Abstract
Antigen 43 (Ag43) is a self-recognizing surface adhesin found in most Escherichia coli strains. Due to its excellent cell-to-cell aggregation characteristics, Ag43 expression confers clumping and fluffing of cells and promotes biofilm formation. Ag43 expression is repressed by the cellular redox sensor OxyR. Here we used mutant versions of OxyR that are locked in either the reduced or the oxidized form as well as the addition of a simple redox-changing chemical to show that the redox state of OxyR influences Ag43 expression. Furthermore, the redox state of OxyR influences the biofilm-forming potential of E. coli. Finally, we demonstrated that Ag43-mediated cell aggregation confers significant protection against hydrogen peroxide killing.
Life in an aerobic environment is intimately connected with the presence of reactive oxygen compounds, and such intermediates are formed whenever molecular oxygen reacts with cellular electron carriers. Reactive oxygen species, such as hydrogen peroxide, nitric oxide, and hydroxyl radicals, can damage nucleic acids, proteins, and cell membranes (reviewed by Storz and Imlay [26]). To counter oxidative stress, cells can take various protective measures, such as the expression of enzymes that detoxify reactive oxygen compounds and repair damage caused by them.
The oxidative stress response in Escherichia coli is orchestrated by two master control systems encoded by the oxyR and soxRS genes. The OxyR arm of the oxidative stress response primarily senses peroxides (31) and affects a regulon of more than 30 genes that are either activated or repressed by OxyR. According to the prototypic two-state model, OxyR can exist in two principal forms, reduced and oxidized, due to reversible disulfide bond formation (31, 32). The two forms have different conformations and recognize different DNA motifs (27). Hydrogen peroxide can act directly with OxyR, generating an oxidized cysteine derivative, which is then presumably able to react and form a disulfide bridge. Alternatively, OxyR can be oxidized independently of peroxide exposure by disulfide bridge formation due to an altered redox state in the cytoplasm, reflected by a shift in the glutathione/glutathione disulfide ratio, i.e., disulfide stress (1). Only the oxidized form of OxyR (OxyROx) can activate transcription (32). Indeed, OxyROx activates several genes whose products have obvious roles in eliminating reactive oxygen compounds, e.g., katG and aphCF, encoding catalase and alkyl hydroperoxide reductase, respectively. Although OxyROx generally acts as an activator, it can repress transcription; for example, it represses gntP, uxuAB, and fhuF (33, 34).
When the cell is not subjected to either oxidative or disulfide stress, a reduced form of OxyR (OxyRRed) is thought to be dominant (1, 31). According to the available literature, OxyRRed, apart from repressing the transcription of oxyR itself, seems to repress only one other indigenous gene in E. coli, i.e., flu (the mom gene of bacteriophage Mu is not normally present). The flu gene (also called agn43), mapping at 43 min on the E. coli chromosome, encodes the surface protein antigen 43 (Ag43), which induces fluffing of cells, a characteristic which led to its original flu designation (7). Ag43 is present in ∼50,000 copies per cell (20). The expression is phase variable, with switching rates of ∼10−3 per cell per generation under normal growth conditions due to the concerted action of the Dam methyltransferase (positive regulation) and OxyR (negative regulation) (9, 13, 30). The flu promoter region contains three GATC sites, which overlap a recognition sequence for OxyR (9, 10, 13). When the GATC sites are methylated, OxyR cannot bind; conversely, bound OxyR prevents methylation (29).
