Abstract
Active immunization with fibrillar β–amyloid peptide (Aβ42) as well as passive transfer of anti-Aβ antibodies significantly reduces Aβ plaque deposition, neuritic dystrophy, and astrogliosis in the brain of mutant amyloid precursor protein (APP)-transgenic mice. Although the mechanism(s) of clearance of Aβ from the brain following active or passive immunization remains to be determined, it is clear that anti-Aβ antibodies are critical for clearance. DNA immunization provides an attractive alternative to direct peptide and adjuvant approaches for inducing a humoral response to Aβ. We constructed a DNA minigene with Aβ fused to mouse interleukin-4 (pAβ42-IL-4) as a molecular adjuvant to generate anti-Aβ antibodies and enhance the Th2-type of immune responses. Gene gun immunizations induced primarily IgG1 and IgG2b anti-Aβ antibodies. Fine epitope analysis with overlapping peptides of the Aβ42 sequence identified the 1–15 region as a dominant B cell epitope. The DNA minigeneinduced anti-Aβ antibodies bound to Aβ plaques in brain tissue from an Alzheimer’s disease patient demonstrating functional activity of the antibodies and the potential for therapeutic efficacy.
Keywords: DNA immunization, Antibodies, Epitopes, Mice, Antigens
Abbreviations: Aβ: β-amyloid peptide, APP: Amyloid precursor protein, APP/TG: APP-transgenic, AD: Alzheimer’s disease, CHO: Chinese hamster ovary, HA: Hemagglutinin, HRP: Horseradish peroxidase, WB: Western blot
1 Introduction
Alzheimer’s disease (AD) is the most common form of dementia in the elderly and is characterized by a progressive loss of memory and a general cognitive decline. The neuropathological features of the disease include neurofibrillary tangles, deposition of β-amyloid peptides (Aβ40 or Aβ42) in plaques, and neuronal loss in affected brain regions [1]. The Aβ peptides cleaved from the amyloid precursor protein (APP) by the β- and γ-secretases [2–5] are believed to play a central role in the onset and progression of AD [6]. Recently, Schenk et al. [7] demonstrated that immunization of APP-transgenic (APP/Tg) mice with Aβ42 induced the generation of antibodies, which dramatically reduced the deposition of amyloid plaques and promoted the clearance of plaques from the brain. Other groups have reported similar observations and have extended these studies by showing that active immunization protected mice from developing functional memory deficits [8–10]. Remarkably, passive administration of an anti-Aβ monoclonal antibody to APP/Tg mice also resulted in decreased Aβ levels in the brain, significantly reducing the AD-like neuropathology [11, 12], and reversing memory deficits [13]. These results suggest that the generation of antibodies against Aβ in humans might provide protection against the onset of AD [14].
Based on these data, Aβ immunotherapy has been moved to the clinical trials. The AN1792 vaccine was comprised of Aβ42 as the antigen and QS21 as the adjuvant. Unfortunately, the vaccine trial was halted because approximately 5% of the participants receiving the vaccine developed some degree of meningoencephalitis [14, 15]. The failure of this clinical trial is disappointing; however, this does not mean that other Aβ immunotherapy strategies will not eventually be successful in treating AD. Because Th1-mediated responses have been implicated in many autoimmune diseases [16], there has been speculation that the adjuvant may have contributed to the adverse response in a subset of the patients receiving the AN1792 vaccine [17, 18]. Alternative, immunotherapy approaches that polarize the immune response to Aβ towards a Th2 phenotype, that in some cases have been shown to inhibit autoimmune diseases, may provide significant advantages [19, 20, 21]. An example of this type of approach is the intranasal immunization of APP/Tg mice with Aβ42 but without a conventional adjuvant, which was shown to induce a Th2-mediated humoral response to Aβ [18, 22].
Another adjuvant-free vaccination approach is DNA immunization, which has been shown to generate potent humoral and cellular immune responses against viral, tumor, and foreign antigens [23–34]. Importantly, it has also been reported that co-administration of IL-4 cytokine gene along with a gene, encoding foreign or “self” antigens, caused the immune response to be driven toward a more Th2-like phenotype [20, 21, 35–39]. Here we describe a novel chimeric DNA minigene encoding Aβ fused with mouse IL-4. Immunization of wild-type mice with the pAβ42-IL-4 generated a potent humoral response to Aβ. Analysis of the IgG isotypes showed that IgG1 and IgG2b were the predominant species, which suggests that the immune response was primarily Th2-mediated. The anti-Aβ antibodies generated were specific for the N-terminal region of Aβ42 and recognized Aβ plaques in human AD brain tissue, which indicated that antibodies are potentially therapeutic.
2 Results
2.1 Expression of chimeric DNA minigenes in vitro
Three different plasmids were constructed (Fig. 1a, b), and expression of all plasmids has been confirmed by analyzing the lysate and supernatants of transiently transfected Chinese hamster ovary (CHO) cells (Fig. 1c–e). The additional bands in the region between 19–24 kDa (lanes 1–2) likely represent different glycosylation states [40] of Aβ42-IL-4 or Aβ28-IL-4 fusion proteins. Importantly, we did not detect any Aβ peptides in the supernatant or lysate of cells transfected with vector DNA (Fig. 1c, lane 2, and data not shown). In addition to Western blot (WB), the expression of Aβ42, Aβ42-IL-4, and Aβ28-IL-4 were confirmed by flow cytometry analysis using immunostaining with monoclonal anti-Aβ42 6E10 and anti-IL-4 antibodies (Fig. 1f–h). Transiently transfected CHO cells expressed Aβ42, Aβ42-IL-4 and Aβ28-IL-4 molecules.
Fig. 1.
