Abstract
The involvement of the c-Myc transcription factor in neoplastic transformation is well documented. However, which of its numerous target genes are crucial for tumorigenesis remains a frequently contested issue. We have recently established a non-transgenic murine model for B-cell lymphoma based on neoplastic conversion of p53-null bone marrow cells by conditionally active Myc. Using this model, we have identified a number of genes whose expression levels are affected by Myc during B-lymphomagenesis. Here we discuss their possible roles in neoplastic processes and describe an experimental approach allowing in vivo validation of these roles. We demonstrate that lymphoma cells overexpressing one of the Myc targets, the interleukin-10 receptor gene, have a very strong selective advantage over low IL10R expressors. Furthermore, Mcl1, a presumptive IL10R effector, also confers selective advantages when overexpressed in Myc-transformed hematopoietic cells. Thus, both IL10R and Mcl1 might be amenable to therapeutic interventions, and new targets can be identified and validated using the selection approach.
Keywords: c-Myc, B-cell lymphoma, target genes, Mcl1, IL10
INTRODUCTION
c-Myc, an oncogenic transcription factor, is a crucial regulator of cell expansion. It was realized early on that activation of Myc forces quiescent fibroblasts to re-enter the cell cycle1 and that rodent fibroblasts with targeted disruption of Myc are severely deficient in cell proliferation.2 Thus, in mice (but curiously not in Drosophila3), decreased expression of Myc results in hypoplasia.4 Predictably, some of the best-recognized Myc targets are crucial regulators of cell homeostasis: cdk45 and telomerase6 (upregulation) and cdk inhibitors7-11 and gadd4512 (downregulation). In addition, Myc regulates, at the transcriptional level, a dazzling array of genes pertaining to numerous physiological and pathological processes, such as cell growth and cell death.13,14 In all, the database of Myc-regulated genes currently contains 1,697 entries (http://www.myccancergene.org/). It is often pointed out that many of these genes are indirect targets and that only a fraction of them might contain bona fide Myc-binding sites. Yet recent research using chromatin immunoprecipitation and other approaches has revealed that Myc binds to and regulates up to 15% of all genes (reviewed in Ref. 15). The abundance of Myc target genes raises the question of how crucial mediators of Myc-induced tumorigenesis could be identified.
Below we describe a simple approach that has allowed us to assign functional significance to Myc-induced variations in interleukin-10 receptor levels in transformed bone marrow cells. According to our data, the il10ra gene is downregulated by Myc,16 and its expression levels become rate-limiting for B-lymphomagenesis. However, in the absence of active Myc, IL10R protein levels rapidly recover. This boosts cell expansion and ultimately correlates with Myc-independent tumor relapse. We envision that the same approach could be used to functionally validate various Myc targets as well as other genes tentatively implicated in B-lymphomagenesis.
MATERIALS AND METHODS
Tumor Production and Analysis
Generation of Myc- and MycER-induced murine B-lymphomas has been described earlier.16-18 Syngeneic C57BL6/J mice (NCI, Frederick, MD) were used as hosts for tumor production. Lymphoma cells (5–10 × 106) were injected subcutaneously into the flanks, and tumor growth was monitored over the course of several weeks. For 4-OHT treatment, the hormone powder (H6278, Sigma, St. Louis, MO) was dispersed in corn oil (Sigma) at the concentration of 10 mg/mL. The administration of 4-OHT was performed daily via intraperitoneal injections (1 mg per mouse). For the selection assay, tumors were harvested and dissected approximately 3 weeks after implantation. Neoplastic cells were used for flow cytometric analyses or subsequent passages in vivo.
Microarray Analysis
Total RNAs from MycER tumors were used for cDNA synthesis. Labeled probes were hybridized to the U74v2 gene chip (Affymetrix, Inc. Santa Clara, CA) using the PENN Microarray Facility's standard protocol (http://www.med.upenn.edu/microarr/Data%20Analysis/Affymetrix/methods.htm). Affymetrix MAS 5 probeset signals and presence/absence flags were calculated. The LPE (Local Pooled Error) test for differential expression as implemented in S+ArrayAnalyzer v 1.1 (Insightful Corporation, Seattle, WA) was applied with 1% Bonferonni multiple testing correction to median IQR normalized MAS 5 signal values. The resulting list of 686 genes was imported into GeneSpring v 6.1 (Silicon Genetics, San Carlos, CA), filtered for presence (per Affymetrix MAS5 analysis) in 2 of 2 samples in one or more conditions (MycON or MycOFF), and then filtered for fold change.
