Abstract
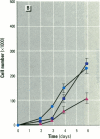
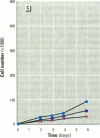
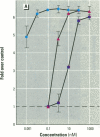
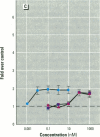
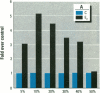
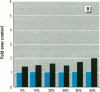
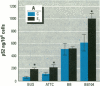
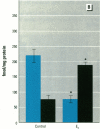
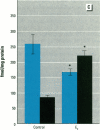
MCF7 human breast cancer cells have been studied extensively as a model for hormonal effects on breast cancer cell growth and specific protein synthesis. Because the proliferative effect of natural estrogen is considered the hallmark of estrogen action, it was proposed that this property be used to determine whether a substance is an estrogen. The E-screen assay, developed for this purpose, is based on the ability of MCF7 cells to proliferate in the presence of estrogens. The aim of our study was to characterize the response of four MCF7 cell stocks (BUS, ATCC, BB, and BB104) and determine which of them performed best in the E-screen test. The four stocks assayed were distinguishable by their biological behavior. In the absence of estrogen, MCF7 BUS cells stopped proliferating and accumulated in the G0/G1 phase of the cell cycle; estrogen receptors increased, progesterone receptors decreased, and small amounts of pS2 protein were secreted. Of all the MCF7 stocks tested, MCF7 BUS cells showed the highest proliferative response to estradiol-17 beta: cell yields increased up to sixfold over those of nontreated cells in a 144-hr period. The differences between estrogen-supplemented and nonsupplemented MCF7 BUS cells were due mostly to G0/G1 proliferative arrest mediated by charcoal dextran-stripped serum. MCF7 BUS cell stocks and others showing a similar proliferative pattern should be chosen for use in the E-screen test, or whenever a proliferative effect of estrogen is to be demonstrated.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arteaga C. L., Tandon A. K., Von Hoff D. D., Osborne C. K. Transforming growth factor beta: potential autocrine growth inhibitor of estrogen receptor-negative human breast cancer cells. Cancer Res. 1988 Jul 15;48(14):3898–3904. [PubMed] [Google Scholar]
- Baker P. R., Wilton J. C., Jones C. E., Stenzel D. J., Watson N., Smith G. J. Bile acids influence the growth, oestrogen receptor and oestrogen-regulated proteins of MCF-7 human breast cancer cells. Br J Cancer. 1992 Apr;65(4):566–572. doi: 10.1038/bjc.1992.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezwoda W. R., Meyer K. Effect of alpha-interferon, 17 beta-estradiol, and tamoxifen on estrogen receptor concentration and cell cycle kinetics of MCF 7 cells. Cancer Res. 1990 Sep 1;50(17):5387–5391. [PubMed] [Google Scholar]
- Bronzert D. A., Triche T. J., Gleason P., Lippman M. E. Isolation and characterization of an estrogen-inhibited variant derived from the MCF-7 breast cancer cell line. Cancer Res. 1984 Sep;44(9):3942–3951. [PubMed] [Google Scholar]
- Brünner N., Frandsen T. L., Holst-Hansen C., Bei M., Thompson E. W., Wakeling A. E., Lippman M. E., Clarke R. MCF7/LCC2: a 4-hydroxytamoxifen resistant human breast cancer variant that retains sensitivity to the steroidal antiestrogen ICI 182,780. Cancer Res. 1993 Jul 15;53(14):3229–3232. [PubMed] [Google Scholar]
- Butler W. B., Berlinski P. J., Hillman R. M., Kelsey W. H., Toenniges M. M. Relation of in vitro properties to tumorigenicity for a series of sublines of the human breast cancer cell line MCF-7. Cancer Res. 1986 Dec;46(12 Pt 1):6339–6348. [PubMed] [Google Scholar]
- Calvo F., Brower M., Carney D. N. Continuous culture and soft agarose cloning of multiple human breast carcinoma cell lines in serum-free medium. Cancer Res. 1984 Oct;44(10):4553–4559. [PubMed] [Google Scholar]
