Abstract
The myotubularin (MTM) family constitutes one of the most highly conserved protein-tyrosine phosphatase subfamilies in eukaryotes. MTM1, the archetypal member of this family, is mutated in X-linked myotubular myopathy, whereas mutations in the MTM-related (MTMR)2 gene cause the type 4B1 Charcot–Marie-Tooth disease, a severe hereditary motor and sensory neuropathy. In this study, we identified a protein that specifically interacts with MTMR2 but not MTM1. The interacting protein was shown by mass spectrometry to be MTMR5, a catalytically inactive member of the MTM family. We also demonstrate that MTMR2 interacts with MTMR5 via its coiled-coil domain and that mutations in the coiled-coil domain of either MTMR2 or MTMR5 abrogate this interaction. Through this interaction, MTMR5 increases the enzymatic activity of MTMR2 and dictates its subcellular localization. This article demonstrates an active MTM member being regulated by an inactive family member.
The myotubularin (MTM) family constitutes one of the largest and most highly conserved protein-tyrosine phosphatase (PTP) subfamilies in eukaryotes (1–3). The human MTM family of phosphatases includes MTM1/MTM-related (MTMR)1/MTMR2, MTMR3/MTMR4, and MTMR6/MTMR7/MTMR8 subgroups (1, 3). The consensus CX5R active site motif is found in the MTM family and the sequence “CSDGWDR” is invariant within all of the enzymatically active members of this family. Most PTPs use phosphoproteins as substrates and specifically dephosphorylate substrates containing only phosphotyrosine sites. Other phosphatases, collectively known as dual-specificity phosphatases, are capable of removing phosphoserines/threonines and phosphotyrosines from protein substrates.
Initially, MTM1 was reported to be a dual-specificity phosphatase (4–6). However, we and others have demonstrated that MTM1 utilizes the lipid second messenger, phosphatidylinositol 3-phosphate [PI(3)P], as a physiological substrate (7, 8). Recent findings demonstrate that other MTMR phosphatases MTMR1, MTMR2, MTMR3, MTMR4, and MTMR6 also dephosphorylate PI(3)P, suggesting that activity toward this substrate is common to all active MTM family members (9–12). MTMR2 and MTMR3 have also been shown to dephosphorylate phosphatidylinositol 3,5-bisphosphate (9, 13). PI(3)P plays a key role in membrane trafficking/vesicular transport processes and serves as a targeting mechanism for proteins containing specific PI(3)P-binding modules such as Fab1/YOTB/Vac1p/EEA1 (FYVE), pleckstrin homology (PH), and Phox homology domains (14–17).
To date, two MTMR proteins have been associated with human diseases. The MTM1 gene on chromosome Xq28 is mutated in X-linked myotubular myopathy, a severe congenital muscular disorder characterized by hypotonia and generalized muscle weakness in newborn males (18, 19). Myogenesis in affected individuals is arrested at a late stage of differentiation/maturation following myotube formation, and the muscle cells have characteristically large centrally located nuclei (20). Mutations in a second MTM gene MTMR2, on chromosome 11q22, have been shown recently to cause the neurodegenerative disorder, type 4B1 Charcot–Marie-Tooth disease (21, 22). Type 4B1 Charcot–Marie-Tooth disease is an autosomal recessive demyelinating neuropathy characterized by abnormally folded myelin sheaths and Schwann cell proliferation in peripheral nerves (23, 24).
MTM1 and MTMR2 are highly similar proteins (64% identity, 76% similarity), use the same physiologic substrate, and have a ubiquitous expression pattern (6, 9–12). However, mutations in MTM1 and MTMR2 cause different diseases with different target tissues and pathological characteristics. Therefore, MTM1 and MTMR2 may be subjected to differential regulatory mechanisms that preclude functional redundancy. In a previous study, we showed that developmental expression and subcellular localization of MTM1 and MTMR2 are differentially regulated, resulting in their utilization of specific cellular pools of PI(3)P (11). However, the physiologic function of MTM1 and related proteins in cell development and signaling processes remains unknown. Therefore, studies directed toward clarifying the regulation of MTMR enzymes, as well as identifying downstream effectors, will be of significant value.
One of the most notable characteristics of the human MTM family is the existence of at least five catalytically inactive forms [MTMR5 (sbf1)/MTMR9 (LIP-STYX)/MTMR10/MTMR11/MTMR12 (3-PAP)], which contain germline substitutions in catalytically essential residues within the PTP active site motif. Although the exact role of MTMR inactive forms is still unclear, previous reports suggest that the “dead” phosphatases may function either as interaction modules or as naturally occurring substrate-trapping mutants (25–27). The most extensively characterized catalytically inactive MTMR protein is MTMR5. Although the cellular function of MTMR5 is unknown, Cleary and colleagues (28) have shown recently that male mice deficient for MTMR5 are infertile and azoospermic, suggesting a role for MTM family proteins in spermatogenesis and germ cell differentiation.