Generation of Recombinant Retroviruses
The murine IL10Rα-encoding retrovirus has been described earlier.16 To generate an IL10Rα mutant lacking the intracellular domain, the PCRII-mIL10Rα plasmid16 was cut with BamH I and Xma I, and the truncated IL10R insert was subcloned into the pSL1180 phagemid vector (Amersham Pharmacia, Piscataway, NJ). In this vector, the polylinker segment between Xma I and Sal I site was replaced with the double-stranded oligonucleotide (CCGGGTTAGAATTACCTCAG) containing a single T-to-G substitution shown in bold. This substitution resulted in a stop-codon (underlined) positioned in-frame with the IL10R coding sequence. The IL10Rstop sequence was cut out with Bgl II and Sal I and inserted into the bicistronic GFP-expressing retroviral vector pMIGRI19 linearized with Bgl II and Xho I. The full length murine mcl-1 cDNA was purchased from the American Type Culture Collection (Manassas, VA, catalog # MGC-13884, IMAGE ID: 4190996). The cDNA was released from the pCMV-SPORT6 vector using Not I and EcoR V restriction enzymes, cloned into pSL1180, and then recloned, using Bgl II and Hpa I restriction sites, into MIGR1.
Cell Propagation, Transfection, and Infection
Myc3 B-lymphoma cells were cultured on monolayers of gamma-irradiated S17 cells as described previously.18 BOSC and GP293 packaging cells were cultured in DMEM with 10% FBS. For in vitro infections, either of these cell lines was transfected with retroviral clones using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Conditioned media were harvested and added to Myc3 cells for 2 to12 h. Three days post infection, the percentages of GFP-positive cells in infected cultures were determined using flow cytometry.
Flow Cytometric Analyses
To detect expression of mIL10Rα and the mIL10Rstop truncated protein, transiently transfected packaging cells were stained on ice for 45 min with a biotinconjugated anti-mouse IL10R antibody (559913, Pharmingen, San Diego, CA), followed by staining with APC-conjugated streptavidin (SA1005, Caltag Laboratories, Burlingame, CA). All samples were analyzed using a FACScalibur flow cytometer (Becton Dickinson, Mountain View, CA) with the CELLQuest software (Becton Dickinson).
RESULTS AND DISCUSSION
Improved Survival of Neoplastic Cells following Myc Inactivation
We have recently established a new mouse model for B-cell lymphoma based on infection of p53-null bone marrow cells with retroviruses encoding either constitutively active Myc17 or a fusion between Myc and the mutant form of the estrogen receptor (MycER),20 requiring the presence of the synthetic estrogen 4-hydroxytamoxifen (4-OHT) for activity.16 The use of conditionally active Myc allowed us to address the role of Myc in tumor maintenance (Fig. 1). To this end, cells obtained from primary tumors were injected subcutaneously into flanks of syngeneic mice, and administration of the estrogen was maintained until palpable neoplasms have formed. Animals were then randomized into three groups. Mice in the first group continued to receive 4-OHT and rapidly developed large tumors (Fig. 2, tumors 1 and 2). In the second group, 4-OHT treatment was permanently discontinued (tumors 3 and 4). In the third group, 4-OHT treatment was suspended for two weeks and resumed on day 21 (tumors 5 and 6). We observed that in animals deprived of 4-OHT, tumor growth decreased dramatically, but the neoplasms did not undergo overt regression. Instead tumors remained stable for approximately two weeks. If 4-OHT was re-administered, extremely rapid neoplastic growth ensued (tumors 5 and 6), attesting to the viability of cells with inactivated Myc. Moreover, even without estrogen re-administration, MycER tumors began to slowly regrow after only two weeks of dormancy (Fig. 2 and Ref. 16).
FIGURE 1.

The non-transgenic mouse model of B-cell lymphoma based on the expression of conditionally active Myc in p53-null bone marrow cells. The use of 4-OHT deprivation allows the analysis of the role of Myc in tumor maintenance.
FIGURE 2.

