Abstract
Estrogens profoundly influence the physiology and pathology of reproductive and other tissues. Consequently, emphasis has been placed on delineating the mechanisms underlying regulation of estrogen levels. Circulating levels of estradiol in women are controlled by follicle-stimulating hormone (FSH), which regulates transcription of the aromatase gene (CYP19A1) in ovarian granulosa cells. Previous studies have focused on two downstream effectors of the FSH signal, cAMP and the orphan nuclear receptor steroidogenic factor-1 (NR5A1). In this report, we present evidence for β-catenin (CTNNB1) as an essential transcriptional regulator of CYP19A1. FSH induction of select steroidogenic enzyme mRNAs, including Cyp19a1, is enhanced by β-catenin. Additionally, β-catenin is present in transcription complexes assembled on the endogenous gonad-specific CYP19A1 promoter, as evidenced by chromatin immunoprecipitation assays. Transient expression and RNAi studies demonstrate that FSH- and cAMP-dependent regulation of this promoter is sensitive to alterations in the level of β-catenin. The stimulatory effect of β-catenin is mediated through functional interactions with steroidogenic factor-1 that involve four acidic residues within its ligand-binding domain, mutation of which attenuates FSH/cAMP-induced Cyp19a1 mRNA accumulation. Together, these data demonstrate that β-catenin is essential for FSH/cAMP-regulated gene expression in the ovary, identifying a central and previously unappreciated role for β-catenin in estrogen biosynthesis, and a potential broader role in other aspects of follicular maturation.
Keywords: ovary, steroidogenic factor-1, granulosa cells, steroidogenesis, estrogen
Estrogens play a central role in regulating homeostatic and pathologic pathways. They influence fertility and sexual behavior, lipid metabolism, bone remodeling, and the development of various endocrine cancers (1). Ovarian follicles are the primary source of local and circulating estrogen in mammals (2). Synthesis of follicular estradiol depends on the coordinated actions of the pituitary gonadotropin hormones, follicle-stimulating hormone (FSH) and luteinizing hormone, cytokines, and growth factors (3, 4).
FSH induces estrogen biosynthesis by triggering cAMP-dependent signaling cascades to regulate transcription of the CYP19A1 gene. This gene encodes the cytochrome P450 enzyme aromatase, which catalyzes the irreversible conversion of androgens to estrogens (5). The CYP19A1 gene contains multiple promoters that dictate tissue-specific patterns of aromatase expression (6). In the ovary, expression of CYP19A1 is directed by the type II promoter (PII) that resides within the immediate 5′ region flanking the translational start site (7). PII is also pathologically activated in adipocytes in malignant breast tissue (8). Therefore, delineating mechanisms that contribute to follicular expression of CYP19A1 is essential for understanding how estrogen levels are regulated in health and disease.
Among the numerous cis-acting elements identified within the CYP19A1 PII regulatory region, two classes of elements are central to hormone responsiveness: a noncanonical cAMP-response element (CRE)-like sequence (CLS) and nuclear receptor motifs. The CLS binds the CRE-binding protein (CREB) and other basic-leucine zipper proteins (9, 10). The nuclear receptor motifs bind steroidogenic factor-1 (SF1, officially designated NR5A1) (11–14), and potentially its close relative, liver receptor homologue-1 (LRH1, officially designated NR5A2) (15, 16).
SF1 is essential for the development and function of the reproductive axis at multiple levels (17). How extracellular tropic hormones, such as FSH, activate SF1-mediated transcription has been extensively debated. In addition to posttranslational modifications (18–21), hormonally regulated interactions with various protein partners, including other transcription factors and coactivators, are among proposed mechanisms (11, 20, 22–27).
Studies in the adrenal gland and developing urogenital ridge suggest SF1 activity can be modulated by the transcription cofactor β-catenin (officially designated CTNNB1) (28–31). These reports emphasize the ability of the Wnt family of secreted glycoproteins to affect SF1-dependent transcription via its association with β-catenin. As a mediator of Wnt signaling, β-catenin regulates numerous genes involved in development and tumorigenesis (32). Recently, mice that express a constitutively active β-catenin mutation only in granulosa cells (GCs) were found to be subfertile and develop GC tumors (33). A role for Wnt signaling and β-catenin in embryonic gonad lineage specification has also been suggested (30, 31). To date, whether β-catenin contributes to hormonal stimulation of SF1 target tissues remains unknown.
To determine whether β-catenin modulates expression of SF1 target genes in the ovarian follicle, we explored its role in transcriptional regulation of gonadotropin-induced steroidogenesis in rat GCs. Our results provide functional evidence that an interaction between β-catenin and SF1 is essential for the FSH and cAMP cascades that regulate Cyp19a1 and additional genes involved in estrogen production in GCs.
Results
β-Catenin Enhances FSH Regulation of Key Steroidogenic Enzyme mRNAs.
