down to nicotinamide mononucleotide (NMN) and recycled to NAD+ by using NMN deamidase and the last two biosynthetic enzymes, NadD and E. Flux through this cycle is independent of DNA ligase and is stimulated ≈4-fold by aeration (16). A possibly related phenomenon is the observation of Swenson et al. (18, 55) that UV irradiation causes E. coli to stop respiring, lose its NAD pools, and release pyridine compounds from the cell. Loss of these pools suggests that de novo pyridine synthesis may be blocked by stress. The pyridine biosynthetic enzyme NadB is known to be oxygen-sensitive (56–58). Another biosynthetic and recycling enzyme (NadE) has sequence motifs that suggest existence of an oxygen-sensitive iron-sulfur cluster. We speculate that heavy stress both blocks de novo NAD synthesis and stimulates nucleotide splitting, as seen in the cycle. Under such conditions, NAD is destroyed by NadK and by splitting of nucleotides to NMN, which can accumulate and escape from the cell as nicotinamide nucleoside (NmR) by using the NmR diffusion facilitator (41). It should be noted that activity of the NAD-dependent repair enzyme DNA ligase can tolerate a severe drop in NAD+ because its Km for NAD+ is surprisingly low (30 nM) (8) compared with the normal NAD+ pool (1 mM).
Comparing Bacterial NadK with Enzymes of Other Organisms.
The NadK kinase of E. coli and S. enterica share a structural fold with a class of distantly related kinases, including 6-phosphofructokinases, diacylglyceride kinases, and sphingosine kinases (59). Interestingly, the structurally related rabbit muscle phosphofructokinase also shows a change in oligomerization state in response to its inhibitor, citrate, which favors the less active dimeric state over the more active tetramer (60).
Although the model presented above is based on data obtained in bacteria, it is likely to be broadly applicable. All organisms share use of NAD and NADP in metabolism and stress response. In yeast, three NadK homologs have been reported and deletion of all three is not lethal (54), suggesting the existence of a nonhomologous NAD kinase. The three yeast enzymes all convert NADH directly to NADPH, achieving in an only slightly different way the decrease in NADH and increase in NADPH inferred to occur in bacteria. Mutants lacking one NadK homologue (Pos5) are sensitive to peroxide (26). Another homologue (Utp1) is needed during growth on low iron to supply NADPH to release metal ions (ferric and cupric) from binding proteins during import (27). Both phenotypes suggest roles of NadK in oxidative stress response consistent with the model proposed here.
Materials and Methods
Construction of a Chromosomal nadK Mutation.
A chromosomal nadK duplication was constructed by two-fragment transduction as described in refs. 41, 61, and 62. One donor carried an argA:MudA (Arg− Lac+ AmpR; Amp, ampicillin) insertion; the other carried a nadB:MudA (Nad− Lac− AmpR) insertion. Transductants (AmpR) made by using a 1:1 mixture of these lysates were screened to identify those with a nadK duplication, which are stably Arg+, Nad+ and unstably Lac+ AmpR (see Fig. 1). One of the two nadK copies was replaced by a CmR by using linear transformation methods (63, 64). The nadK:CmR transformants were verified by PCR and genetic linkage tests.
Cloning the nadK Gene.
The nadK gene (GenBank accession no. 16421206) was amplified by PCR and cloned into vector pTrcHis2-TOPO (Invitrogen) to form plasmids that produce NadK with or without a C-terminal histidine tag (pJG101 or pJG102, respectively). These plasmids carry an ampicillin resistance gene and express NadK from an isopropyl β-d-thiogalactoside-inducible lac promotor.
Expression and Purification of NadK Enzyme.