MycER activity correlates with rapid tumor growth and high rates of apoptosis. Plots refer to tumor sizes in animals continuously treated with the estrogen (with 4OHT), deprived of the estrogen (w/o 4OHT), and retreated with the estrogen after a 2-week hiatus (w/o > with 4OHT). The insets depict TUNEL staining performed on representative tumor samples from each group.
To correlate tumor dormancy with neoplastic cell survival, hematoxylin/eosin and TUNEL stainings of formalin-fixed tumor sections were performed. We observed that while the rate of cell proliferation decreased upon inactivation of Myc (data not shown), so did the rate of cell death. Interestingly, tumors with reactivated Myc exhibited yet higher rates of apoptosis, as judged by the percentage of TUNEL-positive cells (Fig. 2). Conversely, relapsing tumors with inactivated MycER had modest rates of cell proliferation but almost no apoptosis.
Transcriptional Signatures of MycON and MycOFF Tumors
In order to correlate Myc-induced changes in cell proliferation and survival to alterations in gene expression, we performed a series of microarray experiments. In these experiments transcriptional signatures of tumors with active and inactive MycER were compared. Differentially expressed genes were identified as described in Materials and Methods. Forty-four genes were upregulated more than fourfold in MycON cells and 68 genes in MycOFF cells (Tables 1 and 2).
TABLE 1.
Myc-activated genes
| Fold Induction | Affy ID | Common name | Description |
|---|---|---|---|
| 38.2 | 97473_at | Tm4sf7 | transmembrane 4 superfamily member 7 |
| 24.4 | 102632_at | Calmbp1 | calmodulin binding protein 1 |
| 14.3 | 96293_at | 2410015N17Rik | RIKEN cDNA 2410015N17 gene |
| 13.7 | 94971_at | Cdkn3 | cyclin-dependent kinase inhibitor 3 |
| 12.9 | 104423_at | 2810047L02Rik | RIKEN cDNA 2810047L02 gene |
| 7.4 | 96269_at | Idi1 | isopentenyl-diphosphate delta isomerase |
| 6.5 | 97124_at | Fin15 | fibroblast growth factor inducible 15 |
| 6.5 | 103525_at | Hnrpll | heterogeneous nuclear ribonucleoprotein L-like |
| 6.4 | 97411_at | Ect2 | ect2 oncogene |
| 6.1 | 103203_f_at | 4432406C08Rik | RIKEN cDNA 4432406C08 gene |
| 6.1 | 160159_at | Ccnb1 | cyclin B1 |
| 5.6 | 160501_at | Kif20a | kinesin family member 20A |
| 5.4 | 92840_at | Nup54 | nucleoporin 54 |
| 5.3 | 95755_at | Csda | cold-shock domain protein A |
| 5.3 | 160906_i_at | E430003D02Rik | RIKEN cDNA E430003D02 gene |
| 5.3 | 99578_at | Top2a | topoisomerase (DNA) II alpha |
| 5 | 93099_f_at | Plk | polo-like kinase homolog (Drosophila) |
| 5 | 160395_at | D11Ertd603e | DNA segment, Chr 11, ERATO Doi 603, expressed |
| 5 | 93250_r_at | Hmgb2 | high-mobility group box 2 |
| 5 | 101065_at | Pcna | proliferating cell nuclear antigen |
| 4.9 | 160069_at | Gmnn | geminin |
| 4.9 | 94292_at | Strap | serine/threonine kinase receptor–associated protein |
| 4.7 | 97527_at | Cks2 | CDC28 protein kinase regulatory subunit 2 |
| 4.7 | 101589_at | Hmgn2 | high-mobility group nucleosomal binding domain 2 |
| 4.7 | 100612_at | Rrm1 | ribonucleotide reductase M1 |
| 4.6 | 99457_at | Mki67 | antigen identified by monoclonal antibody Ki 67 |
| 4.5 | 92807_at | Txn1 | thioredoxin 1 |
| 4.5 | 100128_at | Cdc2a | cell division cycle 2 homologue A (S. pombe) |
| 4.5 | 94255_g_at | Clic4 | chloride intracellular channel 4 (mitochondrial) |
| 4.4 | 102911_at | Brca2 | breast cancer 2 |
| 4.4 | 97468_at | Cks1 | CDC28 protein kinase 1 |
| 4.3 | 100116_at | 2810417H13Rik | RIKEN cDNA 2810417H13 gene |
| 4.3 | 94274_at | Ube2s | ubiquitin-conjugating enzyme E2S |
| 4.3 | 96784_at | Anln | anillin, actin-binding protein (scraps homolog, Drosophila) |
| 4.3 | 94953_at | Racgap1 | Rac GTPase-activating protein 1 |
| 4.2 | 100512_at | Uchl5 | ubiquitin carboxyl-terminal esterase L5 |
| 4.2 | 160955_at | 2010309E21Rik | RIKEN cDNA 2010309E21 gene |
| 4.2 | 101521_at | Birc5 | baculoviral IAP repeat-containing 5 (survivin) |
| 4.2 | 92790_at | Kpna2 | karyopherin (importin) alpha 2 |
| 4.2 | 104097_at | Bub1 | budding uninhibited by benzimidazoles 1 homologue (S. cerevisiae) |
| 4.1 | 94294_at | Ccnb2 | cyclin B2 |
| 4.1 | 101959_r_at | Tfdp1 | transcription factor Dp 1 |
| 4 | 92639_at | Stk6 | serine/threonine kinase 6 |
| 4 | 99098_at | Fdps | farnesyl diphosphate synthetase |
TABLE 2.