In this study, we have searched for proteins that interact with MTM1 and/or MTMR2 by coimmunoprecipitation. The coimmunoprecipitated proteins were then subjected to proteolytic digestion and analyzed by mass spectrometry. By using this approach, we have found that MTMR2, but not MTM1, directly interacts with MTMR5. Through this interaction, MTMR5 regulates the enzymatic activity and the subcellular localization of MTMR2. This study demonstrates a previously unreported functional role for catalytically inactive MTM family members.
Materials and Methods
Plasmids.
Vectors for mammalian expression of N-terminally FLAG-tagged MTM1 and MTMR2 and enhanced GFP (EGFP)-tagged MTM1 and MTMR2 fusion proteins have been described (7, 11). Bacterial expression vectors for recombinant His-tagged MTM1 and MTMR2 fusion proteins have also been described (7, 11). A PCR product encoding protein fragments that correspond to the MTMR2-ΔPH domain (MTMR2-ΔPH, amino acids 183–643) and the MTMR2-Δcoiled-coil domain (MTMR2-Δcoil, amino acids 1–588) were inserted into the 5′ BglII and 3′ KpnI sites of pEGFP (CLONTECH). Site-directed mutagenesis was carried out by using a PCR-based procedure (29). Vectors for the expression of bacterial recombinant GST-tagged MTMR5 was created by using the pGEX-6P-2 (Amersham Pharmacia). The complete MTMR5 ORF was amplified by PCR by using a pMSCVneo-sbf1 as a template (30). The pGEX-MTMR5 vector was created by inserting the MTMR5 ORF into the 5′-EcoRI and 3′-HindIII sites. Mammalian expression vectors for N-terminally FLAG-tagged full-length (MTMR5-WT, amino acids 1–1,930), Δ-coiled coil (MTMR5-Δcoil, amino acids 1–1,640), and Δ-PH (MTMR5-ΔPH, amino acids 1–1,760) MTMR5 proteins were created by inserting cDNA fragments into the 5′ HindIII and 3′ EcoRI sites of pCDNA3.1-NF. All constructs were confirmed by DNA sequencing.
Cell Culture and Immunoprecipitation.
COS-1 and 293 cells were maintained at 37°C with 5% CO2 in DMEM containing 10% FCS, 50 units/ml penicillin, and 50 μg/ml streptomycin. For immunoprecipitation, 293 cells were transiently transfected by using the FuGENE 6 reagent (Roche Molecular Biochemicals) according to manufacturer protocol. Thirty hours after transfection, the cells were washed twice with PBS and lysed in RIPA buffer (PBS supplemented with 1% Nonidet P-40/0.5% sodium deoxycholate/1 mM PMSF/1 μg/ml aprotinin/1 mM sodium orthovanadate). The cell lysates were harvested and incubated at 4°C for 30 min and cleared by centrifugation at 10,000 × g for 10 min. The supernatant was incubated with anti-FLAG antibody (Sigma) or anti-GFP antibody (Santa Cruz Biotechnology) for 3 h, after which protein G-Sepharose (Amersham Pharmacia) was added and incubated another 1 h. The immunoprecipitates were washed four times with RIPA buffer containing 0.05% SDS and boiled in SDS/PAGE sample buffer. Proteins were resolved by SDS/PAGE and immunoblotted with anti- GFP antibody, anti-FLAG antibody, or anti-MTMR5 (Sbf1) antibody (30).
Protein Identification by Matrix-Assisted Laser Desorption Ionization (MALDI) Mass Spectrometry.
The gel-separated protein of interest was excised and in-gel digested with trypsin as described (31). In-gel digestion was also performed on gel pieces excised from a similar gel mobility region from both the vector control and MTM1 sample. The pools of tryptic peptides from the various samples were analyzed by MALDI/time-of-flight (TOF) mass spectrometry in linear positive mode to generate a peptide mass map by using α-cyano-4-hydroxycinnaminic acid (saturated solution in 50% acetonitrile with 0.1% trifluoroacetic acid) as the UV-absorbing matrix. MALDI/TOF was performed on a Voyager-DE STR TOF instrument (Applied Biosystems) equipped with a nitrogen laser operating at 337 nm. The peptide mass values were used to search a nonredundant database (National Center for Biotechnology Information) by using the software tools ms-fit (http://prospector.ucsf.edu/) and profound (http://prowl.rockefeller.edu/cgi-bin/ProFound). MALDI/post source decay was performed on selected parent ions in reflectron mode.
Protein Expression and Purification.