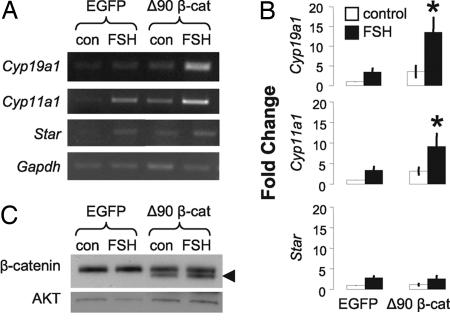
We initially evaluated the effect of β-catenin on induction of mRNAs encoding CYP19A1 and rate-limiting components of steroid synthesis, steroidogenic acute regulatory protein (STAR), and cytochrome P450 side-chain-cleavage enzyme (CYP11A1). Preantral rat GCs were cultured in the absence of added steroids to establish a model where FSH induction of some target genes is modest (34), permitting optimal detection of contributions from β-catenin. GCs were transduced with an adenovirus encoding a β-catenin mutant lacking amino-terminal residues involved in its targeted degradation (Δ90), while retaining interactions with DNA-binding proteins (35). An adenovirus containing EGFP (36) provided a control for estimating the effect of Δ90 β-catenin in untreated and FSH-stimulated cells. Semiquantitative RT-PCR analysis indicated that, when compared with EGFP controls, both 24-h FSH treatment and Δ90 β-catenin alone could induce all three steroidogenic enzyme mRNAs, albeit to extents that varied between experiments (Fig. 1A and B). In contrast, Δ90 β-catenin consistently and significantly enhanced FSH induction of Cyp19a1 and Cyp11a1 mRNAs (P < 0.05), but not Star (Fig. 1 A and B). These data provide evidence that β-catenin can selectively modulate FSH regulation of two key steroidogenic enzymes in GCs, CYP19A1 and CYP11A1. Because CYP19A1 showed a marked sensitivity to β-catenin, subsequent analyses focused on the gene encoding this protein.
Fig. 1.
β-catenin enhances FSH induction of endogenous Cyp19a1 and Cyp11a1 mRNAs. Primary GCs were transduced with Δ90 β-catenin or EGFP adenoviruses [3 × 1010 virus particles per milliliter (vpm)]. Samples were treated with FSH (100 ng/ml, 24 h) where indicated. (A) Representative RT-PCR analysis of Cyp19a1, Cyp11a1, Star, and Gapdh mRNAs. (B) Fold change was calculated by comparison with untreated EGFP controls (mean ± SEM, n = 4; ∗, P < 0.05). (C) Western blot analysis confirmed endogenous and Δ90 β-catenin (◀) expression (upper and lower bands, respectively); AKT was the loading control.
Residues 235–238 in the SF1 Ligand-Binding Domain Mediate a Functional Interaction with β-Catenin.
The ability of β-catenin to augment Cyp19a1 expression could be because of interactions with SF1, because the nuclear receptor has been shown to bind the cofactor via residues 235–238 in the proximal activation domain (Fig. 2A) (30). To test this hypothesis, we used recombinant adenoviruses to coexpress Δ90 β-catenin with either wild-type SF1, or SF1 with its β-catenin interaction domain mutated [SF1(235-4A)] in primary GCs, and measured the accumulation of Cyp19a1 mRNA.
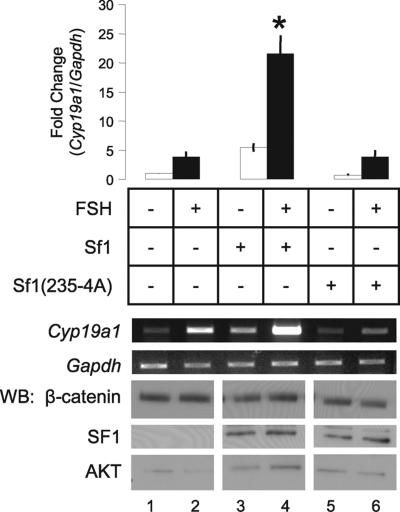
Fig. 2.
Residues 235–238 in the SF1 ligand-binding domain mediate a functional interaction with β-catenin. (A) SF1 schematic: DBD, DNA-binding domain; h, hinge region; LBD, ligand-binding domain (including H1, helix 1); P, Ser-203 phosphorylation site; residues 235–238, β-catenin interaction domain; pAF, proximal activation domain; and AF2, activation function 2 domain. (B) Primary GCs were transduced with adenoviruses encoding EGFP (3 × 1010 vpm), SF1 (1 × 1011 vpm), or SF1(235-4A) (1 × 1010 vpm) alone, or in combination with Δ90 β-catenin (3 × 1010 vpm). RNA and protein were isolated after 48 h, and gene/protein expression was evaluated as described in Fig. 1. Cyp19a1 and Gapdh, RT-PCR analysis; WB, Western blots. (Bar Graph) Fold change was determined by comparison with untreated EGFP controls (mean ± SEM; n = 3; ∗, P < 0.05).
Compared with EGFP controls (Fig. 2B, lane 1), wild-type SF1 increased Cyp19a1 mRNA expression in the absence of FSH (P < 0.05, Fig. 2B, lane 3). Coexpression of Δ90 β-catenin significantly increased the stimulatory effect of SF1 alone (P < 0.05, Fig. 2B, lane 4). In contrast, SF1(235-4A) did not induce Cyp19a1 mRNA, despite levels of expression similar to those of its wild-type counterpart (Fig. 2B, lane 5). The Δ90 β-catenin mutant was unable to rescue gene expression by SF1(235-4A) (Fig. 2B, lane 6). Similar results were observed when lower titers of wild-type and mutant SF1 were used (data not shown). These data suggest that the trans-activation of Cyp19a1 by SF1 requires both an intact β-catenin binding site and a functional interaction with β-catenin.
The Functional Interaction Between FSH and SF1 Requires an Intact β-Catenin-Interaction Domain.
We next determined whether the β-catenin interaction domain of SF1 was required to facilitate the FSH signal in rat GCs. In the absence of added steroids, both FSH and SF1 alone produced small increases in Cyp19a1 mRNA when compared with untreated EGFP controls (Fig. 3, lanes 1–3). The stimulatory effect of FSH was dramatically enhanced by the presence of SF1 (P < 0.05, Fig. 3, lane 4), but not SF1(235-4A) (Fig. 3, lane 6). Thus the β-catenin-interaction domain of SF1 is also essential for the synergistic actions of FSH and SF1 on Cyp19a1.