Myc-repressed genes
| Fold repression | Affy ID | Common name | Description |
|---|---|---|---|
| −21.8 | 99446_at | CD20 (a.k.a. Ms4a1) | membrane-spanning 4 domains, subfamily A, member 1 |
| −21.7 | 96109_at | Klf2 | Kruppel-like factor 2 (lung) |
| −21 | 103061_at | Gad1 | glutamic acid decarboxylase 1 |
| −20.5 | 96515_at | Il4i1 | interleukin-4 induced 1 |
| −20.4 | 98034_at | H2-DMb1 | histocompatibility 2, class II, locus Mb1 |
| −18.9 | 96525_at | Il10ra | interleukin-10 receptor, alpha |
| −17.1 | 96207_at | Rbms1 | RNA binding motif, single stranded interacting protein 1 |
| −16.7 | 160667_at | Evl | Ena-vasodilator stimulated phosphoprotein |
| −16.5 | 103985_at | Map4k2 | mitogen-activated protein kinase kinase kinase kinase 2 |
| −16.1 | 101845_s_at | Csprs | component of Sp100-rs |
| −13.6 | 102940_at | Ltb | lymphotoxin B |
| −13.4 | 103299_at | AI132321 | expressed sequence AI132321 |
| −10.8 | 102378_at | Sspn | Sarcospan |
| −10.2 | 94354_at | Abca1 | ATP-binding cassette, sub-family A (ABC1), member 1 |
| −9.7 | 103422_at | Cd1d1 | CD1d1 antigen |
| −9 | 98859_at | Acp5; TRAP | acid phosphatase 5, tartrate resistant |
| −8.8 | 98974_at | Igh-4 | similar to immunoglobulin delta-chain |
| −8.7 | 93714_f_at | LOC436489 | similar to MHC class I–alpha |
| −8.4 | 100011_at | Klf3 | Kruppel-like factor 3 (basic) |
| −7.8 | 92471_i_at | Slfn2 | schlafen 2 |
| −7.8 | 95373_at | Cd2 | CD2 antigen |
| −7.7 | 101792_at | Mouse-gene fragment for delta-immunoglobulin (exon 8) | |
| −7.6 | 92866_at | H2-Aa | histocompatibility 2, class II antigen A, alpha |
| −7.3 | 103596_at | Dgka | diacylglycerol kinase, alpha |
| −7.3 | 98402_at | Macf1 | microtubule-actin crosslinking factor 1 |
| −7.2 | 103202_at | Gbp3 | guanylate nucleotide binding protein 3 |
| −6.8 | 94224_s_at | Ifi205 | interferon-activated gene 205 |
| −6.6 | 92740_at | Iga | mouse Ig germline D-J-C region alpha gene and secreted tail. |
| −6.6 | 103697_at | A230075M04Rik | RIKEN cDNA A230075M04 gene |
| −6.1 | 99511_at | Prkcb | protein kinase C, beta |
| −6.1 | 160754_at | Pygm | muscle glycogen phosphorylase |
| −5.9 | 100998_at | H2-Ab1 | histocompatibility 2, class II antigen A, beta 1 |
| −5.7 | 101912_at | 1100001G20Rik | RIKEN cDNA 1100001G20 gene |
| −5.5 | 101054_at | Ii | Ia-associated invariant chain |
| −5.5 | 93683_at | Rag1 | recombination activating gene 1 |
| −5.4 | 94834_at | Ctsh | cathepsin H |
| −5.3 | 98035_g_at | H2-DMb1 | histocompatibility 2, class II, locus Mb1 |
| −5.2 | 94713_at | Myo7a | myosin VIIa |
| −5.1 | 104429_at | H2-Ob | histocompatibility 2, O region beta locus |
| −5 | 93026_at | Mgst1 | microsomal glutathione S-transferase 1 |
| −5 | 103531_f_at | 1300013B24Rik | RIKEN cDNA 1300013B24 gene |
| −5 | 93657_at | Spib | Spi-B transcription factor (Spi-1/PU.1 related) |
| −4.9 | 97313_at | Gdi1 | guanosine diphosphate (GDP) dissociation inhibitor 1 |
| −4.8 | 99623_s_at | Olfm1 | olfactomedin 1 |
| −4.8 | 93104_at | Btg1 | B-cell translocation gene 1, anti-proliferative |