Recombinant MTM1 and MTMR2 were expressed as C-terminally His-tagged fusion proteins in Escherichia coli BL21 (DE3) Codon Plus cells (Stratagene) and purified by using Ni2+-agarose affinity resin as described (32). Recombinant MTMR5 was expressed as a fusion protein with N-terminal GST in E. coli BL21 (DE3) Codon Plus cells and purified by using glutathione-agarose affinity resin as described (33).
GST Pull-Down Assay.
GST-MTMR5 (10 μg) was added to equimolar His-tagged MTM1 and MTMR2 in reaction buffer (20 mM Tris-Cl, pH 8.0/200 mM NaCl/0.1% Nonidet P-40/1 mM DTT/1 mM PMSF/1% BSA). After a 2-h incubation at 4°C, glutathione-agarose beads were added and incubated for another 1 h at 4°C. Beads were washed four times with TBST (20 mM Tris-Cl, pH 8.0/200 mM NaCl/0.1% Tween 20) and boiled in SDS/PAGE sample buffer. Proteins were resolved by SDS/PAGE and immunoblotted with anti-His antibody (Qiagen, Valencia, CA).
Immunofluorescence Microscopy.
COS-1 cells were seeded on two-chamber slides (21 × 21 mm) at a density of 5 × 104 cells per well. Cells were cultured overnight and transiently transfected by using FuGENE 6 reagent (Roche Molecular Biochemicals). Thirty hours after transfection, the cells were washed twice with PBS and fixed with 4% paraformaldehyde in PBS for 10 min. After being washed with PBS for two times, cells were incubated with methanol for 2 min. For immunostaining, cells were pretreated for 30 min with 5% BSA containing PBS as a blocking agent. Then, cells were incubated with 2.5% BSA in PBS containing anti-FLAG antibody (Sigma) for 1 h and washed with PBS for 10 min. Cells were incubated for 30 min with Cy3-conjugated anti-mouse secondary antibody (Jackson ImmunoResearch) in 2.5% BSA containing PBS. Finally, the cells were washed twice for 10 min with PBS and then mounted by using ProLong Antifade mounting medium (Molecular Probes). Fluorescence analysis was performed by conventional fluorescence microscopy and by confocal microscopy (Zeiss LSM 510).
Results and Discussion
Identification of an MTMR2-Interacting Protein.
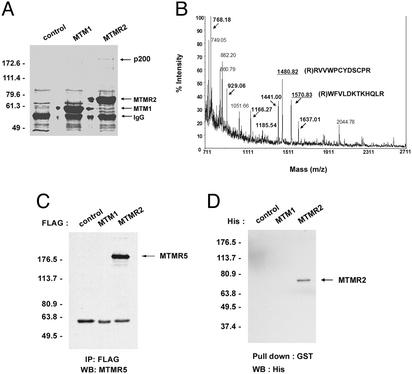
To isolate and identify proteins that interact with MTM1 and/or MTMR2, we transfected 293 cells with either FLAG-tagged MTM1 or MTMR2 mammalian expression vectors. FLAG-tagged fusion proteins were immunoprecipitated and analyzed by SDS/PAGE and Coomassie blue staining. We observed a protein of ≈200 kDa (p200) that associated with MTMR2 but was absent in the vector control and MTM1 samples (Fig. 1A, upper right). To identify this protein, MALDI/TOF mass spectrometry was performed as described in Materials and Methods. Samples corresponding to vector control, MTM1, and MTMR2 gel lanes were individually analyzed by MALDI/TOF. The p200 mass fingerprint is shown in Fig. 1B. Peptide mass values unique to the p200 protein were used to search the National Center for Biotechnology Information nonredundant database by using either ms-fit or profound software tools. Also, MALDI/post source decay was used to obtain partial sequence information on two prominent peptides detected in the MALDI/TOF spectrum. Database searching combined with the MALDI/post source decay data identified MTMR5 (215 kDa) as the 200-kDa protein interacting specifically with MTMR2.
Figure 1.
Identification of an MTMR2-interacting protein. (A) 293 cells were transfected with either FLAG-MTM1 or FLAG-MTMR2 expression vectors. Thirty hours after transfection, cell extracts were subjected to immunoprecipitation by using anti-FLAG antibody. Immunoprecipitated proteins were separated by SDS/PAGE, and the gel was stained with Coomassie blue. The p200 band excised for MALDI/TOF mass spectrometry analysis is marked with an arrow. (B) MALDI/TOF mass spectrometry of the typtic digests of MTMR5. Shown in bold are peaks unique to the p200 band compared with control samples, which correspond to matched peptides to human MTMR5. The peptide masses analyzed by MALDI/post source decay are underlined. (C) 293 cells were transfected with FLAG-MTM1 or FLAG-MTMR2 expression vectors. Thirty hours after transfection, cell extracts were subjected to immunoprecipitation by using anti-FLAG antibody, and endogenous MTMR5 was detected by Western blot analysis by using anti-MTMR5 antibody. (D) Purified GST-tagged bacterial recombinant MTMR5 (10 μg) was incubated with equimolar His-tagged MTM1 or MTMR2 fusion proteins. As a control, equimolar GST was incubated with His-tagged MTMR2. GST or GST-MTMR5 was pulled down by glutathione-agarose beads. Coprecipitated proteins were resolved on SDS/PAGE and detected by Western blot analysis by using anti-His antibody.