To prepare tagged or untagged NadK protein, cells carrying the appropriate plasmid were grown aerobically to A600 = 0.6 at 37°C on LB medium supplemented with 100 mg/ml ampicillin/1% glucose; cells were transferred to LB Amp medium containing 1 mM isopropyl β-d-thiogalactoside and incubated 8 h to express NadK. Cells were washed in 50 mM phosphate buffer (pH 7.8) with NaCl (50 mM) and frozen at −70°C. Frozen-cell pellets were resuspended in 20 mM phosphate buffer containing 50 mM NaCl and 1 mg/ml lysozyme. After 1 h on ice, cells were disrupted by sonication and debris was removed by centrifugation at 30 min at 20,000 × g.
The histidine-tagged proteins were purified from the above extracts by using the ProBond resin (Invitrogen) and eluted by an imidazole step gradient. The active fractions were concentrated by using a Centriprep 50 device (Amicon), and transferred to storage buffer (50 mM phosphate butter, pH 7.3, containing 0.1 mM ATP/0.3 mM MgCl2/1 mM DTT/20% glycerol). The purified protein remained active for months at −70°C in this buffer. Purity was determined by SDS/PAGE. This procedure gave a 10-fold purification of the 1,225-fold overexpressed-tagged NadK protein. This purified, histidine-tagged NadK protein was used to characterize the enzyme unless otherwise stated.
Untagged NadK was partially purified by 5′ AMP affinity chromatography. After cell lysis, DNA was precipitated by streptomycin sulfate (1.5%). The protein in this supernatant was transferred by dialysis to 100 mM Tris·HCl buffer (pH 7.5) with 10 mM MgCl2/1 mM ATP, loaded onto a 5′ AMP Sepharose column (Amersham Pharmacia Biotech) and eluted with 10 mM NAD+. This fractionation resulted in a 4-fold purification of the 600-fold overexpressed-untagged NadK protein.
NAD Kinase Assay.
NAD kinase was assayed as described by Kornberg (65). The reaction was run at 37°C in a 1.0-ml reaction mixture containing 5.0 mM NAD+, 10 mM MgCl2, 5 mM ATP, and 100 mM Tris·HCl (pH 7.5) and was stopped by heating (100°C for 90 seconds) followed by centrifugation to remove denatured protein. The produced NADP+ was reduced to NADPH and quantified by absorbance at 340 nm; reduction was achieved by adding glucose-6-phosphate (G6P; final concentration 10 mM) and 2 units of yeast glucose-6-phosphate dehydrogenase (G6PDH; Sigma). Metal ions were used at 10 mM.
Determination of Kinetic Parameters.
The NAD kinase activity was assayed (as above) by using concentrations of ATP and NAD+ from 0.5 mM to 5.0 mM. The Km values were determined by fitting a Michaelis–Menten curve to the kinetic data by nonlinear least squares with kaleidagraph (Synergy Software, Reading, PA).
Gel Electrophoresis.
Native PAGE and SDS/PAGE were performed according to Laemmli (66) by using Precast 12% (for SDS/PAGE) and 8–16% (for native PAGE) gradient gels (Gradipore, Frenchs Forest, Australia). For electrophoretic fractionation of native proteins, cells were grown aerobically in LB medium to an OD600 of 0.6, pelleted, and resuspended in phosphate buffer (pH 7.8) containing 0.1 mM ATP, 0.1 mM EDTA, and 1.0 mM DTT. Crude extracts were made by sonication, debris was removed by centrifugation, and the soluble extract was desalted by using a pd10 column (Amersham Pharmacia). Electrophoresis was performed on 50 mg of crude extract protein at 4°C by using Tris/glycine buffer (pH 8.8) containing 1 mM DTT and 0.1 mM ATP.
After electrophoresis, NAD kinase activity was detected by incubating gels at 37°C in a 10-ml staining mixture containing 2.25 ml of nitroblue tetrazolium (NBT; 2 mM), 150 μl of phenazine methosulfate (2.7 mM), 300 μl of G6P (0.1 M), 25 μl of G6PDH (0.5 units/μL), 250 μl of NAD (100 mM), 500 μl of ATP (60 mM), 300 μl of MgCl2 (100 mM), and 1.0 ml of Tris·HCl (1.0 M; pH 7.5). In this reaction mixture, NADP produced by the kinase is reduced to NADPH by G6PD. The resulting NADPH was oxidized by phenazine methosulfate, which then reduces the NBT dye, forming a purple precipitate that marks the position of NAD kinase activity.