| −4.8 | 101048_at | Ptprc | protein tyrosine phosphatase, receptor type, C |
| −4.8 | 93688_at | Cmah | cytidine monophospho-N-acetylneuraminic acid hydroxylase |
| −4.7 | 98001_at | Arhgef1 | Rho guanine nucleotide exchange factor (GEF) 1 |
| −4.5 | 92436_at | Stk23 | serine/threonine kinase 23 |
| −4.5 | 160781_r_at | Unc93b1 | unc-93 homologue B1 (C. elegans) |
| −4.4 | 100295_at | Lgl | mouse Ig germline lambda-chain gene Vx-J2-C2-region, clone 30X2. |
| −4.4 | 161819_f_at | Laptm5 | lysosomal-associated protein transmembrane 5 |
| −4.4 | 99953_at | Rgl2 | ral guanine nucleotide dissociation stimulator-like 2 |
| −4.3 | 161005_at | 5730420B22Rik | RIKEN cDNA 5730420B22 gene |
| −4.3 | 94285_at | H2-Eb1 | histocompatibility 2, class II antigen E beta |
| −4.2 | 93321_at | Ifi203 | interferon activated gene 203 |
| −4.2 | 95119_at | 1110038D17Rik | RIKEN cDNA 1110038D17 gene |
| −4.2 | 160812_at | Gga2 | Golgi-associated, gamma adaptin ear containing, ARF-binding protein 2 |
| −4.2 | 98980_at | Cd37 | CD37 antigen |
| −4.1 | 97963_at | Sipa1 | signal-induced proliferation associated gene 1 |
| −4.1 | 160773_at | Zcchc7 | zinc finger, CCHC domain containing 7 |
| −4.1 | 97173_f_at | H2-K2; H-2K2 | similar to MHC class I–alpha |
| −4.1 | 95893_at | Blk | B lymphoid kinase |
| −4.1 | 98931_at | Gns | glucosamine (N-acetyl)-6-sulfatase |
| −4.1 | 101331_f_at | Igk-V8 | immunoglobulin kappa chain variable 8 (V8) |
| −4.1 | 93092_at | H2-DMa | histocompatibility 2, class II, locus DMa |
| −4.1 | 100511_at | 6330406L22Rik | RIKEN cDNA 6330406L22 gene |
| −4 | 103970_at | Dri2 | dead ringer homologue 2 (Drosophila) |
The functions of many Myc-upregulated and Myc-downregulated genes were consistent with the biological findings. Several genes implicated in cell proliferation maintained high transcription levels only in the presence of functional MycER, for example, cyclins B1 and B2, cdc2A, and DP1 (Table 1). On the other hand, many of the genes upregulated in the absence of Myc were known to contribute to B-cell survival and expansion, in particular, in response to antigen or cytokine stimulation. This list included components of B-cell receptor signaling pathways (receptor type protein tyrosine phosphatase C and B-lymphoid kinase),21 NF-κB–activators (lymphotoxin B),22 direct apoptosis inhibitors (Mcl1),23 and cytokine receptors (interleukin 10 receptor)24 (Table 2 and data not shown). While their expression correlated with neoplastic B-cell survival and eventual relapse, some of them were incompletely characterized or even reported to suppress tumorigenesis. For example, while IL10 has been known to stimulate proliferation of normal B cells in vitro,25 its in vivo effects on both normal and neoplastic B cells are not well understood. In addition, anti-angiogenic, and thus anti-neoplastic, effects of IL10 have been reported.26-28 Thus, we were interested in developing an unbiased approach capable of revealing both tumor-promoting and tumor-inhibiting properties of IL10R and other Myc target proteins.