To confirm the interaction between MTMR2 and MTMR5, 293 cells were transfected with expression vectors for FLAG-tagged MTM1 or MTMR2. Cells were harvested, and the cell extracts were subjected to immunoprecipitation by using anti-FLAG antibody, followed by Western blot analysis by using anti-MTMR5 antibody to detect endogenous MTMR5. As shown in Fig. 1C, MTMR5 was efficiently immunoprecipitated in the presence of MTMR2. No corresponding protein was observed in the vector control or MTM1 samples, confirming that the interaction was specific for MTMR2. To examine whether the interaction between these two proteins is direct, we examined the binding in vitro. For the GST pull-down assay, GST-tagged MTMR5 and His-tagged MTM1 and MTMR2 fusion proteins were expressed in bacteria and purified as described in Materials and Methods. As shown in Fig. 1D, MTMR2 specifically interacted with MTMR5, whereas no interaction was detected with MTM1. These data demonstrate that MTMR2 and MTMR5 interact both in vivo and in vitro.
MTMR2 Forms Homo-/Heterodimer Through Its Coiled-Coil Domain.
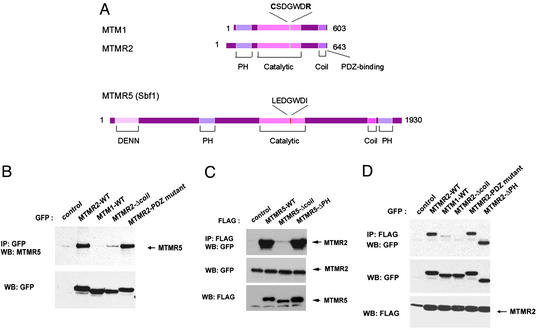
In addition to a phosphatase catalytic domain, MTMR proteins possess several motifs known to mediate protein–protein and/or protein–lipid interactions (Fig. 2A). MTM1 and MTMR2 have coiled-coil domains and PDZ-binding motifs in their C-terminal region that may be involved in protein–protein interactions. MTMR5 also has a coiled-coil domain followed by a PH domain in its C-terminal region (Fig. 2A). To identify the regions mediating the MTMR2/MTMR5 interaction, EGFP-tagged MTMR2 with its coiled-coil domain deleted (MTMR2-Δcoil) and a PDZ-binding motif mutant (MTMR2-PDZ mutant, TVV→AVA) were transiently transfected into 293 cells. MTMR2 was immunoprecipitated by using anti-GFP antibody, and the associated MTMR5 was analyzed by Western blot analysis with anti-MTMR5 antibody. As shown in Fig. 2B Upper, MTMR5 associated with WT MTMR2 and the MTMR2-PDZ mutant but failed to associate with MTMR2-Δcoil. Protein levels were verified by Western blot analysis (Fig. 2B Lower). Inversely, 293 cells were transfected with expression vectors for EGFP-tagged MTMR2 alone, together with FLAG-tagged WT MTMR5, or deletion mutants of MTMR5 as indicated in Fig. 2C. FLAG-MTMR5 proteins were immunoprecipitated by using anti-FLAG antibody and the amount of associated MTMR2 determined by Western blot analysis by using anti-GFP antibody. The MTMR5 C-terminal PH domain deletion mutant (MTMR5-ΔPH) associated with MTMR2 at levels comparable to that of WT MTMR5. However, MTMR5-Δcoil did not associate with MTMR2, suggesting that MTMR2 and MTMR5 interact via their coiled-coil domains.
Figure 2.