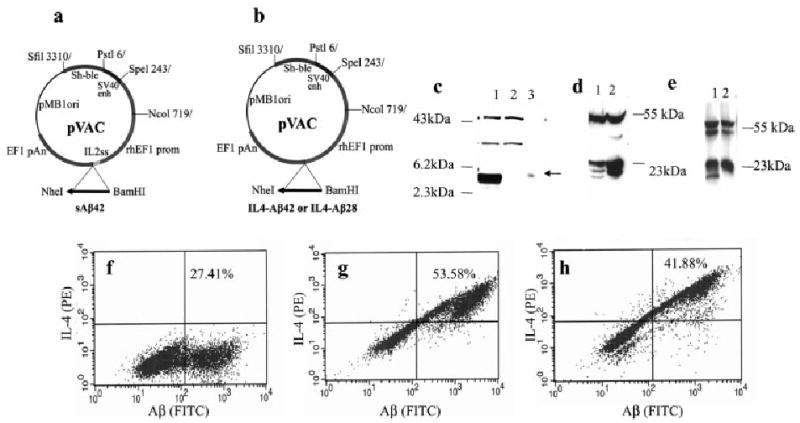
(a, b) Schematic representation of the constructs. (a) DNA sequence encoding Aβ42 peptide was cloned in frame with the IL-2 signal sequence using BamHI and NheI restriction sites; (b) DNA sequences encoding Aβ42 or Aβ28 peptides fused with IL-4 were generated by PCR and cloned using BamHI and NheI restriction sites. (c–e) Expression of Aβ42 (c) and Aβ42/28-IL-4 (d, e) minigene cassettes in CHO cells transfected with appropriate plasmid. Protein was recovered from supernatant or cell lysate by IP and analyzed on 16.5% Tricine-SDS-PAGE (c) or 15% Tris-SDS-PAGE (d, e) followed by WB. For IP and WB the monoclonal anti-Aβ antibody 6E10 was used. (c) Lane 1: supernatant of cells transfected with pAβ42; lane 2: supernatant of cells transfected with vector; lane 3: 5 ng of Aβ42 peptide (HPLC grade, > 95% purity). (d) Lane 1: lysate of cells transfected with pAβ42-IL-4; lane 2: lysate of cells transfected with Aβ28-IL-4. (e) Lane 1: supernatant of cells transfected with pAβ42-IL-4; lane 2, supernatant of cells transfected with pAβ28-IL-4. (f–h) Flow cytometric analysis of the expression of Aβ42 (f), Aβ42-IL-4 (g) and Aβ28-IL-4 (h) proteins in CHO cells transfected with appropriate plasmids. Transfected CHO cells were treated with brefeldin A overnight, incubated with 6E10 antibody alone (f) or 6E10 and rat anti-mouse IL-4-PE (g, h). To detect Aβ expression, anti-mouse Ig-FITC antibodies were added.
2.2 Generation of anti-Aβ antibodies
We immunized B6SJLF1 mice by bombardment of the skin with microscopic gold particles coated with the various DNA minigenes using the Helios gene gun. Mice immunized with pAβ42 did not generate anti-Aβ42 antibodies (data not shown). In contrast, conjugation of Aβ42 with IL-4 resulted in a robust anti-Aβ42 immune response generated by three out of eight experimental mice after only two boosts. Anti-Aβ42 antibody responses in three mice were moderate, whereas the remaining two mice responded weakly (Fig. 2a). Antibody titers of the pooled sera were detected in two separate experiments and were equal to 1:3,200 (Fig. 2b). Mice immunized with vector did not induce anti-Aβ42 antibody production, whereas injection of psHA-mC3d3 generated only antihemagglutinin (HA)-specific antibodies (data not shown). Thus, the presence of the mouse IL-4 molecular adjuvant in the plasmid was critical for generation of anti-Aβ specific antibody responses.
Fig. 2.
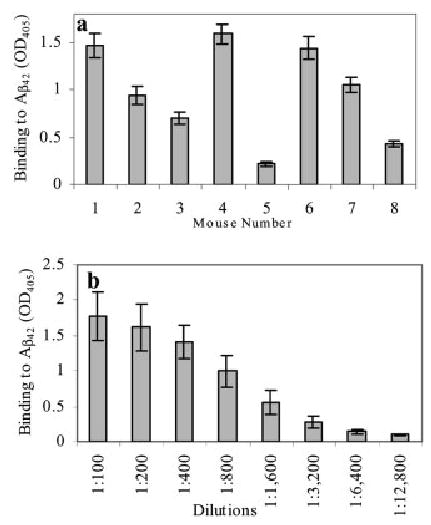
Generation of anti-Aβ42 antibodies after gene gun immunization of eight B6SJLF1 mice with plasmids, encoding Aβ42-IL-4 fusion protein. (a) Serum of each mouse (No. 1–8) was diluted 1:250 and used in ELISA for detection of total Ig (SD represents results from three separate assays with the same sera). (b) Titer of anti-Aβ42 antibodies was detected in the pooled sera from immunized mice (SD represent two separate experiments with sera of mice No. 1–4 and 5–8).
There is a high level of homology between human and rodent Aβ42 peptides [41]. Accordingly, we demonstrated that immunization with pAβ42-IL-4 that encodes human Aβ42 induces cross-reactivity with mouse Aβ42. However, the antisera bound to rodent Aβ42 two times weaker than to human Aβ42 (data not shown). Because we demonstrated the immune response to the self-Aβ42 peptide, it was also interesting to analyze antibody production to the self-IL-4 molecule. Our data indicate that DNA vaccination with pAβ42-IL-4 generated low-titer antibodies to the IL-4 molecular adjuvant (Fig. 3).
Fig. 3.

