Abstract
Objective: The Brugada syndrome (BS) is characterized by an ST segment elevation in the right precordial ECG leads and a high risk of sudden death. The ECG sign of BS is often concealed, but can be unmasked with potent sodium channel blockers. Using canine right ventricular (RV) wedge preparations, we previously developed an experimental model of BS using flecainide to depress the action potential (AP) dome in RV epicardium. We hypothesized that a combination of INa and ICa blockade may be more effective in causing loss of the epicardial AP dome and precipitating the syndrome. The present study was designed to test this hypothesis using terfenadine, an antihistamine known to block both INa and ICa (as well as IKr). Methods: Intracellular APs and a transmural ECG were simultaneously recorded from canine RV wedge preparations. Results: Terfenadine (5-10 μM) caused a heterogeneous loss of the epicardial AP dome, resulting in ST segment elevation, phase 2 reentry (12/16), and spontaneous polymorphic VT/VF (6/16). Flecainide (≤7.5 μM), ajmaline (≤20 μM) and procainamide (≤300 μM) failed to generate polymorphic VT in any preparation except when combined with a calcium channel blocker (verapamil, ≤20 μM). Terfenadine-induced ST segment elevation was normalized and arrhythmias suppressed following Ito block with 4-aminopyridine (0.5 - 2 mM). Conclusion: Our data suggest that combined sodium and calcium channel block may be more effective than sodium channel block alone in unmasking the Brugada syndrome and that pharmacologic agents that inhibit Ito may be useful in preventing lethal arrhythmias in patients with the syndrome.
Keywords: Sodium Channel Blocker, Arrhythmia, Sudden Death, Ventricular Tachycardia, Ventricular Fibrillation
Introduction
The Brugada syndrome, first described as a new clinical entity a decade ago, is characterized by ST segment elevation in right precordial leads V1 to V3 and a high incidence of sudden death.1 ECG characteristics are unrelated to ischemia, electrolyte abnormalities, or structural heart disease. A right bundle branch block morphology, prolonged PR and HV intervals are observed in some patients, particularly those with documented SCN5A mutations. 2 The ECG sign of Brugada syndrome is often concealed, complicating diagnosis, but can be unmasked with potent sodium channel blockers, including Class IA or IC antiarrhythmic agents. 3-6
We have previously proposed a cellular mechanism for the Brugada syndrome in which accentuation of the epicardial action potential notch and eventual loss of the epicardial action potential dome results in ST segment elevation, phase 2 reentry, and polymorphic VT/VF. 7-9 The proposed mechanism involves a rebalancing of the currents available at the end of phase 1 of the epicardial action potential. Diminution of inward currents (INa and ICa) or enhancement of outward currents (Ito, IK-ATP) can result in a slowing of the second upstroke of the epicardial action potential, eventually leading to loss of the action potential dome as a consequence of all-or-none repolarization at the end of phase 1.
Based on this schema, we hypothesized that combined INa and ICa block would be more effective in unmasking the syndrome. The present study is designed to test this hypothesis using terfenadine, a histamine H1-receptor antagonist known to block INa, ICa, as well as IKr; 10-12 and the combination of sodium channel blockers and verapamil.
Methods
The detailed methods employed for isolation, perfusion and recording of transmembrane activity from the arterially perfused canine right ventricular (anterior wall) wedge preparation, as well as the viability and electrical stability of the preparation, have been previously reported. Experiments demonstrating that activity recorded from the cut surface of the perfused wedge preparation is representative of cells within the respective layers of the wall throughout the wedge have also been reported in a number of previous studies. 13-17
Briefly, transmural wedge preparations with dimensions of approximately 2 × 1 × 0.9 cm to 2.5 × 1.5 × 1.2 cm were dissected from the right ventricle of males and females. The preparations were cannulated via a small (diameter ∼100-150 μm) coronary artery (descending branch of the right coronary artery) and perfused with cardioplegic solution (Tyrode’s containing 8 mM KCl). Unperfused tissue was carefully removed using a razor blade. The preparations were then placed in a small tissue bath and arterially-perfused with Tyrode’s solution. The temperature of the coronary perfusate was maintained at 35 ± 0.5 °C. The perfusate was delivered to the artery by a roller pump (Cole Parmer Instrument Co., Niles, IL). Perfusion pressure was monitored with a pressure transducer (World Precision Instruments, Inc., Sarasota, FL) and maintained between 40 and 50 mm Hg by adjustment of the perfusion flow rate.