Ag43 is a member of the autotransporter family of excreted proteins; it is processed into a mature form consisting of two subunits, α and β (10, 12, 13). The α subunit reaches the cell exterior with assistance from the β subunit and remains attached to the cell surface, presumably through a noncovalent interaction with the β subunit. Ag43 is a self-recognizing adhesin and has been found to induce characteristic surface properties on host cells, such as autoaggregation and a frizzy colony morphology (10, 11). Ag43 is found in most E. coli strains and can actually be expressed by a broad spectrum of gram-negative bacteria (15, 16). A key function of Ag43 seems to be the promotion of bacterial biofilm formation due to its excellent cell-to-cell aggregation characteristics (16, 17, 23). Previous studies suggested that the reduced, but not the oxidized, form of OxyR is an efficient repressor of the flu gene (9, 13, 24). However, the precise roles of the redox states of OxyR in controlling the flu promoter have not been elucidated. Here we study such aspects.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. An oxyR-null derivative of E. coli K-12 MG1655 (3) (MGJ1) was constructed by P1 transduction from E. coli N9716 (oxyR::Sp) (a kind gift from G. Storz). MS428 is a fim derivative of MG1655 (16), and MS528 is a fim flu derivative of MG1655 (16). The killing effects of H2O2 were assessed with both MS528 and E. coli reference strain BD1428. Ag43 expression in BD1428 is phase variable; Ag43 phase-on and phase-off derivatives are referred to as BD1512 and BD1511, respectively (7). Cells were grown at 37°C on solid or in liquid Luria-Bertani (LB) medium supplemented with the appropriate antibiotics unless otherwise stated. For induction of the araBAD promoter, 0.2% arabinose was used.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| MG1655 | K-12 reference strain | 3 |
| MS428 | MG1655 fim | 16 |
| MS528 | MG1655 fim flu | 16 |
| MGJ1 | MG1655 oxyR | This study |
| BD1428 | Phase-variable Ag43 expression | 7 |
| BD1511 | Ag43 phase-off variant of BD1428 | 7 |
| BD1512 | Ag43 phase-on variant of BD1428 | 7 |
| Plasmids | ||
| pBAD/myc-HisA | Arabinose-inducible promoter | 8 |
| pMGJ1 | oxyR gene in pBAD/myc-HisA | This study |
| pMAS141 | oxyRRed,C199S gene in pBAD/myc-HisA | This study |
| pMAS142 | oxyROx,H198R gene in pBAD/myc-HisA | This study |
| pHHA147 | flu gene downstream of tet promoter in pBR322 | 11 |
Recombinant DNA techniques.
The oxyR gene was amplified by PCR from MG1655 chromosomal DNA with primers KK71 (5-CGCGCTCGAGATAAGATGGAGGATGGATAATG) and KK72 (5′-CGCGAAGCTTGGAAGCCTATCGGGTAGCTG) containing XhoI and HindIII sites, respectively. The resulting PCR product was digested with XhoI and HindIII and inserted into the XhoI/HindIII site of plasmid pBAD/myc-HisA (8) to produce plasmid pMGJ1. In this construct, the expression of the oxyR gene is under the control of the arabinose-inducible araBAD promoter. Plasmids containing versions of the oxyR gene locked in the oxidized (pMAS141) and reduced (pMAS142) forms were constructed by using overlapping PCR with primers KK71 (upstream primer) and KK72 (downstream primer), together with primers 281 and 282 (locked reduced form; 5′-GGAAGATGGTCACTCTTTGCGCGATCAGG and 5′-CCTGATCGCGCAAAGAGTGACCATCTTCC) or primers 283 and 284 (locked oxidized form; 5′-GCTGGAAGATGGTCGCTGTTTGCGCGATC and 5′-GATCGCGCAAACAGCGACCATCTTCCAGC). These primers incorporate a specific amino acid change into the OxyR protein, C199S for OxyRRed (OxyRRed,C199S) and H198R for OxyROx (OxyROx,H198R). Plasmids containing each of the oxyR genes were introduced into oxyR mutant strain MGJ1.
DNA manipulations.
Plasmid DNA was isolated by using a QIAprep spin miniprep kit (Qiagen). Restriction endonucleases were used according to the manufacturer's specifications (Biolabs). PCRs were carried out as previously described (25). Amplified products were sequenced by using an ABI PRISM BigDye terminator cycle sequencing ready reaction kit (PE Applied Biosystems) to ensure the fidelity of the PCRs. Samples were run on a Perkin-Elmer ABI PRISM 310 genetic analyzer (PE Applied Biosystems) as described in the manufacturer's specifications.
Colony morphology.
Colony morphology was assayed by using a Carl Zeiss Axioplan epifluorescence microscope, and digital images were captured with a 12-bit cooled slow-scan charge-coupled device camera (KAF 1400 chip; Photometrics, Tucson, Ariz.) controlled by PMIS software (Photometrics).
Western immunoblotting.