Fig. 3.
Cooperative induction of Cyp19a1 mRNA by FSH and SF1 requires an intact β-catenin-interaction domain. Primary GCs were transduced with EGFP, SF1, or SF1(235-4A) adenoviruses as described in Fig. 2. FSH treatment and analyses of mRNA/protein expression were described in Fig. 1. (Bar Graph) Fold change was determined by comparison with untreated EGFP controls (mean ± SEM; n = 3; ∗, P < 0.05).
FSH/cAMP Regulation of the CYP19A1 Type II Requires β-Catenin.
Modulation of Cyp19a1 mRNA levels by β-catenin could reflect either enhanced transcription or posttranscriptional stabilization of the message. Transient expression assays and RNA interference were used to explore the effect of β-catenin on activity of a cAMP-responsive fragment of the CYP19A1 PII in primary GCs, as well as a human granulosa tumor cell line (KGN). Like GCs, KGN cells express endogenous SF1 and increase steroidogenesis in response to cAMP signaling (data not shown and ref. 37).
To initially test whether cAMP stimulation of CYP19-luc required β-catenin, a pool of four siRNA duplexes was used to knock down endogenous β-catenin protein (CTNNB1) in primary GCs (Fig. 4A). A similar pool of nontargeting siRNA duplexes controlled for non-sequence-specific effects. FSH or forskolin (24 h) significantly induced CYP19-luc in primary GCs cotransfected with control siRNA compared with vehicle-treated groups (Fig. 4A). In contrast, cotransfection with Ctnnb1-specific siRNA attenuated basal activity of the promoter and compromised the efficacy of FSH and forskolin (P < 0.05, Fig. 4A).
Fig. 4.
β-Catenin mediates FSH/cAMP induction of CYP19A1 PII. (A) Primary GCs were transiently cotransfected with CYP19A1 (100 ng) and phRG-B Renilla luciferase reporters (10 ng) and either nonspecific (control) or Ctnnb1-specific siRNA (50 nM). Western blot analysis confirmed knockdown of endogenous β-catenin protein by Ctnnb1 siRNA as compared with mock or control siRNA-transfected GCs. AKT was the loading control. GCs were treated with 10 nM testosterone plus vehicle, 10 μM forskolin, or 100 ng/ml FSH for 24 h before luciferase assays. Bar graphs represent normalized luciferase activity, firefly/Renilla (FF/Ren); mean ± SEM; ∗, P < 0.05. (B–D) KGN cells were transiently cotransfected with CYP19A1 (200 ng) and phRG-TK Renilla reporters (20 ng), and expression vectors or siRNA, and treated with vehicle or 10 μM forskolin (24 h) before luciferase assays. (B) Nonspecific (control) or CTNNB1-specific siRNA (100 nM) cotransfected into KGN cells (n = 4). (C) Axin1 or empty vector (100 ng) cotransfected into KGN cells (n = 5). (D) Empty vector or Δ90 β-catenin (100 ng) cotransfected (n = 3).
Similar results were observed in KGN cells by using two different approaches to reduce endogenous β-catenin. In addition to RNAi, we overexpressed Axin1, which encodes a rate-limiting component of a cytoplasmic protein complex that degrades β-catenin (38–40) and negatively regulates β-catenin-dependent transcription (39, 41). Because KGN cells have low levels of FSH receptors (37), we used forskolin to activate adenylyl cyclase and elevate cAMP. Cotransfection with either CTNNB1-specific siRNA or an Axin1 expression vector significantly inhibited forskolin stimulation of CYP19-luc (P < 0.05, Fig. 4 B and C).
We also tested whether increasing β-catenin levels affected cAMP responsiveness of CYP19A1 PII. Adenoviral transduction of Δ90 β-catenin in KGN cells synergistically enhanced forskolin stimulation of endogenous CYP19A1 mRNA (data not shown), mimicking results obtained in rat GCs treated with FSH (Fig. 1). Transiently transfected Δ90 β-catenin was insufficient to activate CYP19-luc in KGN cells (Fig. 4D). However, forskolin stimulation of CYP19-luc was significantly enhanced by Δ90 β-catenin (P < 0.05, Fig. 4D). Collectively, these data demonstrate that the actions of β-catenin on CYP19A1 in GCs are dependent on hormone-induced cAMP cascades.
SF1 Requires β-Catenin for Basal and cAMP Regulation of the Type II CYP19A1 Promoter.
Because the CYP19A1 PII region contains both cAMP- and SF1-response elements, we determined whether β-catenin was required for SF1-dependent activation of this promoter. Transient Δ90 β-catenin expression in KGN cells potentiated SF1 stimulation of CYP19-luc (P < 0.05, Fig. 5A). This result is consistent with observed effects on the endogenous gene in primary GCs (Fig. 2B) and emphasizes β-catenin as a transcriptional partner of SF1.
Fig. 5.
SF1 requires β-catenin for basal and cAMP regulation of CYP19A1 PII. (A and B) Transient transfection of KGN cells with CYP19A1 and Renilla reporters was as described for Fig. 4. (A) Sf1 (10 ng) was cotransfected in combination with empty or Δ90 β-catenin vectors (100 ng). Cells were harvested 48 h after transfection for luciferase assays. Data represent fold change compared with empty vector (mean ± SEM, n = 3). (B) Empty or Sf1 vector (10 ng) was cotransfected with control or CTNNB1-specific siRNA (100 nM) (duplicate transfections). Cells were treated with vehicle or 10 μM forskolin (24 h) before luciferase assays. Data represent fold induction relative to forskolin-treated empty vector control cotransfected with control siRNA (mean ± SEM, n = 3; ∗, P < 0.05).