The wedge preparations were equilibrated in the tissue bath until electrically stable, usually 1-2 hours. The preparations were continuously stimulated at a basal cycle length of 2000 msec using bipolar silver electrodes insulated except at the tips and applied to the endocardial surface. A transmural electrocardiogram (ECG) was recorded using electrodes consisting of AgCl half cells attached to Tyrode’s-filled tapered polyethylene electrodes placed in the Tyrode’s solution bathing the preparation, 1.0 to 1.5 cm from the epicardial and endocardial surfaces of the preparation, along the same axis as the transmembrane recordings (Epicardium: “+” pole). Transmembrane action potentials were simultaneously recorded from 2 epicardial and 1 endocardial sites using floating microelectrodes (DC resistance: 10 to 20 Mω) filled with 2.7 M KCl and connected to a high-input impedance amplifier. Impalements were obtained from the epicardial and endocardial surfaces of the preparation at positions approximating the transmural axis of the ECG recording. When two simultaneous epicardial impalements were recorded, the one with the longer APD was designated as Epi1, while the other was designated as Epi 2.
Statistics were performed using either paired or unpaired t test, as appropriate. All data are reported as mean ± SE.
Results
The ability of combined INa and ICa block to cause loss of the epicardial action potential dome and phase 2 reentry in the canine right ventricular wedge preparation is illustrated in Figure 1. High concentrations of terfenadine, in this case 5 μM, produced an accentuation of the epicardial action potential notch following acceleration of the rate from a BCL of 800 msec to 400 msec. The dramatic accentuation of the notch was due to the effect of the drug to depress the phase 0, augment the magnitude of phase 1, and delay the appearance of the second upstroke. The electrocardiographic manifestations of these changes in action potential characteristics include an elevation of J point, augmentation of the J wave, and inversion of the T wave (Figure 1A vs. 1B). With continued rapid pacing, phase 1 became more accentuated, progressing until all-or-none repolarization occurred at the end of phase 1 at some epicardial sites but not others, leading to the development of both epicardial (EDR) and transmural (TDR) dispersion of repolarization (Figure 1C). Propagation of the dome from the region where it was maintained to the region at which it was lost resulted in the development of local phase 2 reentry (Figure 1D).
Figure 1.
Terfenadine-induced ST segment elevation, T wave inversion, transmural and epicardial dispersion of repolarization and phase 2 reentry. Each panel shows transmembrane action potentials from one endocardial (top) and two epicardial sites together with a transmural ECG recorded from a canine arterially-perfused right ventricular wedge preparation. A: Control (BCL 400 msec). B: Terfenadine (5 μM) accentuated the epicardial action potential notch creating a transmural voltage gradient that manifests as an ST segment elevation or exaggerated J wave in the ECG. First beat recorded after changing from BCL 800 msec to BCL 400 msec. C: Continued pacing at BCL 400 msec results in all-or-none repolarization at the end of phase 1 at some epicardial sites but not others, creating a local epicardial dispersion of repolarization (EDR) as well as a transmural dispersion of repolarization (TDR). D: Phase 2 reentry occurs when the epicardial action potential dome propagates from a site where it is maintained to regions where it has been lost. (Note: Panel D was recorded from a different preparation.)
This pharmacologic model of the Brugada syndrome differs from the congenital forms with respect to the rate dependence of ST segment elevation. In the congenital forms, the slow recovery of Ito from inactivation renders this current less available at fast rates. As a consequence, acceleration generally leads to normalization of the ST segment or to no change. In some acquired forms of Brugada syndrome, as in this pharmacologic model, slow dissociation of the drug from the sodium and calcium channels leads to strong use dependent channel block and exacerbation of ST segment elevation at faster rates. An example of this characteristic of the model is illustrated in Figure 2. In the presence of 5 μM terfenadine, acceleration to BCL 400 msec causes a gradual outward shift in the balance of currents available at the end of phase 1, leading to all-or-none repolarization at the end of phase 1 in Epi 2. This progression is closely correlated with an elevation of the ST segment in the ECG (Figure 2B).