MTMR2 forms homo-/heterodimer through a coiled-coil interaction. (A) Structural features of MTM1, MTMR2, and MTMR5. MTM1 and MTMR2 contain a catalytic domain that encompasses the CX5R active site motif of PTP. In addition, MTM proteins possess several other domains/motifs that are likely to facilitate membrane association and protein–protein interactions. MTM1 and MTMR2 contain a PH domain, a coiled-coil domain, and a PDZ-binding motif as indicated. MTMR5 also has a coiled-coil domain followed by a PH domain in its C-terminal region. Domain boundaries were obtained from smart (34) and coil (35). (B) 293 cells were transfected with the indicated expression vectors. Thirty hours after transfection, cell extracts were subjected to immunoprecipitation by using anti-GFP antibody. Coprecipitated endogenous MTMR5 was analyzed by Western blot analysis by using anti-MTMR5 antibody (Upper). The expression level of proteins in the transfected cells was monitored by Western blot analysis (Lower). (C and D) pEGFP-MTMR2 (WT) expression vectors were cotransfected with various FLAG-tagged MTMR5 mutant expression vectors (C), or FLAG-MTMR2 (WT) expression vectors were cotransfected with various EGFP-tagged MTMR2 mutant expression vectors (D) as indicated. Thirty hours after transfection, cell extracts were subjected to immunoprecipitation by using anti-FLAG antibody followed by Western blot analysis by using anti-GFP antibody (Top). The expression level of proteins in the transfected cells was monitored by Western blot analysis (Middle and Bottom).
To further characterize the protein–protein interactions mediated by the coiled-coil domain of MTMR2, ethylene glycol bis-(succinimidylsuccinate) cross-linking experiments were carried out by using bacterial recombinant MTM1 and MTMR2. The cross-linking experiments clearly demonstrate that MTMR2 forms a homodimer whereas MTM1 exists predominantly as a monomer (data not shown). EGFP- or FLAG-tagged MTMR2 variants were also analyzed by coimmunoprecipitation to determine the region important for homodimerization. Overexpressed MTMR2 was immunoprecipitated by using anti-FLAG antibody from each cell lysate, and the associated proteins were analyzed by Western blot analysis with anti-GFP antibody. Consistent with the in vitro ethylene glycol bis-(succinimidylsuccinate) cross-linking study, MTMR2 formed a homodimer (Fig. 2D Top). Furthermore, MTMR2 did not form a heterodimer with MTM1 (Fig. 2D Top). Analogous to the interaction with MTMR5, homodimerization was impaired with MTMR2-Δcoil (Fig. 2D Top). Furthermore, MTMR2-PDZ mutant or MTMR2-ΔPH did not affect MTMR2 dimerization, suggesting homodimerization is mediated by the coiled-coil domain.
The Leucine Heptad Repeat Is Critical for MTMR2 Coiled-Coil Function.
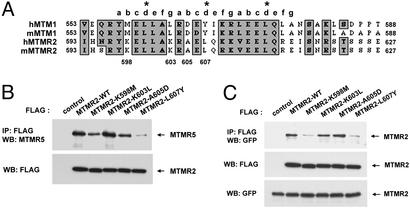
Although MTM1 and MTMR2 both have putative coiled-coil domains and share high sequence similarities, only MTMR2 interacts with MTMR5 and forms a coiled-coil domain-dependent heterodimer. Coiled-coil domains often contain leucine residues or related amino acids spaced every seventh residue. In Fig. 3A, this heptad repeat is denoted with the letters a–g with leucine residues residing at the d position. The MTM1 and MTMR2 coiled-coil domains possess this heptad repeat with some notable differences. Specifically, the second d-position leucine (L607) and several residues (K598, K603, and A605) found in MTMR2 are not conserved in MTM1 (Fig. 3A). Therefore, we created MTMR2 point mutants containing these MTM1 amino acid substitutions and analyzed the ability of these mutants to interact with MTMR5. As shown in Fig. 3B Upper, MTMR2 K603L showed a similar binding affinity to MTMR5 compared with WT MTMR2. The binding affinity of MTMR2 K598M and A605D mutants was diminished. However, the L607Y mutant dramatically impaired the ability of MTMR2 to associate with MTMR5.
Figure 3.
MTMR2 point mutants diminish its homo-/heterodimerization. (A) Sequence alignment of coiled-coil domain of MTM1 and MTMR2. Gray boxes represent conserved amino acids. The heptad repeat is denoted with the letters a–g, with leucine residues residing at the d position. (B and C) 293 cells were transfected with FLAG-tagged MTMR2 point mutant expression vectors alone (B) or combined with EGFP-MTMR2 (WT) expression vectors (C) as indicated. Thirty hours after transfection, cell extracts were subjected to immunoprecipitation by using anti-FLAG antibody followed by Western blot analysis by using anti-MTMR5 (B) or anti-GFP (C) antibody. The expression level of proteins in the transfected cells was monitored by Western blot analysis with anti-FLAG and/or anti-GFP antibody.
When analyzed for their ability to homodimerize, the MTMR2 K603L and A605D mutants did not show any differences compared with WT MTMR2. However, the K598M and L607Y mutants were unable to homodimerize (Fig. 3C Top). The expression level of these mutants and the WT MTMR2 were equivalent (Fig. 3B Lower and 3C Middle and Bottom). These data show that the second leucine repeat residue, L607 of MTMR2, is most critical for both MTMR2 homodimerization and heterodimerization with MTMR5. Furthermore, the K598 and A605 residues are also important for normal coiled-coil function. Interestingly, all MTM family members have a putative coiled-coil domain in their C-terminal regions. Sequences within their coiled-coil domains may drive specificity for protein–protein interactions. Our results raise the possibility that multiple inactive MTM family members may interact with other active MTMs.