Samples were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride microporous membrane filters as described previously (25). Serum raised against the α subunit of Ag43 was used as the primary antibody, and peroxidase-conjugated anti-rabbit serum was used as the secondary antibody.
Hydrogen peroxide sensitivity.
Resistance to hydrogen peroxide was assayed by measurement of zones of inhibition. A suspension of cells from an overnight culture was added to 20 ml of overlay agar, and the mixture was poured into a petri dish and allowed to dry. Five microliters of 30% H2O2 was added to the center of the plate and allowed to adsorb. Zones of growth inhibition were measured after overnight growth at 37°C.
The ability of Ag43-mediated cell aggregation to protect against hydrogen peroxide killing was assessed according to the following protocol. Cells from overnight cultures were washed and resuspended in phosphate-buffered saline to a concentration of approximately 109 cells per ml. For each strain, 100-μl aliquots were incubated at room temperature for 15 min in a microtiter plate. Then, 100 μl of an H2O2 solution (starting at 2.2 M and serially diluted to 0.03 M) was added, and the mixture was incubated for 15 min at room temperature. The cells were washed and then plated in a dilution series to determine the total number of surviving cells.
DTT-induced inhibition of Ag43 expression.
An overnight culture of E. coli strain MS428 was diluted and grown in 100 ml of LB medium with gentle shaking at 37°C. Aliquots (100 μl) of either 1 or 2 M dithiothreitol (DTT) were added every 15 min starting at an optical density at 600 nm (OD600) of 0.1. At an OD600 of 1.0, 10 ml of culture was harvested and washed in phosphate-buffered saline. The α subunit of Ag43 was detached by incubating the cells for 4 min at 60°C. Cells were removed by centrifugation, and the protein in the supernatant was precipitated with 75% acetone. Samples were subjected to Western blotting.
Biofilm assay.
Biofilm formation on plastic surfaces was monitored by using 96-well microtiter plates as previously described (17), the only difference being that arabinose was added for promoter induction.
RESULTS
Construction of locked OxyR variants.
Cysteine residue C199 is pivotal in determining the redox state of OxyR. Thus, out of six cysteine residues, only C199 seems to be essential for activity (19). It is solvent accessible and subject to redox changes (14, 19). The shift between the reduced and the oxidized forms of OxyR involves C199. A mutation, C199S, locks OxyR in the reduced form, OxyRRed,C199S; conversely, a mutation involving position 198, H198R, causes locking of OxyR in the oxidized form, OxyROx,H198R (18). Accordingly, we introduced these changes into the wild-type oxyR gene to create two locked forms: pMAS141 (C199S, locked reduced form) and pMAS142 (H198R, locked oxidized form). The tightly regulated arabinose-inducible araBAD promoter was used for expression of the genes (8). The wild-type oxyR gene, also controlled by the inducible araBAD promoter (pMGJ1), was used for comparison. All three constructs were made in the same manner to ensure that there were no differences in gene expression. As a host we constructed an oxyR derivative of the E. coli K-12 reference strain MG1655, i.e., MGJ1. The oxyR constructs were monitored for their responses to hydrogen peroxide (Fig. 1). As expected, the MGJ1 host harboring the locked reduced form of OxyR showed a response similar to that of reference strain MGJ1 and was unable to activate the oxidative stress response of the OxyR regulon. Conversely, MGJ1 harboring the locked oxidized form of OxyR was slightly more resistant than MGJ1 harboring the wild-type protein, probably due to priming of the oxidative stress response regulon.
FIG. 1.
H2O2 response profiles monitored by adding identical aliquots of H2O2 to the center of plates incubated with oxyR strain MGJ1 harboring various plasmids. (A to D) The plasmids were pBAD/myc-HisA (A), pMGJ1 (wild-type OxyR) (B), pMAS141 (OxyRRed,C199S) (C), and pMAS142 (OxyROx,H198R) (D). Note the presence of bubbles in MGJ1(pMAS142) (D), likely due to enhanced catalase activity resulting from priming of the oxidative stress response regulon. (E) Block diagram showing the mean sizes of the zones of inhibition and standard deviations from eight independent experiments. WT, wild type.