Titer of anti-IL-4 antibodies detected in the sera of individual mice vaccinated with Aβ42 DNA immunogen fused with IL-4 molecualr adjuvant (n=8)
2.3 Characterization of anti-Aβ42 antibodies by isotyping
We analyzed isotypes of anti-Aβ42 antibodies in the sera of six mice, which represent high and moderate responders. All animals generated IgG1 antibodies, whereas the level of IgG2ab was negligible. The production of IgG1 antibodies is an indirect measure of the relative contribution of Th2-type cytokines, whereas IgG2a antibodies reflect the contribution of Th1 to the immune response [32]. Thus our data indicate that pAβ42-IL-4 minigene induces a highly Th2-polarized response. Four mice with the highest production of IgG1 antibodies also generated significant levels of IgG2b (Fig. 4). There was no IgE or IgA antibody production detected in immunized mice (data not shown).
Fig. 4.

Isotyping of anti-Aβ42 antibodies after immunization of mice with pAβ42-IL-4. Each serum from six mice, which represent high and moderate responders (No. 1–4, 6, 7) were diluted 1:250 and used for detection of IgG1, IgG2ab, IgG2b, and IgM subclasses of anti-Aβ42 antibodies. All mice demonstrated IgG1 (Th2)/IgG2ab (Th1) ratio much higher than 1.
2.4 Mapping of B cell epitopes and binding to human β-amyloid plaques
In order to identify epitopes recognized by antibodies induced after DNA immunization, we analyzed binding of immune sera to the six overlapping peptides by competition ELISA. Pre-incubation of sera from high and moderate responders with the full-length Aβ42 peptide resulted in the most complete inhibition of antibody binding to the adsorbed Aβ42 peptide (82–98% inhibition). The peptide Aβ1–15 was also effective, inhibiting the binding of antisera by 49–92%. In addition, Aβ6–20 showed partial inhibition of antibody binding. Notably, a mixture of Aβ16–30, Aβ21–35 and Aβ26–42 peptides (2.5 μM each) was ineffective (Fig. 5).
Fig. 5.

Mapping of B cell epitopes by six overlapping peptides. Serum from each mouse (No. 1–4, 6, 7) was preincubated with indicated peptides or mixture of peptides before binding to Aβ42-coated wells. The percent of inhibition by small peptides as well as by control full-length peptide was calculated based on binding of sera without competing peptides to Aβ42 as 100%.
To analyze the capability of these antibodies to bind to Aβ plaques in human brain tissue, we used pooled sera from six immunized mice. This antiserum bound to Aβ plaques on the brain sections of cortical tissue from a severe AD case (Fig. 6). The antisera were effective at a dilution of 1:1,000, the endpoint dilution used in these experiments. The pre-immune sera from these mice did not bind to the Aβ plaques. Notably, Aβ42 peptide abrogated binding of antisera to the Aβ plaques in human brain tissue (Fig. 6).
Fig. 6.

Binding of immune (Aβ42-IL-4), pre-immune (negative control) sera, and 6E10 monoclonal antibody (positive control) to the 50-μm brain sections of formalin-fixed cortical tissues from an 80-year-old man with neuropathological and behavioral patterns typical to severe AD. Both pre-immune and immune sera were used at a dilution of 1:1,000, whereas the monoclonal antibody 6E10 was used at a dilution of 1:1,500. Binding of antisera to the brain tissue was blocked by Aβ42 peptide. Arrows indicate senile plaques in frontal cortex sections.
To examine inflammation-related pathology in the brain of animals immunized with DNA minigene, we conducted immunohistochemical analysis. To identify microglia activation as well as leukocyte infiltration and accumulation in the brain, we analyzed expression of CD45, CD11b, CD4, CD8, and CD54 molecules. As shown in Fig. 7, immunostaining in sections from the frontoparietal cortex revealed no observable differences between wild-type and immunized mice. CD11b-positive microglial cells exhibited a resting morphology with long fine processes. Importantly, CD4-positive cells were found within blood vessels but had not infiltrated into the neuropil.
Fig. 7.
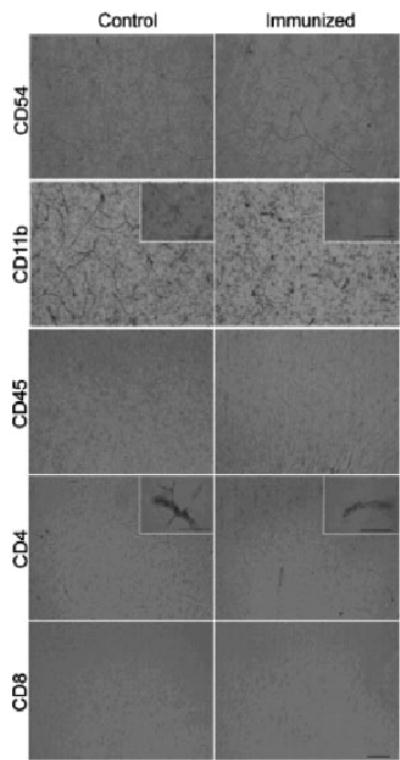
Detection of neuroinflammation in brains of mouse immunized with pAβ42-IL-4 or vector. Markers of neuroinflammation were negative in vaccinated mice compared to control (immunized with vector) animals. The left column shows sections from the frontoparietal cortex of a control animal and the right panel from a vaccinated mouse. Markers used are indicated in each row. No evidence for CD54-, CD45-, or CD8-positive cells was observed. CD11b-positive microglial cells exhibited a resting morphology with long fine processes (illustrated in insets). CD4-positive cells were found within blood vessels (insets), but had not infiltrated into the neuropil. Bars = 50 mm. These data were repeated with two other animals.