MTMR2 Localization Is Regulated Through Its Interaction with MTMR5.
The finding that MTMR2 interacts with MTMR5 raises the possibility that a catalytically inactive MTMR5 may regulate the activity of MTMR2. To examine the effect of MTMR5 on MTMR2 enzymatic activity, equal molar amounts of purified recombinant GST-tagged MTMR5 and His-tagged MTMR2 were mixed and tested for lipid phosphatase activity by using PI(3)P and phosphatidylinositol 3,5-bisphosphate as substrates. MTMR5 consistently increased MTMR2 phosphatase activity 4.6- and 3.4-fold for PI(3)P and phosphatidylinositol 3,5-bisphosphate, respectively (data not shown). Although a 3- to 5-fold change in MTMR2 catalytic activity may seem modest, the effect of MTMR5 on MTMR2 catalytic activity may be more pronounced under conditions that favor a MTMR2/MTMR5 heterointeraction, because we were unable to determine the ratio of MTMR2/MTMR2 homodimer to MTMR2/MTMR5 heterodimer present in our assay.
We next examined the effect of MTMR5 on regulating the subcellular distribution of MTMR2. COS-1 cells were cotransfected with EGFP-tagged MTMR2 expression vectors (WT or Δcoil) and FLAG-tagged MTMR5 expression vectors (WT, ΔPH, or Δcoil). As shown in Fig. 4 A and B, MTMR2 (WT) and MTMR5 (WT) were colocalized in the cytoplasmic region with strong perinuclear staining. In contrast, MTMR2-Δcoil did not colocalize with WT MTMR5, showing a diffuse staining pattern throughout the whole cell, indicating that the coiled-coil domain of MTMR2 is important for its subcellular localization (Fig. 4 C and D). We next examined whether impairing the MTMR5 ability to associate with MTMR2 would affect the subcellular localization of WT MTMR2. MTMR5-ΔPH, a variant that is able to interact with MTMR2, was fully capable of colocalizing with MTMR2 (Fig. 4 E and F). However, MTMR5-Δcoil, a variant that cannot interact with MTMR2, lead to a diffused subcellular distribution of WT MTMR2 (Fig. 4 G and H). A small fraction of MTMR2 still showed the perinuclear staining pattern, presumably because of the presence of endogenous MTMR5. Subcellular localization of MTMR5 was not affected by either the PH domain or the coiled-coil domain deletion. Our data indicate that, through their coiled-coil domain interaction, MTMR5 can regulate the subcellular localization of MTMR2. Although MTM1 could not form a heterodimer with MTMR5, MTM1-Δcoil also showed diffused staining pattern throughout the whole cell, suggesting the coiled-coil domain of MTM1 is also important for its subcellular localization (data not shown). In a previous study, we showed that MTM1 and MTMR2 use different subcellular pools of PI(3)P (11). Here we demonstrate that MTMR2 can be targeted to a specific subcellular localization through a heterointeraction with MTMR5. Therefore, we propose that heterodimerization is a mechanism by which MTMR2 locates its specific substrates.
Figure 4.
MTMR5 regulates the subcellular localization of MTMR2. COS-1 cells were cotransfected with EGFP-MTMR2 (WT or Δ-coil) in combination with FLAG-MTMR5 (WT, Δ-PH, or Δ-coil) expression vectors. Thirty hours after transfection, cells were fixed and immunostained by using anti-FLAG antibody. Green fluorescent staining for EGFP-MTMR2 (WT and Δ-coil) is shown in A, C, E, and G. Red fluorescent staining for Cy3-conjugated anti-mouse secondary antibody bound to the FLAG-MTMR5 (WT, Δ-PH, and Δ-coil) is shown in B, D, F, and H.
Among 13 known MTM family members, 5 are catalytically inactive. A potential role in substrate trapping or docking has been proposed (25, 27), but the exact role of the inactive phosphatase members is still unknown. This study signifies that differential binding partners may be a mechanism to assign different biological roles to MTM1 and MTMR2. Recently, Nandurkar et al. (36) isolated and characterized 3-PAP (MTMR12), another catalytically inactive member of the MTM family. They reported that, although recombinant 3-PAP didn't have enzymatic activity in vitro, immunoprecipitated 3-PAP from human platelets had PI(3)P and phosphatidylinositol 3,4-bisphosphate 3-phosphatase activity, suggesting that 3-PAP is in a complex with an active enzyme in vivo. However, no 3-PAP binding partner was identified in that study.