To further characterize the oxyR derivatives, the protein profiles of the strains were investigated by SDS-polyacrylamide gel electrophoresis. As evidenced in Fig. 2, clear differences could be seen in the protein profiles, which could be ascribed to the oxyR status of the strains, i.e., ΔoxyR, wild-type oxyR, oxyRRed,C199S, and oxyROx,H198R. In addition, bands of equal intensities and migrating at the predicted molecular mass of OxyR (34 kDa) were present in all OxyR-expressing strains but not in the ΔoxyR mutant strain, suggesting that the mutations did not influence the level of OxyR. These observations led us to believe that our oxyR variants encoded type-specific functional gene products and could be used as tools to monitor Ag43 expression.
FIG. 2.
Coomassie brilliant blue-stained SDS-polyacrylamide gel (left panel) and Western immunoblot (right panel) of cell lysates of oxyR strain MGJ1 harboring various plasmids. Lane 1, pBAD/myc-HisA; lane 2, pMGJ1 (wild-type OxyR); lane 3, pMAS141 (OxyRRed,C199S); lane 4, pMAS142 (OxyROx,H198R). Western blotting was performed with a polyclonal serum raised against the α subunit of Ag43. The position of the Ag43 α-subunit protein is indicated. Molecular weight size markers (in thousands) are shown at the left.
Only the reduced form of OxyR represses flu.
Western immunoblotting with Ag43 α-fragment-specific antiserum revealed a band migrating at the expected apparent molecular weight for the Ag43 α fragment in lysates from ΔoxyR and oxyROx,H198R cells but not from oxyRRed,C199S cells; a faint band was observed in a wild-type background (Fig. 2). One of the hallmarks of Ag43 expression is the ability to cause autoaggregation of cells with subsequent settling in liquid media. When the settling profiles of the four oxyR variants were monitored, it appeared that only hosts with oxyRRed,C199S or the wild-type oxyR allele stayed in suspension, whereas the ΔoxyR and oxyROx,H198R hosts flocculated (Fig. 3). These results suggest that only the reduced form of OxyR is able to repress the flu gene.
FIG. 3.
Settling profiles of strain MGJ1 harboring plasmids pBAD/myc-HisA (triangles), pMAS141 (OxyRRed,C199S) (crosses), pMGJ1 (wild-type OxyR) (squares), and pMAS142 (OxyROx,H198R) (circles).
Another phenotypic hallmark associated with Ag43 expression is a characteristic frizzy colony morphology (form 1), contrasting the flat opaque colony morphology (form 3) of Ag43-negative cells (11, 16). When MGJ1 cells containing plasmid pMAS141 (oxyRRed,C199S) or pMAS142 (oxyROx,H198R) were grown on arabinose-containing solid media, distinct frizzy colony morphotypes were observed for the oxyROx,H198R strain. On the contrary, only form 3 colony types were seen for the oxyRRed,C199S strain, indicating that the flu gene was tightly repressed (Fig. 4A).
FIG. 4.
Ag43 expression and associated protection against H2O2. (A) Colony morphology of strains examined by phase-contrast microscopy after overnight growth on solid media with 0.2% arabinose. Colonies were photographed at the same magnification. Panel 1, E. coli strain MGJ1 containing plasmid pMAS141 (OxyRRed,C199S); panel 2, E. coli strain MGJ1 containing plasmid pMAS142 (OxyROx,H198R). (B) Phase-contrast microscopy of cells. Panel 1, E. coli strain MS528 (fim flu) containing the vector control (pBR322); panel 2, E. coli strain MS528 containing the flu-carrying plasmid pHHA147. (C) Ag43-mediated cell aggregation enhances the ability to survive lethal doses of hydrogen peroxide. Approximately 109 cells of each strain were exposed to a series of H2O2 concentrations and plated on solid medium to obtain viable counts. Shown are the numbers of surviving cells after exposure to 0.275 M H2O2. Two strain sets were used, each differing only in their Ag43 expression. The first set included E. coli strain MS528 containing the vector control and plasmid pHHA147 (flu+); the second set included a non-Ag43-expressing variant (BD1511) and an Ag43-expressing variant (BD1512) of E. coli strain BD1428. Error bars indicate standard deviations.
A simple reducing agent, DTT, is able to affect Ag43 expression.