3 Discussion
The development of a safe and effective vaccine requires a delicate balance between providing a specific and adequate immune response to provide protection, and reducing or eliminating unwanted adverse events that may induce excess inflammation or an autoimmune response. While there are significant safety concerns about Aβ immunotherapy as a possible treatment for AD, there is also considerable optimism that these hurdles can be overcome with other vaccination approaches. Alternative strategies have been suggested, such as using different adjuvants, different routes of immunization and the using molecular adjuvant(s), such as cytokines in conjunction with Aβ-based vaccine to drive the Th phenotype toward the desired pathway [18, 19, 22, 42]. In this study we have employed a chimeric DNA minigene to deliver a fusion protein containing Aβ antigen together with IL-4 as a molecular adjuvant to amplify immune response to Aβ and to further polarize the response toward a Th2 phenotype in vivo.
Thirteen years ago, it was demonstrated that a gene could be transferred in situ by inoculation of a plasmid vector into muscle [43]. Now, the generation of potent humoral and cellular immune responses to a broad spectrum of pathogen antigens has been demonstrated in different animal model systems using DNA vaccination [25–30, 34, 44–49]. DNA immunization offers significant advantages over peptide/protein-based immunization. First it offers the capability to modify genes encoding desired antigen(s), to change the cellular localization of an antigen by adding or removing signal sequences or transmembrane domains, and to target the desired type of immune response using the appropriate molecular adjuvants. Previously we used these approaches to generate potent humoral and cellular immune responses against different antigens [25–28, 45, 50]. Now to generate potent antibody production to Aβ antigen we prepared plasmids, encoding antigen (Aβ42 or Aβ28) fused with IL-4 (Fig. 1). We demonstrated that mice immunized with pAβ42 did not generate anti-Aβ42 antibodies even after six boosts (data not shown), whereas plasmids, encoding immunogen and IL-4 induced anti-Aβ42 antibodies after just two boosts (Fig. 2).
We and others have previously mapped B cell epitopes using antisera from mice immunized with fibrillar Aβ42 and small peptides derived from Aβ42 [11, 19, 22, 51, 52]. These results have been confirmed and the linear Aβ42 epitope spanning residues 4–10 was identified using high-resolution mass-spectrometry technique [53]. In this report we demonstrated that antibodies generated after pAβ42-IL-4 immunization also recognized Aβ1–15 peptide. Binding to peptide Aβ6–20 was detectable, but significantly weaker than binding to Aβ1–15 and Aβ42 (Fig. 5). Thus, like fibrillar Aβ42 formulated in different adjuvants [11, 19, 22, 51–54], DNA immunization induced antibodies against linear epitope(s) of Aβ42 that span the N-terminal amino acids of this peptide. Collectively these data suggest that Aβ1–15 represents the major B cell antigenic determinant of Aβ42 regardless of DNA or protein immunizations. Interestingly, binding of antibodies to this region of Aβ42 coincides with the ability of antibodies to bind native plaques in brain tissue [11, 53] and trigger ex vivo phagocytosis [54]. Antibodies generated after gene gun immunization with pAβ42-IL-4 also recognize human Aβ plaques in cortical tissues from a severe AD case (Fig. 6).
Successful DNA immunization with IL-4 as a molecular adjuvant to enhance Th2 type of immune responses to different immunogens has been previously reported [20, 21, 35–39]. However, to our knowledge no one examined whether anti-IL-4 antibody production is generated when IL-4 is used as a fusion protein with the immunogen. In this report we have demonstrated that mice immunized with pAβ42-IL-4 induced low levels of antibodies to self-IL-4 cytokine (Fig. 3). Such a response was not unexpected since gene therapy is used to induce immunological memory against self proinflammatory chemokines [55]. IL-4 cytokine is an antiinflammatory cytokine with pleiotropic effects on immune cells of multiple lineages. Thus, production of antibodies to IL-4, unnaturally expressed after DNA vaccination, may be an important regulatory mechanism that protects an organism from overexpression of this cytokine.
The antibody isotype has been used as an indirect measure of the contribution of Th1 and Th2 cytokines to the humoral response [56]. We recently demonstrated that B6SJLF1 mice produce IgG2ab anti-Aβ42 antibodies instead of IgG2a [57]. Accordingly, we analyzed the IgG1 and IgG2ab profiles of the humoral immune response after DNA immunization. Injections with pAβ42-IL-4 induced predominantly IgG1 and to a lesser extent IgG2b anti-Aβ42 antibodies. Anti-Aβ42 antibodies of IgG2ab isotype were negligible (Fig. 4). Thus, the IgG1/ IgG2a ratio measured following immunization with our chimeric Aβ42-IL-4 minigene implies that a highly polarized Th2-mediated immune response was generated. More importantly, IgG1 and IgG2b isotypes of anti-Aβ42 antibodies may correlate with their therapeutic potential [11, 13, 53, 58]. The antibodies generated after Aβ42-IL-4 minigene immunization are predominantly of the IgG1 isotype and bound to the first 15 amino acids of Aβ42 and to Aβ plaques in the AD brain tissue. Therefore, DNA immunization with minigene encoding Aβ antigen and IL-4 molecular adjuvant induces therapeutically potent antibodies to Aβ42.
Because T lymphocyte-mediated meningoencephalitis was observed in some patients after immunization with Aβ42 [59], we analyzed lymphocyte infiltration into brains of immunized wild-type mice. Immunohistochemical analysis of brain sections did not reveal lymphocytemediated neuroinflammation or microglia activation (Fig. 7). Because antibodies raised against human Aβ42 also recognize rodent Aβ42, and therefore rodent APP, we speculate that pathological hallmarks in this case may be similar to the ones appearing after vaccination with self-antigen. Future studies with APP/Tg mice immunized with different pAβ-IL-4 constructs are underway and these experiments will better define the therapeutic effects, such as reducing the deposition of Aβ plaques, clearance of plaques from the brain, and protection of mice from developing functional memory deficits, following DNA vaccination.