Sedimentation Equilibrium.
Sedimentation equilibrium experiments used a Beckman Optima XL-1 analytical ultracentrifuge equipped with scanning absorbance optics and interference optics by using six-channel, 12-mm-thick, charcoal-epon centerpieces at 20°C. Each of the three sample channels contained a different concentration (between 1–20 μM) of protein in 50 mM KPO4 (pH 7.5)/0.15 M NaCl with or without 100 μM NADPH, whereas reference channels contained the dialysate. Equilibrium was confirmed by no change over four 1-h intervals. Data were collected by using both absorbance and interference optics for the samples without NADPH and interference optics only for the sample with NADPH. Values of ν-bar and the extinction coefficients for each protein were calculated from the amino acid sequence as described in ref. 67.
Sedimentation Data Analysis.
Various structural models (absorbance/interference versus radius) were fit to data by using nonlinear least squares via nonlin (68). For the absorbance data, the final model was chosen based on its fit to protein distributions observed in the centrifuge for three to six different initial protein concentrations.
Determination of the Intracellular Pyridine Nucleotide Concentrations.
Duplicate logarithmic cultures of WT S. enterica strain LT2 (TR10000) were grown aerobically in E media with glucose (100 ml of cells in a 1-liter flask with vigorous shaking) or anaerobically (100 ml of cells in a 125-ml flask with minimal shaking). When the culture reached an OD600 = 0.6, pyridine nucleotides were extracted by the method of Olivera and Lundquist (13) by using acid to extract all pyridine nucleotides (oxidized plus reduced) and a base extraction to obtain only the reduced forms (NADH and NADPH). This extraction method allows no oxidation, reduction, or breakdown of nucleotides during the process of cell lysis and extract preparation (13). The relative amounts of NAD+, NADH, NADP+, and NADPH in each extract were then quantified by the enzymatic methods of Zerez et al. (69) by using an NADP+-specific G6PDH and an NAD+-specific yeast alcohol dehydrogenase (Sigma). The intracellular pool sizes were calculated based on cell number at the time of harvest and cell volume as reported by Ingraham et al. (38).
Supplementary Material
Acknowledgments
We thank Baldomero Olivera for many stimulating conversations and advice on preparation of this manuscript. This work was supported in part by National Institutes of Health Grant GM23408.
Abbreviations
- Amp
ampicillin
- CmR
chloramphenicol-resistance determinant.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Pomposiello P. J., Bennik M. H., Demple B. J. Bacteriol. 2001;183:3890–3902. doi: 10.1128/JB.183.13.3890-3902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farr S. B., Kogoma T. Microbiol. Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juhnke H., Krems B., Kotter P., Entian K. D. Mol. Gen. Genet. 1996;252:456–464. doi: 10.1007/BF02173011. [DOI] [PubMed] [Google Scholar]
- 4.Imlay J. A., Chin S. M., Linn S. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 5.Imlay J. A., Linn S. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 6.Woodmansee A. N., Imlay J. A. J. Biol. Chem. 2002;277:34055–34066. doi: 10.1074/jbc.M203977200. [DOI] [PubMed] [Google Scholar]