Since the locked reduced form, OxyRRed,C199S, was found to repress flu, we investigated whether a reducing environment would cause the repression of flu by driving the wild-type OxyR pool into a more reduced state. For this purpose, we used a Δfim strain, MS428. Since large doses of DTT inhibit cell growth, we modified our experimental conditions to not be growth inhibitory by pulse-wise addition of DTT over several generations (Fig. 5). The DTT-treated cultures and an untreated control were subsequently analyzed by Western blotting (Fig. 5). The data suggest that the addition of DTT reduces the expression of Ag43, likely by driving the cellular pool of OxyR into a more reduced state. These data corroborate the data obtained with the mutant form of OxyR and support the notion that OxyR control of flu is redox dependent.
FIG. 5.
Influence of DTT on cell growth and Ag43 expression. (A) E. coli MG1655 fim grown in LB medium (squares) and the same strain grown in LB medium but pulsed at regular 15-min intervals with 100 μl of either 1.0 M (triangles) or 2.0 M (circles) DTT. (B) Western blot of concentrated heat-extracted cell surface proteins obtained from cultures grown to an OD600 of 1.0. Lane 1, E. coli MG1655 fim; lane 2, E. coli MG1655 fim grown with 100-μl pulses of 1.0 M DTT every 15 min; lane 3, E. coli MG1655 fim grown with 100-μl pulses of 2.0 M DTT every 15 min. Western blotting was performed with a polyclonal serum raised against the α subunit of Ag43. The position of the Ag43 α-subunit protein is indicated.
Ag43-mediated autoaggregation protects against oxidizing agents.
OxyR essentially monitors the redox status of the cell and, together with its associated regulon, constitutes the primary response or defense of the cell against H2O2-induced oxidative stress. The above results suggested a link between the oxidative status of OxyR and Ag43 expression. This suggestion prompted us to investigate more directly the effects of Ag43 expression and associated cell aggregation on the survival of cells exposed to H2O2. When Ag43-expressing and non-Ag43-expressing variants of two different E. coli K-12 strains were exposed to lethal concentrations of H2O2 and subsequently plated to determine viable counts, clear differences in survival (approximately 10-fold) were observed between the two host strain sets (Fig. 4C). The real difference in survival was probably larger because the colony counts of Ag43-expressing cells were underestimated due to the fact that Ag43-positive cells aggregate and thus are not plated singly (Fig. 4B).
Biofilm-forming ability correlates with the redox status of OxyR.
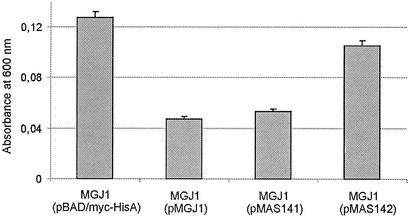
By virtue of its excellent cell-aggregating properties, Ag43 expression enhances bacterial biofilm formation (6, 16, 17). In keeping with the notion that the redox status of OxyR determines its ability to repress Ag43 expression, we investigated the biofilm-forming ability of MGJ1 cells harboring different versions of OxyR. From the results, it became obvious that OxyRRed,C199S hosts were significantly impeded in biofilm formation compared to their OxyROx,H198R counterparts (Fig. 6). Clearly, these results should be attributed to the fact that in OxyRRed,C199S cells, Ag43 expression was virtually abolished (Fig. 2). It appears that Ag43-assisted biofilm formation depends on cellular redox status.
FIG. 6.
Effect of OxyR redox status on biofilm formation by MGJ1 hosts containing various plasmids. Plasmids were as follows, from left to right: pBAD/myc-HisA (vector control), pMGJ1 (wild-type OxyR), pMAS141 (OxyRRed,C199S), and pMAS142 (OxyROx,H198R). Strains were grown on polystyrene microtiter plates. Adherent cells were stained with 0.1% crystal violet. Shown is the quantification of results after the determination of A600 readings. Error bars indicate standard deviations.
DISCUSSION
It is implicit that bacteria such as E. coli, with a diverse subset of environmental niches inside and outside a mammalian host, must be subject to changing redox conditions. To this end, bacteria have developed efficient sensors. The paradigmatic bacterial redox sensor OxyR is one of the main sensors for monitoring the redox level in the cell. The pioneer work of Zheng and coworkers suggested that only two forms of OxyR exist in vivo, differing only in the presence (oxidized) or absence (reduced) of a disulfide bridge spanning C199 and C208 (31).