- Catherino W. H., Jeng M. H., Jordan V. C. Norgestrel and gestodene stimulate breast cancer cell growth through an oestrogen receptor mediated mechanism. Br J Cancer. 1993 May;67(5):945–952. doi: 10.1038/bjc.1993.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correc P., Fondanèche M. C., Bracke M., Burtin P. The presence of plasmin receptors on three mammary carcinoma MCF-7 sublines. Int J Cancer. 1990 Oct 15;46(4):745–750. doi: 10.1002/ijc.2910460432. [DOI] [PubMed] [Google Scholar]
- Devleeschouwer N., Legros N., Olea-Serrano N., Paridaens R., Leclercq G. Estrogen conjugates and serum factors mediating the estrogenic trophic effect on MCF-7 cell growth. Cancer Res. 1987 Nov 15;47(22):5883–5887. [PubMed] [Google Scholar]
- Gyling M., Leclercq G., Heuson J. C. Estrogenic and antiestrogenic down-regulation of estrogen receptor levels: evidence for two different mechanisms. J Recept Res. 1990;10(5-6):217–234. doi: 10.3109/10799899009064667. [DOI] [PubMed] [Google Scholar]
- Karey K. P., Sirbasku D. A. Differential responsiveness of human breast cancer cell lines MCF-7 and T47D to growth factors and 17 beta-estradiol. Cancer Res. 1988 Jul 15;48(14):4083–4092. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Kendra K. L., Norman M. J., Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Res. 1987 Aug 15;47(16):4355–4360. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Norman M. J., Eckert R. L., Peltz S. W., Mangel W. F. Bioactivities, estrogen receptor interactions, and plasminogen activator-inducing activities of tamoxifen and hydroxy-tamoxifen isomers in MCF-7 human breast cancer cells. Cancer Res. 1984 Jan;44(1):112–119. [PubMed] [Google Scholar]
- Krishnan A. V., Stathis P., Permuth S. F., Tokes L., Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993 Jun;132(6):2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Lottering M. L., Haag M., Seegers J. C. Effects of 17 beta-estradiol metabolites on cell cycle events in MCF-7 cells. Cancer Res. 1992 Nov 1;52(21):5926–5932. [PubMed] [Google Scholar]
- Lykkesfeldt A. E., Laursen I., Briand P. Regulation of the secretion of proteins synthesized by the human breast cancer cell line, MCF-7. Mol Cell Endocrinol. 1989 Apr;62(2):287–296. doi: 10.1016/0303-7207(89)90016-6. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Sutherland R. L. Differential effects of tamoxifen and analogs with nonbasic side chains on cell proliferation in vitro. Endocrinology. 1985 Mar;116(3):1071–1078. doi: 10.1210/endo-116-3-1071. [DOI] [PubMed] [Google Scholar]
- Natoli C., Sica G., Natoli V., Serra A., Iacobelli S. Two new estrogen-supersensitive variants of the MCF-7 human breast cancer cell line. Breast Cancer Res Treat. 1983;3(1):23–32. doi: 10.1007/BF01806231. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Hobbs K., Trent J. M. Biological differences among MCF-7 human breast cancer cell lines from different laboratories. Breast Cancer Res Treat. 1987;9(2):111–121. doi: 10.1007/BF01807363. [DOI] [PubMed] [Google Scholar]
- Page M. J., Field J. K., Everett N. P., Green C. D. Serum regulation of the estrogen responsiveness of the human breast cancer cell line MCF-7. Cancer Res. 1983 Mar;43(3):1244–1250. [PubMed] [Google Scholar]
- Pourreau-Schneider N., Berthois Y., Mittre H., Charpin C., Jacquemier J., Martin P. M. Estrogen response of MCF-7 cells grown on diverse substrates and in suspension culture: promotion of morphological heterogeneity, modulation of progestin receptor induction; cell-substrate interactions on collagen gels. J Steroid Biochem. 1984 Dec;21(6):763–771. doi: 10.1016/0022-4731(84)90042-6. [DOI] [PubMed] [Google Scholar]
- Pratt S. E., Pollak M. N. Estrogen and antiestrogen modulation of MCF7 human breast cancer cell proliferation is associated with specific alterations in accumulation of insulin-like growth factor-binding proteins in conditioned media. Cancer Res. 1993 Nov 1;53(21):5193–5198. [PubMed] [Google Scholar]