- 7.Gellert M., Little J. W., Oshinsky C. K., Zimmerman S. B. Cold Spring Harb. Symp. Quant. Biol. 1968;33:21–26. doi: 10.1101/sqb.1968.033.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman S. B., Little J. W., Oshinsky C. K., Gellert M. Proc. Natl. Acad. Sci. USA. 1967;57:1841–1848. doi: 10.1073/pnas.57.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maggio-Hall L. A., Escalante-Semerena J. C. Microbiology. 2003;149:983–990. doi: 10.1099/mic.0.26040-0. [DOI] [PubMed] [Google Scholar]
- 10.Moss J., Garrison S., Oppenheimer N. J., Richardson S. H. J. Biol. Chem. 1979;254:6270–6272. [PubMed] [Google Scholar]
- 11.Starai V. J., Celic I., Cole R. N., Boeke J. D., Escalante-Semerena J. C. Science. 2002;298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- 12.Manlapaz-Fernandez P., Olivera B. M. J. Biol. Chem. 1973;248:5067–5073. [PubMed] [Google Scholar]
- 13.Lundquist R., Olivera B. M. J. Biol. Chem. 1971;246:1107–1116. [PubMed] [Google Scholar]
- 14.Lundquist R., Olivera B. M. J. Biol. Chem. 1973;248:5137–5143. [PubMed] [Google Scholar]
- 15.Wimpenny J. W., Firth A. J. Bacteriol. 1972;111:24–32. doi: 10.1128/jb.111.1.24-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park U. E., Olivera B. M., Hughes K. T., Roth J. R., Hillyard D. R. J. Bacteriol. 1989;171:2173–2180. doi: 10.1128/jb.171.4.2173-2180.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rechsteiner M., Hillyard D., Olivera B. M. J. Cell. Physiol. 1976;88:207–217. doi: 10.1002/jcp.1040880210. [DOI] [PubMed] [Google Scholar]
- 18.Swenson P. A., Schenley R. L. J. Bacteriol. 1970;104:1230–1235. doi: 10.1128/jb.104.3.1230-1235.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brumaghim J. L., Li Y., Henle E., Linn S. J. Biol. Chem. 2003;278:42495–42504. doi: 10.1074/jbc.M306251200. [DOI] [PubMed] [Google Scholar]
- 20.McGuinness E. T., Butler J. R. Int. J. Biochem. 1985;17:1–11. doi: 10.1016/0020-711x(85)90079-5. [DOI] [PubMed] [Google Scholar]
- 21.Filippovich S. I., Afanas'eva T. P., Bachurina G. P., Kritskii M. S. Prikl. Biokhim. Mikrobiol. 2000;36:117–121. [PubMed] [Google Scholar]
- 22.Iwahashi Y., Hitoshio A., Tajima N., Nakamura T. J. Biochem. (Tokyo) 1989;105:588–593. doi: 10.1093/oxfordjournals.jbchem.a122709. [DOI] [PubMed] [Google Scholar]
- 23.Stephan C., Renard M., Montrichard F. Int. J. Biochem. Cell Biol. 2000;32:855–863. doi: 10.1016/s1357-2725(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 24.Hutchison C. A., Peterson S. N., Gill S. R., Cline R. T., White O., Fraser C. M., Smith H. O., Venter C. J. Science. 1999;286:2165–2169. doi: 10.1126/science.286.5447.2165. [DOI] [PubMed] [Google Scholar]
- 25.Lesuisse E., Casteras-Simon M., Labbe P. J. Biol. Chem. 1996;271:13578–13583. doi: 10.1074/jbc.271.23.13578. [DOI] [PubMed] [Google Scholar]
- 26.Krems B., Charizanis C., Entian K. D. Curr. Genet. 1995;27:427–434. doi: 10.1007/BF00311211. [DOI] [PubMed] [Google Scholar]
- 27.Shi F., Kawai S., Mori S., Kono E., Murata K. FEBS J. 2005;272:3337–3349. doi: 10.1111/j.1742-4658.2005.04749.x. [DOI] [PubMed] [Google Scholar]