The reduced form of OxyR, OxyR-SH, has been proposed to be dominant under normal growth conditions, i.e., when the cell is not subjected to oxidative or disulfide stress. The results presented here suggest that the flu gene is repressed by the reduced form of OxyR. Several lines of evidence support this conclusion. First, OxyRRed,C199S cells do not settle from standing cultures, whereas OxyROx,H198R cells settle rapidly, like cells having no oxyR gene (indicative of no repression). Second, OxyROx,H198R cells form frizzy colonies, contrary to OxyRred,C199S cells, which form nonfrizzy colonies. Third, no Ag43 can be detected by Western blotting of lysates from OxyRRed,C199S hosts, but Ag43 can be readily detected in lysates from OxyROx,H198R hosts. Finally, pulse-wise addition of a simple reducing agent, DTT, to Ag43-expressing cells seems to suppress expression (Fig. 5). It was previously demonstrated that the addition of DTT to cells causes skewing of the colony morphology toward the form 3 nonfrizzy morphotype (24). Taken together, these results suggest that only the reduced form is capable of repressing the flu gene and that it does so very tightly.
Interestingly, the reduced form of OxyR has been shown to be dominant in normal growing cells, because the redox potential of OxyR is more positive than the redox potential of the resting cytoplasm by ∼85 mV (1, 2, 31). The addition of a reducing agent such as DTT should not significantly alter the redox status of the OxyR pool and therefore would not be expected to repress flu any further. However, we observed a dramatic difference (Fig. 5). The common view of redox regulation as a simple on-off switch may be too simplistic, according to recent work by Kim et al. (14). They proved that several stable forms of OxyR, all with modifications of C199, occur in vivo. These oxidized forms of OxyR, such as, for example, sulfenic acid (OxyR-S-OH) and glutathione disulfide (OxyR-S-SG), have dissimilar conformations and different DNA affinities (14). This family of OxyR derivatives would be well adapted to showing a graded response to changes in cellular redox status. In view of these facts, it is possible that the DTT-induced repression of Ag43 expression that we observed occurred because the addition of the reducing agent caused skewing of the balance within the cellular OxyR family toward a form, presumably OxyR-SH, which represses the flu gene efficiently. Further work is required to clarify the specific molecular forms of OxyR that are present or dominant under different growth conditions.
The expression of Ag43 is phase variable due to the concerted action of the Dam methyltransferase and OxyR (9, 13, 30). The flu promoter region contains three GATC sites, which overlap a recognition sequence for OxyR (9, 10, 13). When the GATC sites are methylated, OxyR cannot bind; conversely, bound OxyR prevents methylation (29). On the chromosome, the methylation of a newly synthesized GATC target normally takes place within a few minutes (4). When the replication fork has passed the flu promoter region, the race is on between Dam and OxyR to either activate or repress the gene. It is tempting to speculate that a modest increase in the proportion of OxyR in the repression-compatible form (reduced) as a result of the addition of DTT would, over a few generations, change the balance toward a population with fewer Ag43-expressing cells.
What are the biological implications of these findings? A corollary would be that since Ag43 expression seems to be repressed under strongly reducing conditions, it should be less so under oxidizing conditions. This idea prompted us to investigate the possibility of whether Ag43 expression would confer a measure of protection against oxidizing agents. According to this tenet, the tight packing of cells in cellular aggregates connected with Ag43 expression could provide a mechanism for reducing local oxygen concentrations, thereby protecting the cells from damage caused by oxidizing agents. We found strong support for this hypothesis in the fact that Ag43 expression and the ensuing cell-to-cell aggregation were observed to be concomitant with a high degree of protection against H2O2 killing (Fig. 4). Curiously, we found that the addition of sublethal concentrations of H2O2 to cells did not result in any significant increase in Ag43 expression. Protection against H2O2 killing by Ag43-mediated cell aggregation therefore seems to be a side effect of Ag43 expression; the expression itself seems not to be caused directly by H2O2-induced stress. An explanation for this phenomenon could be that only a minor fraction of the OxyR pool is oxidized under such conditions; this amount is sufficient to activate the oxidative stress response, whereupon the cellular redox status returns to normal within a few minutes (2). However, more than enough OxyR likely remains in a reduced state compatible with the repression of flu.