Conversely, depleting endogenous β-catenin by CTNNB1-specific RNAi significantly inhibited the basal trans-activity of SF1 (P < 0.05, Fig. 5B), and compromised the enhanced stimulation of CYP19-luc by forskolin and SF1 together (P < 0.05, Fig. 5B). Similar results were observed with Axin1 (data not shown). These data are in accordance with effects of SF1(235-4A) on the endogenous Cyp19a1 gene (Figs. 2 and 3), and they suggest that functional interactions with β-catenin link the cell surface gonadotropin/cAMP cascade to SF1.
SF1 and β-Catenin Localize to the Endogenous CYP19A1 PII.
Finally, ChIP analysis of KGN cells with a β-catenin antibody revealed modest but consistent association of the cofactor with CYP19A1 PII chromatin (Fig. 6, lane 4). Increased association of SF1 and β-catenin to this regulatory region was detected after 1-h forskolin stimulation in two of three experiments (Fig. 6, lanes 7 and 8). Antibodies to Gαq/11 failed to immunoprecipitate CYP19A1 PII under any condition (Fig. 6, lanes 2 and 6), and none of the antibodies precipitated chromatin distal to the CYP19A1 gene (data not shown). Thus, β-catenin is present in transcription complexes assembled on the endogenous CYP19A1 PII, and its association, along with that of SF1, is likely regulated by cAMP-dependent mechanisms.
Fig. 6.
β-Catenin associates with the endogenous CYP19A1 PII. KGN cells were treated with vehicle or 10 μM forskolin (1 h). ChIP assays were performed with Gαq/11, SF1, or β-catenin antibodies (n = 3). DNA was amplified by using primers against CYP19A1 PII (proximal) and an upstream (distal) region (schematic). CLS, cAMP-response-element-like sequence. Representative PCR using proximal primers is shown. Vehicle data represent three independent experiments. Forskolin results are representative of two of three experiments.
Discussion
The influence of gonadotropin hormones on follicular estrogen synthesis through SF1 and cAMP is well appreciated (11, 14). We now demonstrate that β-catenin is required for this process. Specifically, β-catenin regulates transcription of the central player of estrogen biosynthesis, CYP19A1, via functional interactions with SF1. Prior reports of β-catenin-dependent gene expression in the gonad have been interpreted as evidence for the influence of Wnt signaling (30, 31, 33). We now extend these observations by demonstrating that β-catenin is essential for the ability of SF1 to respond to FSH. Thus, β-catenin may represent an important point of convergence for extracellular signals affecting estrogen production.
Our data suggest that contributions of β-catenin to aromatase expression in GCs are likely funneled, at least in part, through SF1. Decreasing β-catenin levels or disrupting the SF1/β-catenin interaction attenuates FSH/cAMP-induced CYP19A1 promoter activity and mRNA accumulation. These findings are also consistent with a prior report that cooperation between the proximal activation domain and coactivator interaction domains of SF1 are necessary to transmit the stimulatory effect of cAMP (42).
Whereas we have shown that β-catenin is required for full SF1 activity, its involvement in FSH/cAMP signaling appears restricted to a critical subset of genes targeted by the nuclear receptor. Indeed, in contrast to the male, where transcriptional regulation of STAR depends on the coordinated actions of β-catenin and SF1 (31), expression of this gene was unaffected by β-catenin in our studies. This observation may reflect sexually dimorphic actions of β-catenin, or the fact that luteinizing hormone (rather than FSH) is the primary regulator of STAR in GCs (43). Dax1, a putative β-catenin target (30), can also selectively modulate Cyp19a1 over other steroidogenic enzymes (44). Thus, β-catenin signaling may contribute to differential production of estrogen and progesterone by gonadotropins during the reproductive cycle.
The precise molecular mechanism mediating the effect of β-catenin remains unclear, although it is likely to be sensitive to cAMP because association of β-catenin with the endogenous CYP19A1 promoter is enhanced by forskolin. However, we have been unable to detect hormonal regulation of β-catenin accumulation, GSK3-β-dependent phosphorylation, or nuclear localization in vitro (data not shown). Interestingly, phosphorylation of β-catenin by PKA was recently reported to facilitate coactivator binding, without affecting β-catenin accumulation (45). Thus, cAMP/PKA cascades triggered by FSH may induce posttranslational modification of β-catenin. This modification, in turn, could promote its association with select promoters and affect coactivator recruitment, a well-known mechanism for hormonal regulation of SF1 activity (20, 22, 24). Therefore, among proteins that sense cAMP elevation (e.g., cAMP response-binding protein) (24), β-catenin may function to bridge the cyclic nucleotide signal to SF1.
In addition to FSH, other external signals capable of affecting β-catenin or the SF1/β-catenin interaction could regulate CYP19A1 and, subsequently, estrogen production. Activin and Wnt4 are two intriguing candidates. Activin and FSH synergistically promote Cyp19a1 expression (34, 46), predicting cooperative enhancement of β-catenin-dependent transcription. In contrast, the preovulatory luteinizing hormone surge down-regulates Cyp19a1 (47). Concomitant induction of Wnt4 by luteinizing hormone (48) may be a contributing factor, because studies suggest that Wnt4 can attenuate β-catenin/SF1 synergy (31).