Figure 2.
Terfenadine-induced use-dependent block of the sodium and calcium inward currents leads to acceleration-induced loss of the action potential dome and ST segment elevation in the coronary-perfused RV wedge preparation. Traces were recorded immediately after changing stimulation rate from BCL of 800 to 400 msec. A: Control. B: Terfenadine (5 μM)
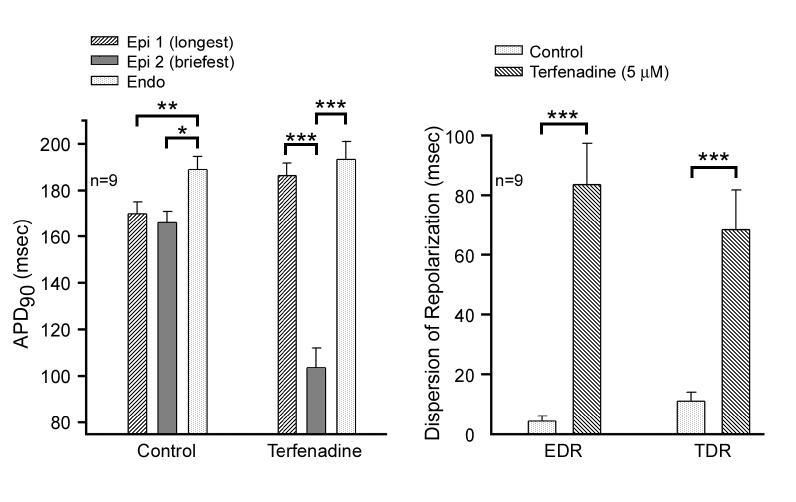
Action potential duration at 90 percent repolarization (APD90) was significantly (p<0.05) shorter in epicardium than the endocardium in control. In the presence of 5 μM terfenadine, APD90 of Epi 2 was significantly shorter than that of both Epi 1 and endocardium (p<0.001, Figure 3A). These differences represent the epicardial dispersion of repolarization (EDR) and transmural dispersion of repolarization (TDR), respectively (Figure 3B). EDR and TDR were 4.3±1.6 and 11.0±2.9 msec in control, and 83.6±13.8 and 68.5±13.2 msec after 5 μM terfenadine at BCL 400 msec (p<0.001).
Figure 3.
Composite data for 9 preparations of the effect of terfenadine (5 μM) to induce increases in transmural (TDR) and epicardial (EDR) dispersion of repolarization. A: APD90 at 2 epicardial and one endocardial site under control conditions (left) and after terfenadine (5 μM) at BCL 400 msec. B: EDR measured as the difference in repolarization time between the shortest and longest simultaneous epicardial action potentials and TDR measured as the difference between repolarization time of the shortest epicardial response and that of the simultaneous endocardial response. APD90= Action potential duration at 90 percent repolarization. * p<0.05, ** p<0.01, *** p<0.001
Terfenadine-induced phase 2 reentry gave rise to a closely coupled extrasystoles in 12/16 preparations. Figure 4A shows an example of this phenomenon. Of note, the phase 2 reentrant extrasystole is not apparent on the ECG because it is buried in the T wave of the preceding beat. The first extrasystole that can be distinguished in the ECG is that of the intramural circus movement reentry, which followed the phase 2 reentrant beat. In 6/16 preparations, the phase 2 reentrant beat spontaneously triggered a polymorphic ventricular tachycardia and fibrillation (VT/VF) (Fig. 4B). When phase 2 reentry did not occur or when it occurred too early to trigger intramural reentry, we were able to induce the arrhythmia by introducing a single extrinsic stimulus to epicardium. Moreover, when phase 2 reentry occurred but failed to induce VT (Fig. 4C), a single premature beat could be introduced to shift phase relationships so as to permit the induction of a long run of polymorphic VT (S1-S2=250 msec, Fig. 4D).
Figure 4.