An in Vivo Selection Assay for Cells Overexpressing Myc Target Genes
The following approach was devised in order to assign functional significance to genes implicated in B-lymphomagenesis, such as IL10R. Its full-length cDNA was inserted into a retroviral vector MIGR1, co-expressing through the IRES element green fluorescent protein (GFP).19 Retroviral constructs were transfected into packaging cells, and viral supernatants were harvested 48 h later. Then Myc-overexpressing B-lymphoma cells (Myc318) were infected in vitro with either MIGR1/IL10R or just MIGR1 (empty vector). By varying the amount of viral supernatants, the infection rate of approximately 10% was achieved, as judged by flow cytometry for GFP. Partially but stably infected cultures were used for tumor production in syngeneic animals. The resulting tumors were analyzed using flow cytometry and percentages of GFP-positive cells were determined again. By enumerating GFP-positive cells before and after passaging in vivo, both positive and negative effects of the gene of interest on B-lymphomagenesis could be revealed (Fig. 3).
FIGURE 3.
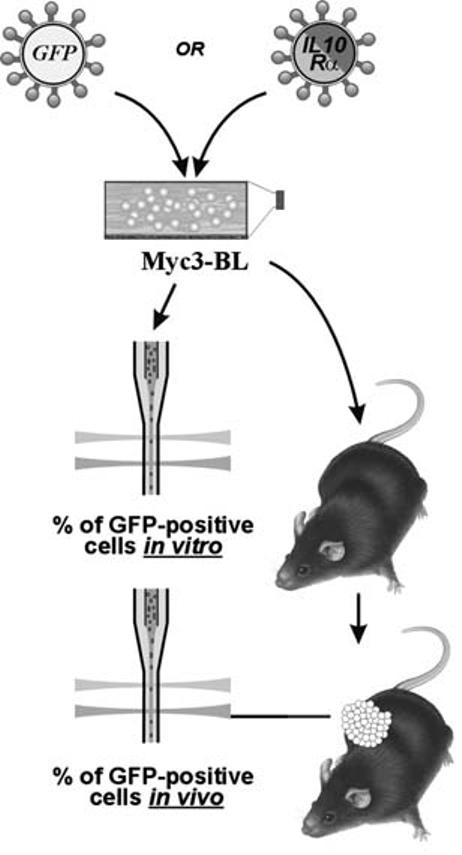
An in vivo selection assay for cells overexpressing Myc target genes. See text for more explanations.
Cells Overexpressing Interleukin 10 Receptor or Its Presumptive Downstream Target Mcl1 Are Selected during B-Lymphomagenesis
The approach described above was applied to determine whether levels of IL10R are rate-limiting during B-lymphomagenesis. IL10Rα full-length cDNA is described in Materials and Methods. As a negative control, we also generated and utilized its truncated version (IL10Rstop) retaining the extracellular and transmembrane domains but lacking the cytoplasmic tail (see Materials and Methods). Transfection of both constructs into the packaging cells resulted in expression of polypeptides of expected lengths (immunoblotting data not shown) located on the cell surface (flow cytometry data in Fig. 4A). The IL10R and IL10Rstop retroviruses were used to infect Myc3 cultures as described in the previous section.
FIGURE 4.

(A) Retrovirus-driven expression of IL10R and IL10Rstop on the surface of transfected BOSC23 cells. Live cells were stained with an anti-IL10R antibody as described in Materials and Methods and analyzed using flow cytometry (APC fluorophor). (B) Positive selection for cells overexpressing IL10R. Percentages of GFP-positive cells in Myc3 B-cell lymphoma cells transduced with either empty vector (GFP) or IL10R/GFP and IL10Rstop/GFP-expressing retroviruses. 0′ refers to cells passaged in vitro. 1′, 2′, and 3′ refer to tumors propagated in vivo for the indicated number of passages. The last panel depicts the IL10R selection experiment performed in IL10-null hosts. (C) Positive selection for cells overexpressing Mcl1. All designations are the same as in B.