4 Materials and methods
4.1 Mice
Eight- or ten-week-old female B6SJLF1 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in the animal facility at the Institute for Brain Aging and Dementia, University of California, Irvine (UCI), CA. All animals were housed in a temperature- and light-cyclecontrolled facility and their care was under the guidelines of the National Institutes of Health and the Institute for Brain Aging and Dementia at UCI.
4.2 DNA constructs
Three different plasmids encoding secreted forms of Aβ peptide were constructed: pAβ42, encoding Aβ42; pAβ28-IL-4 and pAβ42-IL-4, encoding chimeric proteins, where mouse IL-4 is fused with human Aβ28 or Aβ42, respectively (Fig. 1a, b). The pAβ42 was constructed by cloning Aβ42 PCR fragment into pVAC (Invivogen, San Diego, CA) expression vector in frame with IL-2 signal sequence using BamHI and NheI restriction sites. pAβ42-IL-4 and pAβ28-IL-4 were constructed by fusion of Aβ42 or Aβ28 with IL-4 gene via overlapping PCR techniques using appropriate primers and then cloning into pVAC expression vector using the same restriction sites (IL-2 signal sequence was replaced by IL-4 signal sequence). The correct sequences of the generated plasmids were confirmed by nucleotide sequence analysis. The structure of plasmid psHA-mC3d3 encoding HA of influenza and three copies of C3d component of complement was described earlier [28]. All plasmids have been purified by Endofree plasmid maxi kit (Qiagen, Valencia, CA). Purity of the DNA was confirmed by UV spectrophotometry (260 nm/280 nm absorbance ratio >1.7) and gel electrophoresis.
4.3 Transfection of cells and expression of Aβ by psAβ42, pAβ28-IL-4 and pAβ42-IL-4
CHO cells (0.8×106) were transiently transfected with 2 μg of the appropriate plasmid (pAβ42, pAβ42-IL-4, or pAβ28-IL-4) by Lipofectamine Plus Reagent (Invitrogen, Carlsbad, CA) and expressions of these plasmids were analyzed in the supernatants or lysates of cells. Cells transfected with vector were used as a negative control. All proteins were detected by combination of immunoprecipitation (IP) and WB using 6E10 anti-Aβ antibodies (Signet, MA). Tricine-SDS-polyacrylamide gel (16%, for Aβ42) or 15% Tris-SDS-polyacrylamide gel (for fusion proteins) were used for electrophoresis. The antibody-antigen complexes were detected using horseradish peroxidase (HRP)-conjugated anti-mouse antibodies and Luminol reagent (Santa Cruz Biotechnology, Santa Cruz, CA).
4.4 Flow cytometry
The flow cytometry analysis was used for detection of Aβ42 peptide or Aβ42-IL-4 and Aβ28-IL-4 fusion proteins in transfected CHO cells. Transiently transfected CHO cells were treated by inhibitor of intracellular transport brefeldin A (Sigma, St. Louis, MO) at a final concentration of 5 μg/ml for 16 h. Aβ42 expression was analyzed using monoclonal antibodies 6E10 (0.5 μg per 2.5×105 cells) following staining with FITC-conjugated goat anti-mouse polyclonal antibodies (BD PharMingen, San Diego, CA). Double staining with both 6E10 and PE-conjugated anti-mouse IL-4 (0.2 μg per 2.5×105 cells; BD PharMingen) antibodies were used for analyses of Aβ42-IL-4 and Aβ28-IL-4 expression. Flow cytometry analyses were performed with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA), and data were analyzed by CellQuest software (Becton Dickinson).
4.5 Immunization
Immunizations of mice with experimental plasmids (two independent experiments with eight animals for each plasmid) were performed on shaved abdominal skin using the Helios gene gun (Bio-Rad, Hercules, CA) as we described [28]. Briefly, mice were bombarded two times with doses containing 1 μg of DNA per 0.5mg of ~1-μm gold beads (DeGussa-Huls Corp., Ridefield Park, NJ) at a helium pressure setting of 400 psi. Mice were immunized and boosted by the same method biweekly and sera were collected 7–8 days after the final boost. As a control we used animals (eight in each group) immunized by gene gun bombardment with psHA-mC3d3 (positive), or with vector (negative) [28].
4.6 ELISA
Total anti-Aβ42 and anti-HA antibodies have been detected as described previously [7, 19, 28, 57]. Briefly, wells of 96- well plates (Immulon II; Dynatech) were coated with 2.5 μM of human or mouse Aβ42 in bicarbonate coating buffer (pH 9.7) and incubated 3 h at 37°C or overnight at 4°C. They were then washed and blocked with 3% non-fat dry milk in Tween-20 Tris buffer solution (TTBS) for 1–2 h at 37°C. After washing of the wells primary sera from experimental and control mice were added in duplicate at the indicated dilutions. After incubation (O/N at 4°C) and washing, HRPconjugated anti-mouse IgG was added as recommended by manufacturer (Jackson Laboratories). Plates were incubated for 1 h at 37°C, washed, and freshly prepared OPD substrate solution (o-phenylendiamine in 0.05 M phosphatecitrate buffer, pH 5.0; Sigma) was added to develop reaction. All plates were analyzed spectrophotometrically at 405 nm. Anti-HA antibodies have been detected by ELISA exactly as we described earlier [28]. Anti-IL-4 antibodies were detected as described above except that plates were coated with 200 pg/ml recombinant mouse IL-4 (BD Phar-Mingen).
4.7 Antibody isotyping
To determine the specific isotypes, sera from individual mice were diluted 1:250 and tested in duplicate as described above. To detect mouse IgG1, IgG2b, or IgM isotypes, we used anti-mouse Ig-subclass-specific HRP-conjugated secondary antibodies (Zymed, San Francisco, CA). To detect mouse IgG2c (IgG2ab) isotype, we used biotin-conjugated mouse anti-mouse IgG2ab (Igh-1b) monoclonal antibody (PharMingen, San Diego, CA), followed by incubation with HRP-conjugated streptavidin (Vector Laboratories, Burlingame, CA). For detection of IgE and IgA isotypes we used appropriate isotype-specific anti-mouse antibodies (Zymed).