During the preparation of this manuscript, Zerres and colleagues (37) identified a MTMR5 (sbf1)-related gene, which they refer to as sbf2. Interestingly, mutations in this gene produce another form of Charcot–Marie-Tooth disease, type 4B2, an autosomal recessive disease that displays focally folded myelin sheaths. Sbf2 shares 59% amino acid sequence identity with MTMR5. Mutations in MTMR2 have been shown to be responsible for type 4B1 Charcot–Marie-Tooth disease, which also displays focally folded myelin sheaths (23, 24). How do mutations in a dead phosphatase (sbf2) and in MTMR2 both result in a disease that shares a common phenotype? Our results have shown that MTMR2 associates with MTMR5. This association effects MTMR2 activity as well as the subcellular localization of the active phosphatase. The data described in this paper suggest that MTMR2 may also associate with sbf2, and the absence of an intact sbf2 gene product may result in disease by effecting the activity and/or subcellular localization of MTMR2. It will now be interesting and important to determine whether other active and inactive members of the MTM family interact to achieve their specific biological function.
Acknowledgments
We thank Dr. C. D. Worby and Dr. G. S. Taylor for critical review of this manuscript and Dr. S.-G. Ahn for helpful discussion. We also thank members of the PTP group in Dixon laboratory for valuable discussions. This work was supported in part by a grant from the National Institutes of Health and the Walther Cancer Institute (to J.E.D.).
Abbreviations
- MTM
myotubularin
- PTP
protein-tyrosine phosphatase
- MTMR
MTM-related
- PI(3)P
phosphatidylinositol 3-phosphate
- PH
pleckstrin homology
- EGFP
enhanced GFP
- MALDI
matrix-assisted laser desorption ionization
- TOF
time of flight
References
- 1.Laporte J, Blondeau F, Buj-Bello A, Mandel J-L. Trends Genet. 2001;17:221–228. doi: 10.1016/s0168-9525(01)02245-4. [DOI] [PubMed] [Google Scholar]
- 2.Maehama T, Taylor G S, Dixon J E. Annu Rev Biochem. 2001;70:247–279. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 3.Wishart M J, Taylor G S, Slama J T, Dixon J E. Curr Opin Cell Biol. 2001;13:172–181. doi: 10.1016/s0955-0674(00)00195-2. [DOI] [PubMed] [Google Scholar]
- 4.Denu J M, Dixon J E. Curr Opin Chem Biol. 1998;2:633–641. doi: 10.1016/s1367-5931(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 5.Cui X, De Vivo I, Slany R, Miyamoto A, Firestein R, Cleary M L. Nat Genet. 1998;18:331–337. doi: 10.1038/ng0498-331. [DOI] [PubMed] [Google Scholar]
- 6.Laporte J, Blondeau F, Buj-Bello A, Tentler D, Kretz C, Dahl N, Mandel J-L. Hum Mol Genet. 1998;7:1703–1712. doi: 10.1093/hmg/7.11.1703. [DOI] [PubMed] [Google Scholar]
- 7.Taylor G S, Maehama T, Dixon J E. Proc Natl Acad Sci USA. 2000;97:8910–8915. doi: 10.1073/pnas.160255697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blondeau F, Laporte J, Bodin S, Superti-Furga G, Payrastre B, Mandel J-L. Hum Mol Genet. 2000;9:2223–2229. doi: 10.1093/oxfordjournals.hmg.a018913. [DOI] [PubMed] [Google Scholar]
- 9.Walker D M, Urbe S, Dove S K, Tenza D, Raposo G, Clague M J. Curr Biol. 2001;11:1600–1605. doi: 10.1016/s0960-9822(01)00501-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhao R, Qi Y, Chen J, Zhao Z J. Exp Cell Res. 2001;265:329–338. doi: 10.1006/excr.2001.5185. [DOI] [PubMed] [Google Scholar]
- 11.Kim S-A, Taylor G S, Torgersen K M, Dixon J E. J Biol Chem. 2002;277:4526–4531. doi: 10.1074/jbc.M111087200. [DOI] [PubMed] [Google Scholar]
- 12.Laporte J, Liaubet L, Blondeau F, Tronchere H, Mandel J-L, Payrastre B. Biochem Biophys Res Commun. 2002;291:305–312. doi: 10.1006/bbrc.2002.6445. [DOI] [PubMed] [Google Scholar]
- 13.Berger P, Bonneick S, Willi S, Wymann M, Suter U. Hum Mol Genet. 2002;11:1569–1579. doi: 10.1093/hmg/11.13.1569. [DOI] [PubMed] [Google Scholar]