Spontaneous and programmed electrical stimulation-induced polymorphic VT in RV wedge preparations pretreated with terfenadine (5-10 μM). A: Phase 2 reentry in epicardium gives rise to a closely coupled extrasystole. B: Phase 2 reentrant extrasystole triggers a brief episode of polymorphic VT. C: Phase 2 reentrant extrasystole triggers brief reentry. D: Same impalements and pacing conditions as C, however an extrastimulus (S1-S2 = 250 msec) applied to epicardium triggers a polymorphic VT.
In 5 of the 16 wedge preparations studied we observed the development of gross T wave alternans as a consequence of concealed phase 2 reentry occurring after alternate beats. An example is illustrated in Figure 5. The cellular or ionic basis for the success or failure of phase 2 reentry in this example is not obvious, but may be related to small alternation in the refractoriness of the region devoid of the action potential dome.
Figure 5.
T wave alternans. Transmembrane action potentials from one endocardial (top) and two epicardial sites together with a transmural ECG recorded from a canine arterially-perfused right ventricular wedge preparation pretreated with 5 μM terfenadine. T wave alternans occurs as a consequence of concealed phase 2 reentry following alternate beats.
Figure 6 shows the effect of the transient outward current (Ito) blocker 4-aminopyridine (4-AP, 0.5 mM) to normalize the terfenadine-induced ST segment elevation, and suppress the drug-induced phase 2 reentry and polymorphic VT/VF. 4-AP (0.5 - 2.0 mM) affected these changes by restoring the dome of epicardial action potential in 4/4 perfused wedge preparations.
Figure 6.
Inhibition of the transient outward current (Ito) with 4-aminopyridine restores the epicardial action potential dome, dramatically reducing transmural and epicardial heterogeneity and aborting arrhythmogenesis in perfused RV wedge preparations exposed to terfenadine (7 μM). A: Control at BCL of 2000 and 250 msec. B: Terfenadine (7 μM) accentuates the epicardial action potential notch and electrocardiographic J wave at BCL 2000 msec (left) and causes heterogeneous loss of the action potential dome and phase 2 reentry (see arrow) at a BCL of 390 msec (right). C: 4-aminopyridine (4-AP, 0.5 mM), in the continued presence of terfenadine, normalizes the action potential and ECG at both rates and prevents development of arrhythmias. D: Washout of 4-AP restores the Brugada phenotype, leading to development of spontaneous polymorphic VT triggered by a phase 2 reentrant beat at a BCL of 1000 msec.
In another series of experiments, we evaluated the effects of three sodium channel blockers (ajmaline, ≤20 μM, flecainide, ≤7.5 μM, and procainamide, ≤300 μM) traditionally used to unmask the Brugada syndrome in the clinic (Figure 7). Phase 2 reentry was observed in 1/17 preparations (5.9%) exposed to ajmaline, flecainide, or procainamide compared with 15/20 (75%) exposed to terfenadine or the combination of sodium channel block with procainamide or ajmaline and calcium channel block with verapamil. Polymorphic VT/VF was not observed with ajmaline, flecainide, or procainamide alone (0/17), but was induced in 7/20 preparations (35%) exposed to terfenadine or sodium channel blockade (procainamide or ajmaline) and verapamil. In the example illustrated in Figure 8, ajmaline (2 μM) accentuated the epicardial action potential notch, but did not lead to loss of the dome,. Addition of verapamil (0.1 μM) further accentuated the epicardial action potential notch, permitting loss of the epicardial action potential dome and the development of phase 2 reentry, thus providing further proof of concept that a combination of INa and ICa block is more effective than sodium channel block alone. Sodium channel blockade (procainamide or ajmaline) and verapamil induced phase 2 reentry in 3 out of 4 preparations while verapamil alone (≤20 μM) induced phase 2 reentry in only 1 out of 4 preparations.
Figure 7.
Incidence of phase 2 reentry and polymorphic ventricular tachycardia/fibrillation (VT/VF) with sodium channel blockade alone (n=17): ajmaline (≤ 20 μM), flecainide(≤ 7.5 μM), or procainamide (≤300 μM) vs. sodium and calcium channel blockade (n=20) terfenadine (5-10 μM) or sodium channel blockade (procainamide (≤ 300 μM) or ajmaline (≤ 20 μM)) + verapamil (≤20 μM) .
Figure 8.