- 28.Kawai S., Mori S., Mukai T., Suzuki S., Yamada T., Hashimoto W., Murata K. Biochem. Biophys. Res. Commun. 2000;276:57–63. doi: 10.1006/bbrc.2000.3433. [DOI] [PubMed] [Google Scholar]
- 29.Kawai S., Suzuki S., Mori S., Murata K. FEMS Microbiol. Lett. 2001;200:181–184. doi: 10.1111/j.1574-6968.2001.tb10712.x. [DOI] [PubMed] [Google Scholar]
- 30.Kawai S., Mori S., Mukai T., Hashimoto W., Murata K. Eur. J. Biochem. 2001;268:4359–4365. doi: 10.1046/j.1432-1327.2001.02358.x. [DOI] [PubMed] [Google Scholar]
- 31.Garavaglia S., Galizzi A., Rizzi M. J. Bacteriol. 2003;185:4844–4850. doi: 10.1128/JB.185.16.4844-4850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerner F., Niere M., Ludwig A., Ziegler M. Biochem. Biophys. Res. Commun. 2001;288:69–74. doi: 10.1006/bbrc.2001.5735. [DOI] [PubMed] [Google Scholar]
- 33.Anderson P., Roth J. Proc. Natl. Acad. Sci. USA. 1981;78:3113–3117. doi: 10.1073/pnas.78.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth J. R., Benson N., Galitski T., Haack K., Lawrence J., Miesel L. In: Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Neidhardt F., editor. Vol. 2. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 2256–2276. [Google Scholar]
- 35.Cheng W., Roth J. R. J. Bacteriol. 1994;176:4260–4268. doi: 10.1128/jb.176.14.4260-4268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zerez C. R., Moul D. E., Gomez E. G., Lopez V. M., Andreoli A. J. J. Bacteriol. 1987;169:184–188. doi: 10.1128/jb.169.1.184-188.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bark K., Kampfer P., Sponner A., Dott W. FEMS Microbiol. Lett. 1993;107:133–138. doi: 10.1111/j.1574-6968.1993.tb06019.x. [DOI] [PubMed] [Google Scholar]
- 38.Ingraham J. L., Maaloe O., Neidhardt F. C. Growth of the Bacterial Cell. Sunderland, MA: Sinauer; 1983. [Google Scholar]
- 39.Raffaelli N., Finaurini L., Mazzola F., Pucci L., Sorci L., Amici A., Magni G. Biochemistry. 2004;43:7610–7617. doi: 10.1021/bi049650w. [DOI] [PubMed] [Google Scholar]
- 40.Penfound T., Foster J. W. J. Bacteriol. 1999;181:648–655. doi: 10.1128/jb.181.2.648-655.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grose J. H., Bergthorsson U., Roth J. R. J. Bacteriol. 2005;187:2774–2782. doi: 10.1128/JB.187.8.2774-2782.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi K., Tagawa S. FEBS Lett. 1999;451:227–230. doi: 10.1016/s0014-5793(99)00565-7. [DOI] [PubMed] [Google Scholar]
- 43.Lundberg B. E., Wolf R. E., Jr., Dinauer M. C., Xu Y., Fang F. C. Infect. Immun. 1999;67:436–438. doi: 10.1128/iai.67.1.436-438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eisenstark A., Calcutt M. J., Becker-Hapak M., Ivanova A. Free Radical Biol. Med. 1996;21:975–993. doi: 10.1016/s0891-5849(96)00154-2. [DOI] [PubMed] [Google Scholar]
- 45.Storz G., Jacobson F. S., Tartaglia L. A., Morgan R. W., Silveira L. A., Ames B. N. J. Bacteriol. 1989;171:2049–2055. doi: 10.1128/jb.171.4.2049-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mustacich D., Powis G. Biochem. J. 2000;346:1–8. [PMC free article] [PubMed] [Google Scholar]