β-Catenin-dependent regulation of CYP19A1 may be significant in pathologic states as well. cAMP activation of the gonad-specific CYP19A1 promoter in the adipose compartment of malignant breast tissue contributes to high local estradiol levels (8, 49). The orphan nuclear receptor responsive to the stimulatory effects of cAMP in this tissue is LRH1 (50). Interestingly, the β-catenin interaction domain is highly conserved between SF1 and LRH1 (30), and synergistic coactivation of β-catenin and LRH1 occurs in colon cancer (51). Therefore β-catenin may be an important mediator of cAMP stimulation of orphan nuclear receptors, promoting ectopic activation of the gonad-specific CYP19A1 promoter in breast cancer and its physiologic regulation in the ovary.
In summary, this study highlights that FSH/cAMP regulation of CYP19A1 requires a functional interaction between SF1 and β-catenin. The β-catenin-dependent actions of FSH are likely to extend beyond orphan nuclear receptors, impacting genes dependent on other transcription factors that similarly require β-catenin. It also raises the possibility that β-catenin may be pivotal for an array of extracellular signals that dictate when and where the CYP19A1 gene is expressed, impacting estrogen levels in health and disease.
Materials and Methods
Plasmids and siRNA.
Expression and reporter vectors were generously donated as follows: murine Δ90 β-catenin in the pUHD10–3 vector by W. J. Nelson (Stanford University School of Medicine, Palo Alto, CA) (52); murine Axin1 in a pCS2+MT vector by F. Costantini (Columbia University, New York, NY) (53); murine Sf1 and Sf1(235-4A) in the pCMX vector by K. Morohashi (University of Tsukuba, Tsukuba, Japan) (30); and human CYP19A1-517 promoter fragment in pGL3-basic by S. Bulun (Northwestern University, Chicago, IL) (54). Full-length murine Sf1 in pcDNA3 was described in ref. 26. Silencing RNA SMARTpools, CTNNB1 and Ctnnb1, and siCONTROL nontargeting siRNA were purchased from Dharmacon (Lafayette, CO).
Recombinant Adenovirus Construction.
Adenovirus vectors encoding SF1, SF1(235-4A), and Δ90 β-catenin were constructed by using the AdMax system (Microbix Biosystems, Toronto, ON, Canada). Full-length Sf1 and Sf1(235-4A) were excised from pcDNA3 and pCMX, respectively, and subcloned into the EcoRI site of the pDC316(io) adenovirus shuttle vector. Δ90 β-catenin was excised from pUHD10–3 with SacII and XbaI, blunt ends were generated, and the gene was subcloned into the SmaI site of pDC316. Constructs were confirmed by restriction digestion and sequencing. Recombinant adenoviruses were generated by cotransfecting HEK293 cells with adenovirus shuttle vectors and pBHGloxΔE,3Cre plasmid (circularized adenovirus genome) as described (55). Sample absorbance at 260 nm was used to estimate viral content by using the relationship 1012 virus particles per ml (vpm)/A260 unit (56). Titers used were determined empirically by Western blot and functional assays.
Cell Culture.
Female Sprague–Dawley rats (20 days old), purchased from Charles River Laboratories (Hollister, CA), were maintained in accordance with Washington State University Institutional Animal Care and Use Committee guidelines. At 23 days, rats were injected daily with 0.15 mg of 17β-estradiol s.c. for 3 days. Ovaries were harvested and GCs were isolated as described (46). Cells were plated (2–3 × 106 per 60-mm dish) in DMEM/F12 medium (Invitrogen, Carlsbad, CA) supplemented with 10% (vol/vol) FBS and 1% (vol/vol) penicillin/streptomycin (Invitrogen, Grand Island, NY) for 4 h at 37°C in 5% CO2 before adenovirus transduction. KGN cells (RIKEN, Kobe, Japan) were cultured in DMEM supplemented with 10% FBS/1% penicillin/streptomycin at 37°C in 5% CO2.
Adenovirus Transduction and Hormone Stimulation.
Primary GCs were exposed to recombinant adenoviruses diluted in serum-free medium supplemented with 1% penicillin/streptomycin for 13 h, and the medium was then replaced with fresh DMEM/F12/penicillin/streptomycin. At 24 h after transduction, cells were incubated in the presence or absence of 100 ng/ml purified human FSH (National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health) under serum-free conditions (24 h), then harvested for total RNA and protein isolation.
RT-PCR.
Total RNA was isolated and DNase-treated to remove genomic DNA contamination by using RNeasy Kits (Qiagen, Valencia, CA). First-strand cDNA was synthesized (1 μg total RNA) by using oligo(dT) primers and SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). Primer sequences for Cyp19a1 (NM_017085) and Cyp11a1 (NM_017286) were published previously (46). Additional sequences include the following: Gapdh [NM_017008; (F) 5′-ATGGTGAAGGTCGGTGTGAACG-3′, (R) 5′-GTTGTCATGGATGACCTTGGCC-3′], and Star [NM_031558; (F) 5′-GCCAGCAGGAGAATGGAG-3′, (R) 5′-AGCCAGCTCATGGGTGAT-3′]. Preliminary studies identified the linear signal range from 15 to 40 cycles. PCR was performed on 20 ng of total cDNA, and PCR products were resolved by agarose gel electrophoresis and ethidium bromide staining. Quantity One 4.5.0 1-D Analysis software (Bio-Rad, Hercules, CA) was used for image capture and densitometry. Gapdh signal was used to normalize for cDNA content variability between samples.
Western Blots.