Calcium channel block enhances the effect of sodium channel block to create the Brugada phenotype. Effect of ajmaline (2 μM) alone and in combination with verapamil (0.1 μM) in a canine right ventricular wedge. A. Control, BCL=2000 msec. B. 2 μM ajmaline, BCL=2000 msec C. Addition of verapamil (0.1 μM) produces a marked accentuation of the epicardial action potential notch and J wave on the ECG, allowing for heterogeneous loss of the epicardial action potential dome and phase 2 reentry when the pacing rate is increased to BCL 600 msec. Similar results were observed in 3/4 preparations while phase 2 reentry was only inducible in 1/4 preparations with verapamil alone (≤20 μM).
Discussion
Mutations in SCN5A associated with a loss of function of the sodium channel have been shown to underlie some forms of the Brugada syndrome. Linkage to SCN5A is consistent with the clinical finding that potent sodium channel blockers including Class IC antiarrhythmic agents such as flecainide, ajmaline or pilsicainide or Class IA agents such as procainamide and disopyramide, can unmask the Brugada syndrome when the electrocardiographic phenotype is concealed. 3;4;18 These findings prompted the development of the first experimental model of the Brugada syndrome in 1999 using flecainide to block the sodium channel.7 Because sodium channel block alone was not consistently effective and because muscarinic agonists and vagotonic maneuvers were known to accentuate ST segment elevation in patients with the Brugada syndrome, 5 we used acetylcholine to facilitate the induction of phase 2 reentry and polymorphic VT in those studies. Among its many actions, acetylcholine is known to inhibit ICa.19 These early studies provided the initial suggestion that combined INa and ICa block may be more effective. The present study provides a proof of concept for this hypothesis, showing that a combination of sodium and calcium channel block is much more effective in precipitating the Brugada syndrome experimentally, suggesting that this combination may be more effective in unmasking the syndrome in the clinic.
Electrophysiologic distinctions between ventricular epicardium and endocardium have long been appreciated (see 20 for references). The differential and dynamic response of epicardium and endocardium to sodium channel block was first reported in 1991 21 and the ability of sodium channel blockers to induce phase 2 reentry was demonstrated in 1993 in experiments involving tissue slices. 22 Subsequent studies involving the arterially perfused canine right ventricular wedge preparation, showed that INa block as well as augmentation of IK-ATP can give rise to closely coupled extrasystoles via a phase 2 reentrant mechanism in the intact wall of the canine right ventricle. Accentuation of the action potential notch and eventual loss of the action potential dome in epicardium but not endocardium, leads to the development of an ST segment elevation. These electrophysiologic changes give rise to a vulnerable window across the ventricular wall that provides the substrate for the development of reentrant arrhythmias thought to underlie the Brugada syndrome. 7;23;24
Block of INa accentuates the epicardial action potential notch initially by limiting the amplitude of phase 0. Phase 1 therefore begins at a less positive potential and proceeds to more negative potentials. The balance of currents at the end of phase 1 determines whether the repolarization process will continue, or whether the inward currents (principally ICa) will overcome the outward current forces and permit the generation of a second upstroke and action potential dome, thus giving rise to an action potential with a notched configuration in the early phases. A strong ICa is critically important in the generation of a normal action potential because it opposes the robust Ito normally present in right ventricular epicardium. This decisive role of ICa is the basis for the synergistic action of ICa block in causing loss of the action potential dome and in generating the Brugada phenotype in our experimental models.
While combined INa and ICa inhibition can cause a very significant shift in the balance of current at the end of phase 1 of the right ventricular action potential, it should be pointed out that an increase in the contribution of any one of a number of outward currents may effect similar changes, as outlined in the scheme shown in Table 1. The schema summarizes our current understanding of the mechanisms that contribute to the development of electrocardiographic and arrhythmic manifestations of the Brugada syndrome.
Table 1.
Proposed mechanism for ST segment elevation and arrhythmogenesis in the Brugada syndrome.
 |
A number of recent case reports have highlighted the development of T wave alternans in Brugada patients following fever or challenge with a potent sodium channel blocker. 25-27 The alternans takes the form of a marked accentuation in the amplitude of the negative T wave after every other beat and is commonly attended by the appearance of premature ventricular contractions26 and in some cases the development of polymorphic VT. 25 Our data suggest that the appearance of an exaggerated negative T wave is an indication of phase 2 reentry (Figs. 1 and 4) and that alternation of this electrocardiographic sign is indicative of the development of concealed phase 2 reentry following alternate beats (Fig. 5). The association of phase 2 reentry with the markedly negative T wave in the model is consistent with it being a marker of arrhythmogenicity in the clinic.