- 14.Gaullier J M, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H, Aasland R. Nature. 1998;394:432–433. doi: 10.1038/28767. [DOI] [PubMed] [Google Scholar]
- 15.Dowler S, Currie R A, Campbell D G, Deak M, Kular G, Downes C P, Alessi D R. Biochem J. 2000;351:19–31. doi: 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Hortsman H, Seet L, Wong S H, Hong W. Nat Cell Biol. 2001;3:658–666. doi: 10.1038/35083051. [DOI] [PubMed] [Google Scholar]
- 17.Kanai F, Liu H, Field S J, Akbary H, Matsuo T, Brown G E, Cantley L C, Yaffe M B. Nat Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- 18.Laporte J, Hu L J, Kretz C, Mandel J-L, Kioschis P, Coy J F, Klauck S M, Poustka A, Dahl N. Nat Genet. 1996;13:175–182. doi: 10.1038/ng0696-175. [DOI] [PubMed] [Google Scholar]
- 19.Laprote J, Biancalana V, Tanner S M, Kress W, Schneider V, Wallgren-Pettersson C, Herger F, Buj-Bello A, Blondeau F, Liechti-Gallati S, et al. Hum Mutat. 2000;15:393–409. doi: 10.1002/(SICI)1098-1004(200005)15:5<393::AID-HUMU1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Spiro A J, Shy G M, Gonatas N K. Arch Neurol. 1966;14:1–14. doi: 10.1001/archneur.1966.00470070005001. [DOI] [PubMed] [Google Scholar]
- 21.Bolino A, Muglia M, Conforti F L, LeGuern E, Salih M A M, Georgiou D-M, Christodoulou K, Hausmanowa-Petrusewicz I, Mandich P, Schenone A, et al. Nat Genet. 2000;25:17–19. doi: 10.1038/75542. [DOI] [PubMed] [Google Scholar]
- 22.Houlden H, King R H, Wood N W, Thomas P K, Reilly M M. Brain. 2001;124:907–915. doi: 10.1093/brain/124.5.907. [DOI] [PubMed] [Google Scholar]
- 23.Bolino A, Brancolini V, Bono F, Bruni A, Gambardella A, Romeo G, Quattrone A, Devoto M. Hum Mol Genet. 1996;5:1051–1054. doi: 10.1093/hmg/5.7.1051. [DOI] [PubMed] [Google Scholar]
- 24.Gambardella A, Muglia M, Quattrone A. Brain. 1997;120:2113–2115. doi: 10.1093/brain/120.11.2113. [DOI] [PubMed] [Google Scholar]
- 25.Flint A J, Tiganis T, Barford D, Tonks N K. Proc Natl Acad Sci USA. 1997;94:1680–1685. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter T. Nat Genet. 1998;18:303–305. doi: 10.1038/ng0498-303. [DOI] [PubMed] [Google Scholar]
- 27.Wishart M J, Dixon J E. Trends Biochem Sci. 1998;23:301–306. doi: 10.1016/s0968-0004(98)01241-9. [DOI] [PubMed] [Google Scholar]
- 28.Firestein R, Nagy P L, Daly M, Huie P, Conti M, Cleary M L. J Clin Invest. 2002;109:1165–1172. doi: 10.1172/JCI12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michael S F. BioTechniques. 1994;16:410–412. [PubMed] [Google Scholar]
- 30.Firestein R, Cleary M L. J Cell Sci. 2001;114:2921–2927. doi: 10.1242/jcs.114.16.2921. [DOI] [PubMed] [Google Scholar]
- 31.Vacratsis P O, Phinney B S, Gage D A, Gallo K A. Biochemistry. 2002;41:5613–5624. doi: 10.1021/bi016075c. [DOI] [PubMed] [Google Scholar]
- 32.Maehama T, Taylor G S, Slama J T, Dixon J E. Anal Biochem. 2000;279:248–250. doi: 10.1006/abio.2000.4497. [DOI] [PubMed] [Google Scholar]
- 33.Taylor G S, Liu Y, Baskerville C, Charbonneau H. J Biol Chem. 1997;272:24054–24063. doi: 10.1074/jbc.272.38.24054. [DOI] [PubMed] [Google Scholar]
- 34.Schultz J, Milpetz F, Bork P, Ponting C P. Proc Natl Acad Sci USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lupas A, Van Dyke M, Stock J. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 36.Nandurkar H H, Caldwell K K, Whisstock J C, Layton M J, Gaudet E A, Norris F A, Majerus P W, Mitchell C A. Proc Natl Acad Sci USA. 2001;98:9499–9504. doi: 10.1073/pnas.171306098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Senderek J, Bergmann C, Weber S, Ketelsen U-P, Schorle H, Rudnik-Schöneborn S, Büttner R, Buchheim E, Zerres K. Hum Mol Genet. 2003;12:349–356. doi: 10.1093/hmg/ddg030. [DOI] [PubMed] [Google Scholar]