4.8 Mapping of Aβ42 antigenic determinants by competition ELISA
Previously, we have detected antigenic determinants in Aβ42 peptide using a competition ELISA assay where the small overlapping peptides mixed with antisera compete with binding to the Aβ42-coated plates [19]. Briefly, plates were coated with Aβ42, washed, and blocked, as described above. Sera from immunized individual mice (diluted 1:250) were mixed with 2.5 μMof Aβ42 peptide or small overlapping peptides spanning amino acids 1–15 (Aβ1–15), 6–20 (Aβ6–20), 11–25 (Aβ11–25), 16–30 (Aβ16–30), 21–35 (Aβ21–35), and 16–42 (Aβ16–42) of Aβ42 and incubated 1 h at 37°C. The peptidetreated sera were added in duplicate to Aβ42-coated plates and antibody binding was analyzed by ELISA as described above. The percent of inhibition by small peptides and by control full-length peptide was calculated based on binding of sera without competing peptides to Aβ42 as 100%.
4.9 Detection of Aβ plaques in human brain tissues
Sera from immunized mice were screened for an ability to bind to Aβ plaques in the human brain. Briefly, pooled sera were added to the serial 50-μm brain sections of formalinfixed frontal cortical tissue from a patient (an 80-year-old man with neuropathological and behavioral patterns typical for severe AD). Sections were pretreated with 90% formic acid, and exogenous peroxidase was quenched. Three different titers of mice antisera were tested (1:500, 1:750, and 1:1,000 dilutions). As a negative control, we used the same dilutions of pre-immune sera. As a positive control, monoclonal anti-human Aβ antibody 6E10 (dilution 1:1,500) was used to immunostain plaques in AD brain sections. Binding of antisera to the plaques was blocked by soluble Aβ42 peptide in a concentration of 2.5 μM. Binding of antibodies to the brain sections was determined via VECTASTAIN Elite ABC Mouse IgG /DAB substrate biotin-avidin system (both kits from Vector Laboratories), according to manufacturer’s recommendations. Digital camera (Olympus, Japan) was used to view the plaques at 20× image magnification.
4.10 Detection of neuroinflammation in mouse brains
Mice were sacrificed and brains were removed, fixed in 4% paraformaldehyde for 24 h at 4°C, and subsequently cut in serial 50-μm-thick sections. Sections were pretreated with 0.3% H2O2 for 30 min to eliminate endogenous peroxidase activity. The following primary antibodies were used after blocking of sections: CD54 (ICAM) (3E2), CD45 (30-F11), CD11b (M1/70), CD4 (GK1.5), CD8a (53–6.7) (all from BD PharMingen). All antibodies were optimally diluted in 0.1 M Tris, 0.1% Triton X-100 and 2% BSA as recommended by the manufacturer. Bound antibodies were detected using biotinylated anti-hamster (Vector Laboratories) or preabsorbed biotinylated anti-rat (Jackson Immunoresearch) antibodies followed by incubation with avidin:biotin peroxidase complex (ABC), using the Vectastain Elite kit (Vector Laboratories). Staining reactions were performed with 3,3-diaminobenzidine (DAB; Sigma) according to manufacturers’ protocols. For negative control, the primary antibody was omitted from the diluent.
Acknowledgments
We thank Mr. Tom D. Green and Mike Tran for technical support in preparation of the bullets for gene gun immunization and performing of ELISA. In addition, we appreciate the technical assistance of Ms. Mihaela Nistor in immunohistochemical experiments. We acknowledge Dr. D. Stephen Snyder from NIA, for helping us with licensing of gene gun. This work was supported by R01 grants from NIH (AG-20241 for D. H. Cribbs and M. G. Agadjanyan; AI-44809 for M. G. Agadjanyan).
Footnotes
The last two authors are joint senior authors and they contributed equally to this work.