Use of class IA agents such as quinidine has been shown to be effective in reversing the electrocardiographic abnormalities of the Brugada syndrome both experimentally and clinically. 7;28;29 The efficacy of quinidine has been attributed to its ability to inhibit Ito at plasma levels within the therapeutic range. 7;29 In the present study, ST segment elevation and arrhythmias are prevented and/or suppressed with 4-aminopyridine (4-AP, 0.5 -2.0 mM), a specific Ito blocker (Figure 6). Ito inhibition prevents all-or-none repolarization at the end of phase 1 by shifting the balance of currents available at the end of phase 1 to more positive voltages. EDR and TDR are thus reduced or abolished, eliminating the substrate for the development of phase 2 reentry and VT/VF.
Previous studies with terfenadine demonstrated tonic and use dependent block of both INa 11 and ICa 12 . The use dependent nature of inward current blockade is apparent in our model, as loss of the epicardial action potential dome and resultant arrhythmias were only observed after more rapid pacing (Figures 1, 2, and 6). Work from our laboratory indicates that terfenadine blocks late INa and ICa in canine left ventricular myocytes with an IC50 of 1.3 μM and 1.1 μM, respectively (Zygmunt and Antzelevitch, unpublished observations). The importance of combined sodium and calcium channel blockade to induce loss of the epicardial action potential dome and arrhythmias in our model is further supported by the actions of ajmaline or procainamide and verapamil (Figure 8). Similar to terfenadine, loss of the epicardial action potential dome occurs in a use dependent manner with this combination. In contrast, neither ajmaline alone nor procainamide or flecainide were capable of consistently producing the phenomena in our model (Figure 7 and 8).
Clinical Implications
Our data demonstrate that terfenadine can induce loss of the epicardial action potential dome, phase 2 reentry and resulting polymorphic ventricular tachycardia and fibrillation in the canine right ventricular wedge, highlighting the potential adverse effects of agents with combined sodium and calcium channel blocking effects. Whether terfenadine itself may be useful as a diagnostic tool is questionable because of its actions to block the rapidly activating delayed rectifier current, IKr, at relatively low plasma levels.30;31 Indeed, terfenadine was withdrawn from the market in the United States in 1997 because of its proclivity to prolong the QT interval and to induce Torsade de Pointes arrhythmias. Although the mechanism is not fully understood, potent blockade of IKr at nM concentrations is thought underlie the drug’s effect to prolong QT and induce TdP. 32 It is noteworthy that an asymptomatic Brugada patient was recently unmasked during infusion with dimenhydrinate, a first generation histaminic H1 receptor antagonist.{Pastor, 2001 7322/id} Although little is known about the drug’s effects on cardiac ion channels, drugs of this class have anesthetic properties similar to lidocaine.{Giotti, 1976 8334/id}
Our terfenadine data coupled with the demonstration of an enhanced effectiveness of ajmaline or procainamide in the presence of verapamil suggest that addition of calcium channel blockade may improve the sensitivity of sodium channel blockers like procainamide in unmasking the Brugada syndrome. Extrapolation of these findings to the clinic should be done with great caution. A clinical test of the hypothesis, including assessment of the sensitivity and specificity of combined sodium and calcium channel block, appears warranted.
Our data also point to markedly negative T waves in the setting of Brugada syndrome and the development of T wave alternans as markers of phase 2 reentry, a mechanism capable of inducing premature beats that in turn could trigger more ominous arrhythmias.
Study Limitation
As with all basic studies involving animal experimentation, we must exercise caution in extrapolating these finding to the clinic. Although inhibition of INa and ICa by terfenadine generates electrocardiographic characteristic and arrhythmic manifestations very similar to those observed clinically, to what extent this model mimics the various forms of congenital and acquired Brugada syndrome remains to be established.