When transduced cells were used for tumor production in vivo, a steady increase in the percentages of IL10R-overexpressing, GFP-positive cells was apparent: after only three passages in vivo such cells accounted for over 90% of total neoplastic cells, up from 10% observed after infection in vitro (Fig. 4B). No increase was observed with GFP- or IL10Rstop-overproducing cells. Additionally, no increase was observed when the experiment was performed in IL10-deficient mice,29 suggesting that the positive selection for IL10R-overexpressing cells depends on the availability of its cognate ligand.
IL-10 is known to signal predominantly through the STAT3 pathway (reviewed in Ref. 30), which activates expression of several anti-apoptotic genes including Bcl-2, Bcl-xl, survivin, and Mcl1.31 Consistent with this, Mcl1 was upregulated in MycOFF tumors, albeit slightly less than fourfold (data not included in Table 2). We thus asked whether positive selection for Mcl1-overexpressing cells would also be in place. We generated a murine Mcl1/GFP-encoding retrovirus and repeated the basic experiment. As evidenced by data in Figure 4C, after only two passages in vivo, Mcl1-transduced cells accounted for almost 100% of the total cell population. Thus, it is possible that the contribution of the IL10 pathway to B-lymphomagenesis is based on its ability to inhibit apoptosis and improve tumor-cell survival. Consequently, targeting of the IL10 pathway using soluble IL10Rα32 or a dominant-negative STAT333 could block expansion of lymphoma cells. Finally, functional validation of other Myc-regulated genes, which is under way in our laboratory, might yield new therapeutic targets.
ACKNOWLEDGMENTS
We thank Drs. Emma Wilson and Christopher Hunter (University of Pennsylvania) for providing us with IL10-null mice and Dr. Michael Dews for stimulating discussions and helpful comments on the manuscript. This work was supported by National Institutes of Health Grants CA 097932 and CA 102709 and the Commonwealth of Pennsylvania Health Research Formula Fund #4100020574 to A.T.-T.
REFERENCES
- 1.Eilers M, Picard D, Yamamoto KR, Bishop JM. Chimaeras of Myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature. 1989;340:66–68. doi: 10.1038/340066a0. [DOI] [PubMed] [Google Scholar]
- 2.Mateyak MK, Obaya AJ, Adachi S, Sedivy JM. Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth & Differentiation. 1997;8:1039–1048. [PubMed] [Google Scholar]
- 3.Johnston LA, Prober DA, Edgar BA, et al. Drosophila myc regulates cellular growth during development. Cell. 1999;98:779–790. doi: 10.1016/s0092-8674(00)81512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trumpp A, Y. Refaeli, Oskarsson T, et al. c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature. 2001;414:768–773. doi: 10.1038/414768a. [DOI] [PubMed] [Google Scholar]
- 5.Hermeking H, Rago C, Schuhmacher M, et al. Identification of CDK4 as a target of c-MYC. Proc. Natl. Acad. Sci. USA. 2000;97:2229–2234. doi: 10.1073/pnas.050586197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Xie LY, Allan S, et al. Myc activates telomerase. Genes Dev. 1998;12:1769–1774. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell KO, El-Deiry WS. Overexpression of c-Myc inhibits p21WAF1/CIP1 expression and induces S-phase entry in 12-O-tetradecanoylphorbol-13-acetate (TPA)-sensitive human cancer cells. Cell Growth Differ . 1999;10:223–230. [PubMed] [Google Scholar]
- 8.Claassen GF, Hann SR. A role for transcriptional repression of p21CIP1 by c-Myc in overcoming transforming growth factor beta -induced cell-cycle arrest. Proc. Natl. Acad. Sci. USA. 2000;97:9498–9503. doi: 10.1073/pnas.150006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gartel AL, Ye X, Goufman E, et al. Myc represses the p21(WAF1/CIP1) promoter and interacts with Sp1/Sp3. Proc. Natl. Acad. Sci. USA. 2001;98:4510–4515. doi: 10.1073/pnas.081074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seoane J, Pouponnot C, Staller P, et al. TGFβ influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nature Cell. Biol. 2001;3:400–408. doi: 10.1038/35070086. [DOI] [PubMed] [Google Scholar]