References
- 1.Price DL, Sisodia SS. Cellular and molecular biology of Alzheimer’s disease and animal models. Annu Rev Med. 1994;45:435–446. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- 2.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 4.Esler WP, Wolfe MS. A Portrait of alzheimer secretases – new features and familiar faces. Science. 2001;293:1449–1454. doi: 10.1126/science.1064638. [DOI] [PubMed] [Google Scholar]
- 5.Sisodia SS. An accomplice for gamma-secretase brought into focus. Science. 2000;289:2296–2297. doi: 10.1126/science.289.5488.2296. [DOI] [PubMed] [Google Scholar]
- 6.Small DH, Mok SS, Bornstein JC. Alzheimer’s disease and Aβ-toxicity. From top to bottom. Nat Rev Neurosci. 2001;2:595–598. doi: 10.1038/35086072. [DOI] [PubMed] [Google Scholar]
- 7.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimerdisease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 8.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 9.Janus C, Chishti MA, Westaway D. Transgenic mouse models of Alzheimer’s disease. Biochim Biophys Acta. 2000;1502:63–75. doi: 10.1016/s0925-4439(00)00033-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RGM. A learning deficit related to age and betaamyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 11.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 12.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverse memory deficits without reducing brain Ab burden in Alzheimer’s disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 14.Schenk D. Amyloid-β immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 15.Birmingham K, Frantz S. Set back Alzheimer vaccine studies. Nat Med. 2002;8:199–200. doi: 10.1038/nm0302-199b. [DOI] [PubMed] [Google Scholar]
- 16.O’Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 17.Town T, Vendrame M, Patel A, Poetter D, DelleDonne A, Mori T, Smeed R, Crawford F, Klein T, Tan J, Mullan M. Reduced Th1 and enhanced Th2 immunity after immunization with Alzheimer’s β-amyloid. J Neuroimmunol. 2002;132:49–59. doi: 10.1016/s0165-5728(02)00307-7. [DOI] [PubMed] [Google Scholar]
- 18.Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Issazadeh S, Hancock WW, Selkoe DJ. Nasal administration of amyloid-beta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Ann Neurol. 2000;48:567–579. [PubMed] [Google Scholar]
- 19.Cribbs DH, Ghochikyan A, Tran M, Vasilevko V, Petrushina I, Sadzikava N, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with betaamyloid. Int Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz PJ, Garren H, Ruiz IU, Hirshberg DL, Nguyen LT, Karpuj MV, Cooper MT, Mitchell DJ, Fathman CG, Steinman L. Suppressive immunization with DNA encoding a self-peptide prevents autoimmune disease: modulation of T cell costimulation. J Immunol. 1999;162:3336–3341. [PubMed] [Google Scholar]
- 21.Garren H, Ruiz PJ, Watkins TA, Fontoura P, Nguyen LT, Estline ER, Hirschberg DL, Steinman L. Combination of gene delivery and DNA vaccination to protect from and reverse Th1 autoimmune disease via deviation to the Th2 pathway. Immunity. 2001;15:15–22. doi: 10.1016/s1074-7613(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 22.Lemere CA, Maron R, Selkoe DJ, Weiner HL. Nasal vaccination with beta-amyloid peptide for the treatment of Alzheimer’s disease. DNA Cell Biol. 2001;20:705–711. doi: 10.1089/10445490152717569. [DOI] [PubMed] [Google Scholar]
- 23.Ertl HC, Xiang ZQ. Genetic immunization. Viral Immunol. 1996;9:1–9. doi: 10.1089/vim.1996.9.1. [DOI] [PubMed] [Google Scholar]
- 24.Kim JJ, Bagarazzi ML, Trivedi N, Hu Y, Kazahaya K, Wilson DM, Ciccarelli R, Chattergoon MA, Dang K, Mahalingam S, Chalian AA, Agadjanyan MG, Boyer JD, Wang B, Weiner DB. Engineering of in vivo immune responses to DNA immunization via codelivery of costimulatory molecule genes. Nat Biotechnol. 1997;15:641–646. doi: 10.1038/nbt0797-641. [DOI] [PubMed] [Google Scholar]
- 25.Agadjanyan MG, Trivedi NN, Kudchodkar S, Bennett M, Levine W, Lin A, Boyer J, Levy D, Ugen KE, Kim JJ, Weiner DB. An HIV type 2 DNA vaccines induces crossreactive immune responses against HIV type 2 and SIV. AIDS Res Hum Retroviruses. 1997;13:1561–1572. doi: 10.1089/aid.1997.13.1561. [DOI] [PubMed] [Google Scholar]
- 26.Agadjanyan, M. G., Ugen, K., Wang, B., Villafana, T., Merva, M., Petrushina, I., Williams, W. W. andWeiner, D. B., DNA inoculation with an HTLV-1 envelope DNA construct elicits immune responses in rabbits. In Chanock, R. M., Ginsberg, H.S., Brown, F. and Lerner, R.A. (Eds.), Vaccines ’94: modern approaches to new vaccines including prevention of AIDS. Cold Spring Harbor Laboratory Press, Cold Spring Harbor 1994, pp 47–53.
- 27.Agadjanyan MG, Kim JJ, Trivedi N, Wilson DM, Monzavi-Karbassi B, Morrison LD, Nottingham LK, Dentchev T, Tsai A, Dang K, Chalian AA, Maldonado MA, Williams WV, Weiner DB. CD86 (B7–2) can function to drive MHC-restricted antigen-specific CTL responses in vivo. J Immunol. 1999;162:3417–3427. [PubMed] [Google Scholar]
- 28.Ross TM, Xu Y, Bright RA, Robinson HL. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol. 2000;1:127–131. doi: 10.1038/77802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang B, Ugen KE, Srikantan V, Agadjanyan MG, Dang K, Refaeli Y, Sato AI, Boyer J, Williams WV, Weiner DB. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowsk ISH, Deck RR, DeWitt CM, Friedman A, Hawe LA, Leander KR, Martinez D, Perry HC, Shiver JW, Montgomery DL, Liu MA. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman SL, Doolan DL, Sedegah M, Gramzinski R, Wang H, Gowda K, Hobart P, Margalith M, Norman J, Hedstrom RC. Nucleic acid malaria vaccines. Ann N Y Acad Sci. 1995;772:88–94. doi: 10.1111/j.1749-6632.1995.tb44734.x. [DOI] [PubMed] [Google Scholar]