- Prévost G., Foehrlé E., Thomas F., Pihan I., Veber N., Starzec A., Israël L. Growth of human breast cancer cell lines is inhibited by the somatostatin analog BIM23014. Endocrinology. 1991 Jul;129(1):323–329. doi: 10.1210/endo-129-1-323. [DOI] [PubMed] [Google Scholar]
- Reddel R. R., Sutherland R. L. Effects of pharmacological concentrations of estrogens on proliferation and cell cycle kinetics of human breast cancer cell lines in vitro. Cancer Res. 1987 Oct 15;47(20):5323–5329. [PubMed] [Google Scholar]
- Resnicoff M., Medrano E. E., Podhajcer O. L., Bravo A. I., Bover L., Mordoh J. Subpopulations of MCF7 cells separated by Percoll gradient centrifugation: a model to analyze the heterogeneity of human breast cancer. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7295–7299. doi: 10.1073/pnas.84.20.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedl C., Cappelletti V., Coradini D., Granata G., Di Fronzo G. Influence of culture conditions on the estrogenic cell growth stimulation of human breast cancer cells. J Steroid Biochem Mol Biol. 1990 Oct;37(2):195–200. doi: 10.1016/0960-0760(90)90327-h. [DOI] [PubMed] [Google Scholar]
- Ruenitz P. C., Thompson C. B., Srivatsan V. Characterization of MCF 7 breast cancer cell growth inhibition by the antiestrogen nitromifene (CI 628) and selected metabolites. J Steroid Biochem. 1989 Sep;33(3):365–369. doi: 10.1016/0022-4731(89)90325-7. [DOI] [PubMed] [Google Scholar]
- Schütze N., Vollmer G., Tiemann I., Geiger M., Knuppen R. Catecholestrogens are MCF-7 cell estrogen receptor agonists. J Steroid Biochem Mol Biol. 1993 Dec;46(6):781–789. doi: 10.1016/0960-0760(93)90319-r. [DOI] [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990 Jul 4;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Justicia H., Wray J. W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C. The role of estrogens on the proliferation of human breast tumor cells (MCF-7). J Steroid Biochem. 1985 Jul;23(1):87–94. doi: 10.1016/0022-4731(85)90265-1. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Taylor C. M., Blanchard B., Zava D. T. Estrogen receptor-mediated and cytotoxic effects of the antiestrogens tamoxifen and 4-hydroxytamoxifen. Cancer Res. 1984 Apr;44(4):1409–1414. [PubMed] [Google Scholar]
- Whang-Peng J., Lee E. C., Kao-Shan C. S., Seibert K., Lippman M. Cytogenetic studies of human breast cancer lines: MCF-7 and derived variant sublines. J Natl Cancer Inst. 1983 Oct;71(4):687–695. [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Zajchowski D. A., Sager R., Webster L. Estrogen inhibits the growth of estrogen receptor-negative, but not estrogen receptor-positive, human mammary epithelial cells expressing a recombinant estrogen receptor. Cancer Res. 1993 Oct 15;53(20):5004–5011. [PubMed] [Google Scholar]
- Zugmaier G., Knabbe C., Fritsch C., Simpson S., Ennis B., Lippman M., Dickson R. B. Tissue culture conditions determine the effects of estrogen and growth factors on the anchorage independent growth of human breast cancer cell lines. J Steroid Biochem Mol Biol. 1991 Nov;39(5A):681–685. doi: 10.1016/0960-0760(91)90367-e. [DOI] [PubMed] [Google Scholar]
- del Moral R., Ruiz de Almodóvar J. M., Fernández J. C., López-González J. D., Villalba J., Olea N., Pedraza V. Relationship between proliferative activity and cellular hormono-dependence in the MCF-7 breast cancer cell line. Rev Esp Fisiol. 1990 Sep;46(3):247–253. [PubMed] [Google Scholar]
- van der Burg B., Kalkhoven E., Isbrücker L., de Laat S. W. Effects of progestins on the proliferation of estrogen-dependent human breast cancer cells under growth factor-defined conditions. J Steroid Biochem Mol Biol. 1992 Jun;42(5):457–465. doi: 10.1016/0960-0760(92)90257-j. [DOI] [PubMed] [Google Scholar]