- 47.Moskovitz J., Rahman M. A., Strassman J., Yancey S. O., Kushner S. R., Brot N., Weissbach H. J. Bacteriol. 1995;177:502–507. doi: 10.1128/jb.177.3.502-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S. M., Koh H. J., Park D. C., Song B. J., Huh T. L., Park J. W. Free Radical Biol. Med. 2002;32:1185–1196. doi: 10.1016/s0891-5849(02)00815-8. [DOI] [PubMed] [Google Scholar]
- 49.Salvemini F., Franze A., Iervolino A., Filosa S., Salzano S., Ursini M. V. J. Biol. Chem. 1999;274:2750–2757. doi: 10.1074/jbc.274.5.2750. [DOI] [PubMed] [Google Scholar]
- 50.Pomposiello P. J., Demple B. Trends Biotechnol. 2001;19:109–114. doi: 10.1016/s0167-7799(00)01542-0. [DOI] [PubMed] [Google Scholar]
- 51.Chabes A., Georgieva B., Domkin V., Zhao X., Rothstein R., Thelander L. Cell. 2003;112:391–401. doi: 10.1016/s0092-8674(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 52.Rondon M. R., Horswill A. R., Escalante-Semerena J. C. J. Bacteriol. 1995;177:7119–7124. doi: 10.1128/jb.177.24.7119-7124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rondon M. R., Kazmierczak R., Escalante-Semerena J. C. J. Bacteriol. 1995;177:5434–5439. doi: 10.1128/jb.177.19.5434-5439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penrod J. T., Roth J. R. J. Bacteriol. 2006;188:2865–2874. doi: 10.1128/JB.188.8.2865-2874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schenley R. L., Swenson P. A., Joshi J. G. Radiat. Res. 1979;79:611–621. [PubMed] [Google Scholar]
- 56.Draczynska-Lusiak B., Brown O. R. Free Radical Biol. Med. 1992;13:689–693. doi: 10.1016/0891-5849(92)90042-f. [DOI] [PubMed] [Google Scholar]
- 57.Gardner P. R., Fridovich I. Arch. Biochem. Biophys. 1991;284:106–111. doi: 10.1016/0003-9861(91)90270-s. [DOI] [PubMed] [Google Scholar]
- 58.Ollagnier-de Choudens S., Loiseau L., Sanakis Y., Barras F., Fontecave M. FEBS Lett. 2005;579:3737–3743. doi: 10.1016/j.febslet.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 59.Labesse G., Douguet D., Assairi L., Gilles A. M. Trends Biochem. Sci. 2002;27:273–275. doi: 10.1016/s0968-0004(02)02093-5. [DOI] [PubMed] [Google Scholar]
- 60.Hesterberg L. K., Lee J. C. Biochemistry. 1982;21:216–222. doi: 10.1021/bi00531a003. [DOI] [PubMed] [Google Scholar]
- 61.Grose J. H., Bergthorsson U., Xu Y., Sterneckert J., Khodaverdian B., Roth J. R. J. Bacteriol. 2005;187:4521–4530. doi: 10.1128/JB.187.13.4521-4530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hughes K. T., Roth J. R. Genetics. 1985;109:263–282. doi: 10.1093/genetics/109.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Datsenko K. A., Wanner B. L. Proc. Natl. Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu D., Ellis H. M., Lee E.-C., Jenkins N. A., Copeland N. G., Court D. L. Proc. Natl. Acad. Sci. USA. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kornberg A. J. Biol. Chem. 1950;182:805–813. [PubMed] [Google Scholar]
- 66.Laemmli U. K. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 67.Starovasnik M. A., Blackwell T. K., Laue T. M., Weintraub H., Klevit R. E. Biochemistry. 1992;31:9891–9903. doi: 10.1021/bi00156a006. [DOI] [PubMed] [Google Scholar]
- 68.Zimmerman J. J. J. Pharm. Sci. 1983;72:138–142. doi: 10.1002/jps.2600720211. [DOI] [PubMed] [Google Scholar]
- 69.Zerez C. R., Lee S. J., Tanaka K. R. Anal. Biochem. 1987;164:367–373. doi: 10.1016/0003-2697(87)90506-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.