Primary GCs were scraped into PBS, pelleted (400 × g, 4 min), and lysed in radioimmunoprecipitation assay buffer [0.2 mM NaVO3, Complete Mini Protease Inhibitor tablets (Roche Diagnostics, Indianapolis, IN) added fresh]. Protein concentrations were estimated by using Coomassie Plus Protein Assays (Pierce, Rockford, IL). Samples (15 μg) were separated by SDS/4–20% PAGE Tris·HCl gels (Bio-Rad) as described by Laemmli (57) and transferred to PVDF membrane (Bio-Rad) in Towbin buffer. Primary antibodies against β-catenin (BD Transduction Laboratories, Lexington, KY), SF1 (Upstate Biotechnology, Lake Placid, NY), and Akt (Cell Signaling Technology, Beverly, MA) were incubated in 5% milk/0.05% Tween 20/Tris-buffered (pH 8.0) saline blocking solution overnight at 4°C. The SF1 antibody cross-reacts with LRH1 (58), but the antigens are distinguished by molecular weight. After HRP-conjugated secondary antibody incubations (Pierce), antigen–antibody complexes were detected by chemiluminescence (Immobilon; Millipore, Billerica, MA).
Transient Cotransfection and Luciferase Assays.
KGN cells (6 × 104) were plated in complete medium to achieve 70–80% confluence, and primary GCs (3 × 105) were incubated in DMEM/F12/10% FBS for 3–4 h before transfection. Transient transfections using Lipofectamine (KGN) or Lipofectamine 2000 (GC) (Invitrogen, Carlsbad, CA) followed manufacturer’s recommendations. Total DNA transfected was equalized with empty pcDNA3 vector where indicated. All groups were cotransfected with pHRG-TK Renilla luciferase reporter vector (Promega, Madison, WI) to normalize for transfection efficiency differences. Cells were treated for 24 h with vehicle (0.25% DMSO), FSH (100 ng/ml), or forskolin (10 μM) (Sigma–Aldrich, St. Louis MO) in serum-free medium 24 h after transfection for transient expression assays, and 48 h after transfection for RNAi studies. Testosterone propionate (10 nM) was added in GC treatments. Cells were harvested for luciferase assays by using the Dual-Luciferase Reporter Assay System (Promega).
ChIP Assays.
ChIPs were performed as detailed by Weck and Mayo (58). Briefly, KGN cells (1.5 × 106 per 60-mm dish) were plated overnight. Cells were treated with vehicle or 10 μM forskolin for 1 h (37°C, 5% CO2), and cross-linked with 1% formaldehyde for 15 min at room temperature. Cells were lysed (250 μl final volume), and sonicated with a Fisher Sonic Dismembrator Model 100 set at 1 (six times, 15 s each). One tenth of the sample was removed for input and one tenth was removed to evaluate sonication efficiency. Remaining samples were divided into three groups, diluted in 1.5 ml of immunoprecipitation buffer, precleared with salmon sperm DNA/Protein A agarose (Upstate Biotechnology) (45 min, 4°C), and immunoprecipitated overnight at 4°C with 1.0 μg of each antibody [Gαq/11 and β-catenin (Santa Cruz Biotechnology, Santa Cruz, CA), and SF1 (Upstate Biotechnology)]. Eluted protein/DNA complexes were digested with proteinase K, and DNA was purified with the Qiaquick PCR purification kit (Qiagen, Valencia, CA). PCR was performed on purified DNA by using primers upstream [(F) 5′-GCATCAGGGAACACTGGTTT-3′; (R) 5′-GGTACCTGGACCACAGCACT-3′] or within PII of the human CYP19A1 gene [(F) 5′-TTTCCACACTACCGTTGGCCG-3′; (R) 5′-GGCAATCTTCTTCCCTTGAAGC-3′]. Products were separated on agarose/ethidium bromide gels and visualized as indicated above.
Statistical Analyses.
All experiments were repeated at least three times on independent days. Transient transfection experiments were performed in triplicate wells each day unless otherwise noted. All experiments were analyzed by using general linear model procedures of SAS (SAS Institute, Cary, NC), for a completely randomized design. Least-square means are reported. Statistical significance was set at P < 0.05, indicated by asterisks in figures.
Acknowledgments
We thank Jodi Jackson and Patricia Hunt for critical evaluation of the manuscript and Travis Salisbury for useful discussion, and we acknowledge Y. Nishi, T. Yanase, and H. Nawata for use of KGN cells. This work was supported by National Institutes of Health Grants CA 086387 (to J.H.N.) and P01 HD21921 (to M.H.-D.) and National Institutes of Health Medical Scientist Training Program Grant T32-GM07250 (to T.N.P.).