- 32.Raz E, Carson DA, Parker SE, Parr TB, Abai AM, Aichinger G, Gromkowski SH, Singh M, Lew D, Yankauckas MA, Baird SM, Rhodes GH. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994;91:9519–9523. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson HL. Nucleic acid vaccines: an overview. Vaccine. 1997;15:785–787. doi: 10.1016/s0264-410x(96)00249-6. [DOI] [PubMed] [Google Scholar]
- 34.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 35.Kim JJ, Simbiri KA, Sin JI, Dang K, Oh J, Dentchev T, Lee D, Nottingham LK, Chalian AA, McCallus D, Ciccarelli R, Agadjanyan MG, Weiner DB. Cytokine molecular adjuvants modulate immune responses induced by DNA vaccine constructs for HIV-1 and SIV. J Interferon Cytokine Res. 1999;19:77–84. doi: 10.1089/107999099314441. [DOI] [PubMed] [Google Scholar]
- 36.Kim JJ, Trivedi NN, Nottingham LK, Morrison L, Tsai A, Hu Y, Mahalingam S, Dang K, Ahn L, Doyle NK, Wilson DW, Chattergoon M, Chalian AA, Boyer J, Agadjanyan MG, Weiner DB. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur J Immunol. 1998;28:1089–1103. doi: 10.1002/(SICI)1521-4141(199803)28:03<1089::AID-IMMU1089>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Chow YH, Chiang BL, Lee YL, Chi WK, Lin WC, Chen YT, Tao MH. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320–1329. [PubMed] [Google Scholar]
- 38.Geissler M, Gesien A, Tokushige K, Wands JR. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J Immunol. 1997;158:1231–1237. [PubMed] [Google Scholar]
- 39.Ishii KJ, Weiss WR, Ichino M, Verthelyi D, Klinman DM. Activity and safety of DNA plasmids encoding IL-4 and IFN gamma. Gene Ther. 1999;6:237–244. doi: 10.1038/sj.gt.3300799. [DOI] [PubMed] [Google Scholar]
- 40.Thor G, Brian AA. Glycosylation variants of murine interleukin-4: evidence for different functional properties. Immunol. 1992;75:143–149. [PMC free article] [PubMed] [Google Scholar]
- 41.Hilbich C, Kisters-Woike B, Reed J, Masters CL, Beyreuther K. Human and rodent sequence analogs of Alzheimer’s amyloid beta A4 share similar properties and can be solubilized in buffers of pH 7.4. Eur J Biochem. 1991;201:61–69. doi: 10.1111/j.1432-1033.1991.tb16256.x. [DOI] [PubMed] [Google Scholar]
- 42.Bowers WJ, Federoff HJ. Amyloid immunotherapyengendered CNS inflammation. Neurobiol Aging. 2002;23:683–674. doi: 10.1016/s0197-4580(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 43.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 44.Muthumani K, Kudchodkar S, Zhang D, Bagarazzi ML, Kim JJ, Boyer JD, Ayyavoo V, Pavlakis GN, Weiner DB. Issues for improving multiplasmid DNA vaccines for HIV-1. Vaccine. 2002;20:1999–2003. doi: 10.1016/s0264-410x(02)00086-5. [DOI] [PubMed] [Google Scholar]
- 45.Kim JJ, Ayyavoo V, Bagarazzi ML, Chattergoon MA, Dang K, Wang B, Boyer JD, Weiner DB. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol. 1997;158:816–826. [PubMed] [Google Scholar]
- 46.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol. 2001;1:209–219. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 47.Kieber-Emmons T, Monzavi-Karbassi B, Wang B, Luo P, Weiner DB. Cutting edge: DNA immunization with minigenes of carbohydrate mimotopes induces functional anticarbohydrate antibody response. J Immunol. 2000;165:623–627. doi: 10.4049/jimmunol.165.2.623. [DOI] [PubMed] [Google Scholar]
- 48.Doolan DL, Hoffman SL. DNA-based vaccines against malaria: status and promise of the multi-stage malaria DNA vaccine operation. Int J Parasitol. 2001;31:753–762. doi: 10.1016/s0020-7519(01)00184-9. [DOI] [PubMed] [Google Scholar]
- 49.Parker SE, Monteith D, Horton H, Hof R, Hernandez P, Vilalta A, Hartikka J, Hobart P, Bentley CE, Chang A, Hedstrom R, Rogers WO, Kumar S, Hoffman SL, Norman JA. Safety of a GM-CSF adjuvant-plasmid DNA malaria vaccine. Gene Ther. 2001;8:1011–1023. doi: 10.1038/sj.gt.3301491. [DOI] [PubMed] [Google Scholar]
- 50.Green TD, Montefiori DC, Ross TM. Enhancment of antibodies to the human immunodeficiency virus type I envelope by using the molecular adjuvant C3d. J Virol. 2003;77:2046–2055. doi: 10.1128/JVI.77.3.2046-2055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dickey CA, Morgan DG, Kudchodkar S, Weiner DB, Bai Y, Cao C, Gordon MN, Ugen KE. Duration and specificity of humoral immune responses in mice vaccinated with the Alzheimer’s disease-associated beta-amyloid 1–42 peptide. DNA Cell Biol. 2001;20:723–729. doi: 10.1089/10445490152717587. [DOI] [PubMed] [Google Scholar]
- 52.Town T, Tan J, Sansone N, Obregon D, Klein T, Mullan M. Characterization of murine immunoglobulin G antibodies against human amyloid-b 1–42. Neurosci Lett. 2001;307:101–104. doi: 10.1016/s0304-3940(01)01951-6. [DOI] [PubMed] [Google Scholar]
- 53.McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MH, Darabie AA, Brown ME, Janus C, Chishti MA, Horne P, Westaway D, Fraser PE, Mount HT, Przybylski M, St George-Hyslop P. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- 54.Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, Seubert P, Yednock T. Epitope and isotype specificities of antibodies to beta-amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci USA. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karin N. Gene therapy for T cell mediated autoimmunity: teaching the immune system how to restrain its own harmful activities by targeted DNA vaccine. Isr Med Assoc J. 2000;2:63–68. [PubMed] [Google Scholar]
- 56.Finkelman FD, Holmes J, Katona IM, Urban JF, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mossmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Ann Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 57.Petrushina I, Tran M, Sadzikava N, Ghochikyan A, Vasilevko V, Agadjanyan MG, Cribbs DH. Importance of IgG2c isotype in the immune response to b-amyloid in APP/Tg mice. Neurosci Lett. 2003;338:5–8. doi: 10.1016/s0304-3940(02)01357-5. [DOI] [PubMed] [Google Scholar]
- 58.Solomon B, Koppel R, Hanan E, Katzav T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer beta-amyloid peptide. Proc Natl Acad Sci USA. 1996;93:452–455. doi: 10.1073/pnas.93.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]