Several investigators have demonstrated that Ag43 expression confers excellent biofilm-forming properties (6, 16, 17). Since the OxyRRed,C199S form virtually abolishes Ag43 expression, we surmised that cells harboring this version would be significantly handicapped in biofilm formation; this turned out to be the case. Ag43 is expressed in a wide range of E. coli strains. Interestingly, it is expressed by many pathogenic strains. Thus, a survey of enteropathogenic and urinary tract infection E. coli strains showed that 77 and 60%, respectively, of these strains were capable of Ag43 expression (21). Although E. coli K-12 has only one copy of the flu gene, it appears that the situation for wild-type strains is more complex and that two or more copies are present in enteropathogenic E. coli strains and some enterohemorrhagic E. coli strains (22, 28). Data available from databases indicate that multiple flu genes have similar promoter regions, suggesting that control is similar to that of the flu gene in E. coli K-12. In view of the fact that flu is found in so many pathogenic strains, the findings reported here could be important for understanding how bacteria behave during infection. Biofilms are highly complex, structurally organized sessile communities. Numerous bacterial infections occur as biofilms. It has been shown that such communities are highly resistant to antimicrobial agents (5). Here we have demonstrated that a simpler form of cooperative behavior, namely, Ag43-induced bacterial clumping, can also be a highly efficient defense mechanism.
Acknowledgments
We thank Birthe Jul J/orgensen for expert technical assistance.
This work was supported by the Danish Technical Research Council (grant 26-02-0183) and the Natural Sciences Research Council (grant 21-01-0296).
REFERENCES
- 1.Åslund, F., and J. Beckwith. 1999. Bridge over troubled waters: sensing stress by disulfide bond formation. Cell 96:751-753. [DOI] [PubMed] [Google Scholar]
- 2.Åslund, F., M. Zheng, J. Beckwith, and G. Storz. 1999. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc. Natl. Acad. Sci. USA 96:6161-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann, B. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhardt, R. Curtis III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 4.Campbell, J. L., and N. Kleckner. 1988. The rate of Dam-mediated DNA adenine methylation in Escherichia coli. Gene 74:189-190. [DOI] [PubMed] [Google Scholar]
- 5.Costerson, J. W., P. S. Steward, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Danese, P. N., I. A. Pratt, S. Dove, and R. Kolter. 2000. The outer-membrane protein, Ag43, mediates cell-to-cell interactions within E. coli biofilms. Mol. Microbiol. 37:424-432. [DOI] [PubMed] [Google Scholar]
- 7.Diderichsen, B. 1980. flu, a metastable gene controlling surface properties of Escherichia coli. J. Bacteriol. 141:858-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haagmans, W., and M. van der Woude. 2000. Phase variation of Ag43 in Escherichia coli: Dam-dependent methylation abrogates OxyR binding and OxyR-mediated repression of transcription. Mol. Microbiol. 35:877-887. [DOI] [PubMed] [Google Scholar]
- 10.Hasman, H., T. Chakraborty, and P. Klemm. 1999. Antigen 43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 181:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasman, H., M. A. Schembri, and P. Klemm. 2000. Antigen 43 and type 1 fimbriae determine colony morphology of Escherichia coli K-12. J. Bacteriol. 182:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 13.Henderson, I. R., and P. Owen. 1999. The major phase-variable outer membrane protein of Escherichia coli structurally resembles the immunoglobulin A1 protease class of exported protein and is regulated by a novel mechanism involving Dam and OxyR. J. Bacteriol. 181:2132-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, S. O., K. Merchant, R. Nudelman, W. F. Bayer, Jr., T. Keng, J. DeAngelo, A. Hausladen, and J. S. Stamler. 2002. OxyR: a molecular code for redox-related signaling. Cell 109:383-396. [DOI] [PubMed] [Google Scholar]