Glossary
Abbreviations
- Cyp11a1
cytochrome P450 side-chain-cleavage enzyme
- Cyp19a1
cytochrome P450 aromatase
- FSH
follicle-stimulating hormone
- GC
granulosa cell
- LRH1
liver receptor homologue-1
- SF1
steroidogenic factor-1
- STAR
steroidogenic acute regulatory protein
- vpm
virus particles per milliliter.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Simpson E. R., Misso M., Hewitt K. N., Hill R. A., Boon W. C., Jones M. E., Kovacic A., Zhou J., Clyne C. D. Endocr. Rev. 2005;26:322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- 2.Findlay J. K., Britt K., Kerr J. B., O’Donnell L., Jones M. E., Drummond A. E., Simpson E. R. Reprod. Fertil. Dev. 2001;13:543–547. doi: 10.1071/rd01071. [DOI] [PubMed] [Google Scholar]
- 3.Richards J. S. Endocrinology. 2001;142:2184–2193. doi: 10.1210/endo.142.6.8223. [DOI] [PubMed] [Google Scholar]
- 4.Richards J. S., Russell D. L., Ochsner S., Hsieh M., Doyle K. H., Falender A. E., Lo Y. K., Sharma S. C. Recent Prog. Horm. Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- 5.Thompson E. A., Jr., Siiteri P. K. J. Biol. Chem. 1974;249:5373–5378. [PubMed] [Google Scholar]
- 6.Bulun S. E., Sebastian S., Takayama K., Suzuki T., Sasano H., Shozu M. J. Steroid Biochem. Mol. Biol. 2003;86:219–224. doi: 10.1016/s0960-0760(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins C., Michael D., Mahendroo M., Simpson E. Mol. Cell. Endocrinol. 1993;97:R1–R6. doi: 10.1016/0303-7207(93)90227-b. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal V. R., Bulun S. E., Leitch M., Rohrich R., Simpson E. R. J. Clin. Endocrinol. Metab. 1996;81:3843–3849. doi: 10.1210/jcem.81.11.8923826. [DOI] [PubMed] [Google Scholar]
- 9.Michael M. D., Michael L. F., Simpson E. R. Mol. Cell. Endocrinol. 1997;134:147–156. doi: 10.1016/s0303-7207(97)00178-0. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S., Wu Y., Li R., Hu Y. Oncogene. 2005;24:2236–2246. doi: 10.1038/sj.onc.1208415. [DOI] [PubMed] [Google Scholar]
- 11.Carlone D. L., Richards J. S. Mol. Endocrinol. 1997;11:292–304. doi: 10.1210/mend.11.3.9900. [DOI] [PubMed] [Google Scholar]
- 12.Falender A. E., Lanz R., Malenfant D., Belanger L., Richards J. S. Endocrinology. 2003;144:3598–3610. doi: 10.1210/en.2002-0137. [DOI] [PubMed] [Google Scholar]
- 13.Lynch J. P., Lala D. S., Peluso J. J., Luo W., Parker K. L., White B. A. Mol. Endocrinol. 1993;7:776–786. doi: 10.1210/mend.7.6.8395654. [DOI] [PubMed] [Google Scholar]
- 14.Michael M. D., Kilgore M. W., Morohashi K., Simpson E. R. J. Biol. Chem. 1995;270:13561–13566. doi: 10.1074/jbc.270.22.13561. [DOI] [PubMed] [Google Scholar]
- 15.Hinshelwood M. M., Repa J. J., Shelton J. M., Richardson J. A., Mangelsdorf D. J., Mendelson C. R. Mol. Cell. Endocrinol. 2003;207:39–45. doi: 10.1016/s0303-7207(03)00257-0. [DOI] [PubMed] [Google Scholar]
- 16.Clyne C. D., Speed C. J., Zhou J., Simpson E. R. J. Biol. Chem. 2002;277:20591–20597. doi: 10.1074/jbc.M201117200. [DOI] [PubMed] [Google Scholar]
- 17.Parker K. L., Rice D. A., Lala D. S., Ikeda Y., Luo X., Wong M., Bakke M., Zhao L., Frigeri C., Hanley N. A., et al. Recent Prog. Horm. Res. 2002;57:19–36. doi: 10.1210/rp.57.1.19. [DOI] [PubMed] [Google Scholar]
- 18.Hammer G. D., Krylova I., Zhang Y., Darimont B. D., Simpson K., Weigel N. L., Ingraham H. A. Mol. Cell. 1999;3:521–526. doi: 10.1016/s1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- 19.Winnay J. N., Hammer G. D. Mol. Endocrinol. 2006;20:147–166. doi: 10.1210/me.2005-0215. [DOI] [PubMed] [Google Scholar]
- 20.Chen W. Y., Juan L. J., Chung B. C. Mol. Cell. Biol. 2005;25:10442–10453. doi: 10.1128/MCB.25.23.10442-10453.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M. B., Lebedeva L. A., Suzawa M., Wadekar S. A., Desclozeaux M., Ingraham H. A. Mol. Cell. Biol. 2005;25:1879–1890. doi: 10.1128/MCB.25.5.1879-1890.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borud B., Hoang T., Bakke M., Jacob A. L., Lund J., Mellgren G. Mol. Endocrinol. 2002;16:757–773. doi: 10.1210/mend.16.4.0799. [DOI] [PubMed] [Google Scholar]
- 23.Crawford P. A., Dorn C., Sadovsky Y., Milbrandt J. Mol. Cell. Biol. 1998;18:2949–2956. doi: 10.1128/mcb.18.5.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito M., Park Y., Weck J., Mayo K. E., Jameson J. L. Mol. Endocrinol. 2000;14:66–81. doi: 10.1210/mend.14.1.0410. [DOI] [PubMed] [Google Scholar]
- 25.Ito M., Yu R. N., Jameson J. L. Mol. Endocrinol. 1998;12:290–301. doi: 10.1210/mend.12.2.0059. [DOI] [PubMed] [Google Scholar]
- 26.Jorgensen J. S., Nilson J. H. Mol Endocrinol. 2001;15:1505–1516. doi: 10.1210/mend.15.9.0691. [DOI] [PubMed] [Google Scholar]
- 27.Mouillet J. F., Sonnenberg-Hirche C., Yan X., Sadovsky Y. J. Biol. Chem. 2004;279:7832–7839. doi: 10.1074/jbc.M312574200. [DOI] [PubMed] [Google Scholar]