- 11.Staller P, Peukert K, Kiermaier A, et al. Repression of p15INK4b expression by Myc through association with Miz-1. Nature Cell. Biol. 2001;3:392–399. doi: 10.1038/35070076. [DOI] [PubMed] [Google Scholar]
- 12.Marhin WW, Chen S, Facchini LM, et al. Myc represses the growth arrest gene gadd45. Oncogene. 1997;14:2825–2834. doi: 10.1038/sj.onc.1201138. [DOI] [PubMed] [Google Scholar]
- 13.Oster SK, Ho CS, Soucie EL, Penn LZ. The myc oncogene: marvelously complex. Adv. Cancer Res. 2002;84:81–154. doi: 10.1016/s0065-230x(02)84004-0. [DOI] [PubMed] [Google Scholar]
- 14.O'Connell BC, Cheung AF, Simkevich CP, et al. A large scale genetic analysis of c-Myc-regulated gene expression patterns. J. Biol. Chem. 2003;278:12563–12573. doi: 10.1074/jbc.M210462200. [DOI] [PubMed] [Google Scholar]
- 15.Patel JH, Loboda AP, Showe MK, et al. Analysis of genomic targets reveals complex functions of MYC. Nat. Rev. Cancer. 2004;4:562–568. doi: 10.1038/nrc1393. [DOI] [PubMed] [Google Scholar]
- 16.Yu D, Dews M, Park A, et al. Inactivation of Myc in two-hit B-lymphomas causes dormancy with elevated levels of interleukin-10 receptor and CD20: implications for adjuvant therapies. Cancer Res. 2005;65:5454–5461. doi: 10.1158/0008-5472.CAN-04-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu D, Thomas-Tikhonenko A. A non-transgenic mouse model for B-cell lymphoma: in vivo infection of p53-null bone marrow progenitors by a Myc retrovirus is sufficient for tumorigenesis. Oncogene. 2002;21:1922–1927. doi: 10.1038/sj.onc.1205244. [DOI] [PubMed] [Google Scholar]
- 18.Yu D, Allman D, Goldscmidt M, et al. Oscillation between B-lymphoid and myeloid lineages in Myc-induced hematopoietic tumors following spontaneous silencing/reactivation of the EBF/Pax5 pathway. Blood. 2003;101:1950–1955. doi: 10.1182/blood-2002-06-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pear WS, Miller JP, Xu L, et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 20.Littlewood TD, Hancock DC, Danielian PS, et al. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat. Rev. Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 22.Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-κB signal transduction pathway. Immunol. Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 23.Packham G, Stevenson FK. Bodyguards and assassins: Bcl-2 family proteins and apoptosis control in chronic lymphocytic leukaemia. Immunology. 2005;114:441–449. doi: 10.1111/j.1365-2567.2005.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 25.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S, Xie K, Bucana CD, et al. Interleukin 10 suppresses tumor growth and metastasis of human melanoma cells: potential inhibition of angiogenesis. Clin. Cancer Res. 1996;2:1969–1979. [PubMed] [Google Scholar]
- 27.Silvestre JS, Mallat Z, Duriez M, et al. Antiangiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Circ. Res. 2000;87:448–452. doi: 10.1161/01.res.87.6.448. [DOI] [PubMed] [Google Scholar]
- 28.Cervenak L, Morbidelli L, Donati D, et al. Abolished angiogenicity and tumorigenicity of Burkitt lymphoma by interleukin-10. Blood. 2000;96:2568. [PubMed] [Google Scholar]
- 29.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 30.Gamero AM, Young HA, Wiltrout RH. Inactivation of Stat3 in tumor cells: releasing a brake on immune responses against cancer? Cancer Cell. 2004;5:111–112. doi: 10.1016/s1535-6108(04)00028-5. [DOI] [PubMed] [Google Scholar]
- 31.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 32.Ho AS, Liu Y, Khan TA, et al. A receptor for interleukin 10 is related to interferon receptors. Proc. Natl. Acad. Sci. USA. 1993;90:11267–11271. doi: 10.1073/pnas.90.23.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu G, Heller R, Catlett-Falcone R, et al. Gene therapy with dominant-negative Stat3 suppresses growth of the murine melanoma B16 tumor in vivo. Cancer Res. 1999;59:5059–5063. [PubMed] [Google Scholar]