ACKNOWLEDGEMENTS
We gratefully acknowledge the expert technical assistance of Judy Hefferon, Robert Goodrow, Di Hou and Andrew Pitoniak.
Footnotes
Supported by grant HL47678 from NHLBI (CA) and grants from the American Heart Association (JF and CA) and NYS and Florida Grand Lodges F. & A.M.
Reference List
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Smits JP, Eckardt L, Probst V, Bezzina CR, Schott JJ, Remme CA, Haverkamp W, Breithardt G, Escande D, Schulze-Bahr E, LeMarec H, Wilde AA. Genotype-phenotype relationship in Brugada syndrome: electrocardiographic features differentiate SCN5A-related patients from non-SCN5A-related patients. J Am Coll Cardiol. 2002;40:350–356. doi: 10.1016/s0735-1097(02)01962-9. [DOI] [PubMed] [Google Scholar]
- 3.Brugada R, Brugada J, Antzelevitch C, Kirsch GE, Potenza D, Towbin JA, Brugada P. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510–515. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- 4.Priori SG, Napolitano C, Gasparini M, Pappone C, Della BP, Brignole M, Giordano U, Giovannini T, Menozzi C, Bloise R, Crotti L, Terreni L, Schwartz PJ. Clinical and genetic heterogeneity of right bundle branch block and ST-segment elevation syndrome : A prospective evaluation of 52 families [In Process Citation] Circulation. 2000;102:2509–2515. doi: 10.1161/01.cir.102.20.2509. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol. 1996;27:1061–1070. doi: 10.1016/0735-1097(95)00613-3. [DOI] [PubMed] [Google Scholar]
- 6.Brugada P, Brugada R, Brugada J. Sudden death in high-risk family members: Brugada syndrome. Am J Cardiol. 2000;86:K40–K43. doi: 10.1016/s0002-9149(00)01300-x. [DOI] [PubMed] [Google Scholar]
- 7.Yan GX, Antzelevitch C. Cellular basis for the Brugada Syndrome and other mechanisms of arrhythmogenesis associated with ST segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 8.Antzelevitch C. The Brugada syndrome: Ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001;12:268–272. doi: 10.1046/j.1540-8167.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 9.Antzelevitch C, Yan GX. Cellular and ionic mechanisms responsible for the Brugada syndrome. J Electrocardiol. 2000;33(Suppl):33–39. doi: 10.1054/jelc.2000.20321. [DOI] [PubMed] [Google Scholar]
- 10.Ming Z, Nordin C. Terfenadine blocks time-dependent Ca2+, Na+, and K+ channels in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 1995;26:761–769. doi: 10.1097/00005344-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Wang Z. Terfenadine block of sodium current in canine atrial myocytes. J Cardiovasc Pharmacol. 1999;33:507–513. doi: 10.1097/00005344-199903000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Melchert RB, Kennedy RH. Inhibition of L-type Ca2+ channel current in rat ventricular myocytes by terfenadine. Circ Res. 1997;81:202–210. doi: 10.1161/01.res.81.2.202. [DOI] [PubMed] [Google Scholar]
- 13.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu W, Antzelevitch C. Cellular basis for the electrocardiographic features of the LQT1 form of the long QT syndrome: Effects of β-adrenergic agonists, antagonists and sodium channel blockers on transmural dispersion of repolarization and Torsade de Pointes. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing Torsade de Pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 1997;96:2038–2047. doi: 10.1161/01.cir.96.6.2038. [DOI] [PubMed] [Google Scholar]
- 16.Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M cells in arterially-perfused canine left ventricular wedge preparations. Circulation. 1998;98:1921–1927. doi: 10.1161/01.cir.98.18.1921. [DOI] [PubMed] [Google Scholar]
- 17.Emori T, Antzelevitch C. Cellular basis for complex T waves and arrhythmic activity following combined I(Kr) and I(Ks) block. J Cardiovasc Electrophysiol. 2001;12:1369–1378. doi: 10.1046/j.1540-8167.2001.01369.x. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu W, Antzelevitch C, Suyama K, Kurita T, Taguchi A, Aihara N, Takaki H, Sunagawa K, Kamakura S. Effect of sodium channel blockers on ST segment, QRS duration, and corrected QT interval in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:1320–1329. doi: 10.1046/j.1540-8167.2000.01320.x. [DOI] [PubMed] [Google Scholar]