- 28.Gummow B. M., Winnay J. N., Hammer G. D. J. Biol. Chem. 2003;278:26572–26579. doi: 10.1074/jbc.M212677200. [DOI] [PubMed] [Google Scholar]
- 29.Hossain A., Saunders G. F. J. Biol. Chem. 2003;278:26511–26516. doi: 10.1074/jbc.M300804200. [DOI] [PubMed] [Google Scholar]
- 30.Mizusaki H., Kawabe K., Mukai T., Ariyoshi E., Kasahara M., Yoshioka H., Swain A., Morohashi K. Mol. Endocrinol. 2003;17:507–519. doi: 10.1210/me.2002-0362. [DOI] [PubMed] [Google Scholar]
- 31.Jordan B. K., Shen J. H., Olaso R., Ingraham H. A., Vilain E. Proc. Natl. Acad. Sci. USA. 2003;100:10866–10871. doi: 10.1073/pnas.1834480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyoshi K., Hennighausen L. Breast Cancer Res. 2003;5:63–68. doi: 10.1186/bcr566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boerboom D., Paquet M., Hsieh M., Liu J., Jamin S. P., Behringer R. R., Sirois J., Taketo M. M., Richards J. S. Cancer Res. 2005;65:9206–9215. doi: 10.1158/0008-5472.CAN-05-1024. [DOI] [PubMed] [Google Scholar]
- 34.El Hefnawy T., Zeleznik A. J. Endocrinology. 2001;142:4357–4362. doi: 10.1210/endo.142.10.8438. [DOI] [PubMed] [Google Scholar]
- 35.Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. W. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 36.Somers J. P., DeLoia J. A., Zeleznik A. J. Mol. Endocrinol. 1999;13:1364–1372. doi: 10.1210/mend.13.8.0329. [DOI] [PubMed] [Google Scholar]
- 37.Nishi Y., Yanase T., Mu Y., Oba K., Ichino I., Saito M., Nomura M., Mukasa C., Okabe T., Goto K., et al. Endocrinology. 2001;142:437–445. doi: 10.1210/endo.142.1.7862. [DOI] [PubMed] [Google Scholar]
- 38.Behrens J., Jerchow B. A., Wurtele M., Grimm J., Asbrand C., Wirtz R., Kuhl M., Wedlich D., Birchmeier W. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda S., Kishida S., Yamamoto H., Murai H., Koyama S., Kikuchi A. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakanaka C., Weiss J. B., Williams L. T. Proc. Natl. Acad. Sci. USA. 1998;95:3020–3023. doi: 10.1073/pnas.95.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones W. M., Bejsovec A. Curr. Biol. 2003;13:R479–R481. doi: 10.1016/s0960-9822(03)00407-x. [DOI] [PubMed] [Google Scholar]
- 42.Crawford P. A., Polish J. A., Ganpule G., Sadovsky Y. Mol. Endocrinol. 1997;11:1626–1635. doi: 10.1210/mend.11.11.9970. [DOI] [PubMed] [Google Scholar]
- 43.Ronen-Fuhrmann T., Timberg R., King S. R., Hales K. H., Hales D. B., Stocco D. M., Orly J. Endocrinology. 1998;139:303–315. doi: 10.1210/endo.139.1.5694. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z. J., Jeffs B., Ito M., Achermann J. C., Yu R. N., Hales D. B., Jameson J. L. Proc. Natl. Acad. Sci. USA. 2001;98:7988–7993. doi: 10.1073/pnas.141543298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taurin S., Sandbo N., Qin Y., Browning D., Dulin N. O. J. Biol. Chem. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 46.Park Y., Maizels E. T., Feiger Z. J., Alam H., Peters C. A., Woodruff T. K., Unterman T. G., Lee E. J., Jameson J. L., Hunzicker-Dunn M. J. Biol. Chem. 2005;280:9135–9148. doi: 10.1074/jbc.M409486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fitzpatrick S. L., Carlone D. L., Robker R. L., Richards J. S. Steroids. 1997;62:197–206. doi: 10.1016/s0039-128x(96)00181-x. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh M., Johnson M. A., Greenberg N. M., Richards J. S. Endocrinology. 2002;143:898–908. doi: 10.1210/endo.143.3.8684. [DOI] [PubMed] [Google Scholar]
- 49.Bulun S. E., Price T. M., Aitken J., Mahendroo M. S., Simpson E. R. J. Clin. Endocrinol. Metab. 1993;77:1622–1628. doi: 10.1210/jcem.77.6.8117355. [DOI] [PubMed] [Google Scholar]
- 50.Zhou J., Suzuki T., Kovacic A., Saito R., Miki Y., Ishida T., Moriya T., Simpson E. R., Sasano H., Clyne C. D. Cancer Res. 2005;65:657–663. [PubMed] [Google Scholar]
- 51.Botrugno O. A., Fayard E., Annicotte J. S., Haby C., Brennan T., Wendling O., Tanaka T., Kodama T., Thomas W., Auwerx J., et al. Mol. Cell. 2004;15:499–509. doi: 10.1016/j.molcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Barth A. I. M., Pollack A. L., Altschuler Y., Mostov K. E., Nelson W. J. J. Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fagotto F., Jho E.-h., Zeng L., Kurth T., Joos T., Kaufmann C., Costantini F. J. Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou J., Gurates B., Yang S., Sebastian S., Bulun S. E. Cancer Res. 2001;61:2328–2334. [PubMed] [Google Scholar]
- 55.Bebia Z., Somers J. P., Liu G., Ihrig L., Shenker A., Zeleznik A. J. Endocrinology. 2001;142:2252–2259. doi: 10.1210/endo.142.6.8017. [DOI] [PubMed] [Google Scholar]
- 56.Mittereder N., March K. L., Trapnell B. C. J. Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laemmli U. K. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 58.Weck J., Mayo K. E. Mol. Endocrinol. 2006;20:1090–1103. doi: 10.1210/me.2005-0199. [DOI] [PubMed] [Google Scholar]