- 15.Kjaergaard, K., H. Hasman, M. A. Schembri, and P. Klemm. 2002. Antigen 43-mediated autotransporter display, a versatile bacterial cell surface presentation system. J. Bacteriol. 184:4197-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kjaergaard, K., M. A. Schembri, H. Hasman, and P. Klemm. 2000. Antigen 43 from Escherichia coli induces inter- and intraspecies cell aggregation and changes in colony morphology of Pseudomonas fluorescens. J. Bacteriol. 182:4789-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kjaergaard, K., M. A. Schembri, C. Ramos, S. Molin, and P. Klemm. 2000. Antigen 43 facilitates formation of multispecies biofilms. Environ. Microbiol. 2:695-702. [DOI] [PubMed] [Google Scholar]
- 18.Kullik, I., J. Stevens, M. B. Toledano, and G. Storz. 1995. Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for DNA binding and multimerization. J. Bacteriol. 177:1285-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kullik, I., M. B. Toledano, L. A. Tartaglia, and G. Storz. 1995. Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for oxidation and transcriptional activation. J. Bacteriol. 177:1275-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen, P. 1992. The gram-negative outer membrane: structure, biochemistry and vaccine potential. Biochem. Soc. Trans. 20:1-6. [DOI] [PubMed] [Google Scholar]
- 21.Owen, P., M. Meehan, H. de Loughry-Doherty, and I. Henderson. 1996. Phase-variable outer membrane proteins in Escherichia coli. FEMS Immunol. Med. Microbiol. 16:63-76. [DOI] [PubMed] [Google Scholar]
- 22.Roche, A. J., J. P. McFadden, and P. Owen. 2001. Antigen 43, the major phase-variable protein of the Escherichia coli outer membrane, can exist as a family of proteins encoded by multiple alleles. Microbiology 147:161-169. [DOI] [PubMed] [Google Scholar]
- 23.Schembri, M. A., M. Givskov, and P. Klemm. 2002. An attractive surface: gram-negative bacterial biofilms. Sci. STKE 2002:RE6. [Online.] http://www.stke.org/cgi/content/full/OC_sigtrans;2002/132/re6. [DOI] [PubMed]
- 24.Schembri, M. A., and P. Klemm. 2001. Coordinate gene regulation by fimbriae-induced signal transduction. EMBO J. 20:3074-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stentebjerg-Olesen, B., L. Pallesen, L. B. Jensen, G. Christiansen, and P. Klemm. 1997. Authentic display of a cholera toxin epitope by chimeric type 1 fimbriae: effects of insert position and host background. Microbiology 143:2027-2038. [DOI] [PubMed] [Google Scholar]
- 26.Storz, G., and J. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:88-94. [DOI] [PubMed] [Google Scholar]
- 27.Toledano, M. B., I. Kullik, F. Trinh, P. T. Baird, T. D. Sneider, and G. Storz. 1994. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site:a mechanism for differential promoter selection. Cell 78:897-909. [DOI] [PubMed] [Google Scholar]
- 28.Torres, A. G., N. T. Perna, V. Burland, A. Ruknudin, F. R. Blattner, and J. B. Kaper. 2002. Characterization of Cah, a calcium-binding and heat-extractable autotransporter of enterohaemorrhagic E. coli. Mol. Microbiol. 45:951-966. [DOI] [PubMed] [Google Scholar]
- 29.Waldron, D. E., P. Owen, and C. J. Dorman. 2002. Competitive interaction of the OxyR DNA-binding protein and the Dam methylase at the antigen 43 gene regulatory region in Escherichia coli. Mol. Microbiol. 44:509-520. [DOI] [PubMed] [Google Scholar]
- 30.Warne, S. R., J. M. Varley, G. J. Boulnois, and M. G. Norton. 1990. Identification and characterization of a gene that controls colony morphology and auto-aggregation in Escherichia coli K12. J. Gen. Microbiol. 136:455-462. [DOI] [PubMed] [Google Scholar]
- 31.Zheng, M., F. Åslund, and G. Storz. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718-1721. [DOI] [PubMed] [Google Scholar]
- 32.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng, M., X. Wang, B. Doan, K. Lewis, T. D. Schneider, and G. Storz. 2001. Computation-directed identification of OxyR DNA binding sites in Escherichia coli. J. Bacteriol. 183:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng, M., X. Wang, L. Templeton, D. R. Smulski, R. A. LaRossa, and G. Storz. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 183:4562-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]