- 19.Litovsky SH, Antzelevitch C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol. A direct effect of acetylcholine in ventricular myocardium. Circ Res. 1990;67:615–627. doi: 10.1161/01.res.67.3.615. [DOI] [PubMed] [Google Scholar]
- 20.Antzelevitch C, Dumaine R. Electrical heterogeneity in the heart: Physiological, pharmacological and clinical implications. In: Page E, Fozzard HA, Solaro RJ, editors. Handbook of Physiology. The Heart. Oxford University Press; New York: 2001. [Google Scholar]
- 21.Krishnan SC, Antzelevitch C. Sodium channel blockade produces opposite electrophysiologic effects in canine ventricular epicardium and endocardium. Circ Res. 1991;69:277–291. doi: 10.1161/01.res.69.2.277. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan SC, Antzelevitch C. Flecainide-induced arrhythmia in canine ventricular epicardium: Phase 2 Reentry. Circulation. 1993;87:562–572. doi: 10.1161/01.cir.87.2.562. [DOI] [PubMed] [Google Scholar]
- 23.Antzelevitch C. The Brugada syndrome: ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001;12:268–272. doi: 10.1046/j.1540-8167.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 24.Antzelevitch C. Late potentials and the Brugada syndrome. J Am Coll Cardiol. 2002;39:1996–1999. doi: 10.1016/s0735-1097(02)01887-9. [DOI] [PubMed] [Google Scholar]
- 25.Morita H, Nagase S, Kusano K, Ohe T. Spontaneous T wave alternans and premature ventricular contractions during febrile illness in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13:816–818. doi: 10.1046/j.1540-8167.2002.00816.x. [DOI] [PubMed] [Google Scholar]
- 26.Takagi M, Doi A, Takeuchi K, Yoshikawa J. Pilsicanide-induced marked T wave alternans and ventricular fibrillation in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13:837. doi: 10.1046/j.1540-8167.2002.00837.x. [DOI] [PubMed] [Google Scholar]
- 27.Ohkubo K, Watanabe I, Okumura Y, Yamada T, Masaki R, Kofune T, Oshikawa N, Kasamaki Y, Saito S, Ozawa Y, Kanmatsuse K. Intravenous administration of class I antiarrhythmic drug induced T wave alternans in an asymptomatic Brugada syndrome patient. PACE. 2003;26:1900–1903. doi: 10.1046/j.1460-9592.2003.00288.x. [DOI] [PubMed] [Google Scholar]
- 28.Belhassen B, Viskin S, Fish R, Glick A, Setbon I, Eldar M. Effects of electrophysiologic-guided therapy with Class IA antiarrhythmic drugs on the long-term outcome of patients with idiopathic ventricular fibrillation with or without the Brugada syndrome [see comments] J Cardiovasc Electrophysiol. 1999;10:1301–1312. doi: 10.1111/j.1540-8167.1999.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 29.Alings M, Dekker L, Sadee A, Wilde A. Quinidine induced electrocardiographic normalization in two patients with Brugada syndrome. PACE. 2001;24:1420–1422. doi: 10.1046/j.1460-9592.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 30.Crumb WJ, Wible BA, Arnold DJ, Payne JP, Bown AM. Blockade of multiple human cardiac potassium currents by the antihistamine terfenadine: possible mechanism of terfenadine-asssociated cardiotoxicity. Mol Pharmacol. 1995;47:181–190. [PubMed] [Google Scholar]
- 31.Roy ML, Dumaine R, Brown AMHERG. a primary human ventricular target of the nonsedating antihistamine terfenadine. Circulation. 1996;94:817–823. doi: 10.1161/01.cir.94.4.817. [DOI] [PubMed] [Google Scholar]
- 32.Salata JJ, Jurkiewicz NK, Wallace AA, Stupienski RF, Guinosso PJ, Lynch JJ. Cardiac electrophysiological actions of the histamine H1-receptor antagonists astemizole and terfenadine compared with chlorpheniramine and pyrilamine. Circ Res. 1995;76:110–119. doi: 10.1161/01.res.76.1.110. [DOI] [PubMed] [Google Scholar]