Abstract
Drug exporters contribute to the intrinsic drug resistance in many organisms. Although there are at least 20 exporter genes in Escherichia coli, most of them apparently do not confer drug resistance in complex laboratory media except for the AcrAB, EmrE, and MdfA efflux systems. In this study, we comprehensively investigated the growth phase-dependent expression of drug exporter genes. The expression of acrAB, emrAB, emrD, emrE, emrKY, mdfA, and ydgFE is stable at moderate levels during any growth phase, whereas mdtEF promoter activity greatly increased with cell growth and reached the maximum level at the late stationary phase. The growth phase-dependent increase in mdtEF expression was also observed on quantitative reverse transcription-PCR analysis. As expected from the transporter expression, the stationary-phase cells actually showed MdtEF-dependent tolerance to drugs and toxic dyes. Growth phase-dependent elevation of mdtEF expression was found to be mediated by the stationary-phase σ factor rpoS and the RpoS-dependent signaling pathway, Hfq, GadY, and GadX. The induction level was decreased by tnaAB deletion, suggesting that indole sensing stimulates this process.
Bacterial intrinsic tolerance to a wide range of antimicrobial agents is often caused by active efflux systems, such as AcrAB in Escherichia coli and MexAB in Pseudomonas aeruginosa (17, 18, 23), and multidrug-resistant mutants due to the overexpression of efflux pumps have been isolated in clinical settings (15). Our previous studies revealed that there are at least 20 drug exporter genes in E. coli that confer drug resistance when they are overexpressed (19); however, the previous study showed that most of them do not contribute to drug tolerance in complex laboratory media, probably due to their low expression levels, except for acrAB, emrE, and mdfA (29). In that study, drug resistance was determined as MICs, which would not reflect the actual expression levels of drug exporters at different growth phases.
In E. coli, drug exporter gene expression is affected by various environmental stresses. For instance, the acrAB gene is known to be induced by ethanol, osmotic shock, oxidizing agents (11), and bile salts and fatty acids (24). Throughout bacterial growth, the bacterial cell density, nutrient conditions, pH, and other factors are changing. Therefore, it would be important to study the growth phase dependency of the expression of drug exporters that may facilitate understanding of drug exporter-mediated multidrug resistance at actual infection sites.
The growth phase-dependent expression of various genes in E. coli has been reported. For instance, quorum-sensing signal molecule autoinducer 2, which is produced and secreted into the culture medium at the logarithmic phase, influences the expression of type III secretion system-related genes (27) and motility-related genes (28). The σ factor, RpoS, and RpoS-dependent genes are known to be induced at the stationary phase (7). However, there is little information available on growth phase-dependent expression of drug exporter genes.
In this study, we comprehensively investigated the expression of the 20 drug exporter genes at different growth phases and their contribution to growth phase-dependent drug tolerance. We found that out of the 20 drug exporter genes in E. coli, the expression levels of the emrA, emrD, emrK, and ydgF genes are relatively stable at moderate levels without a significant change throughout the bacterial growth phase as well as those of the acrA, emrE, and mdfA genes. In contrast, mdtEF gene expression was significantly increased at the late cell growth phase, followed by mdtEF-dependent drug tolerance. A possible regulation scheme is discussed.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are presented in Table 1. The construction of gene deletion mutants of E. coli MC4100 (2) was performed by the gene replacement method previously described, using the pKO3 plasmid (9). E. coli cells were cultured in Luria-Bertani (LB) medium, supplemented with appropriate antibiotics when necessary.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli strains | ||
| MG1655 | Wild type, chromosomal DNA used for PCR amplification | 1 |
| MC4100 | F−araD139Δ(argF-lac)U169 rpsL150(Strr) relA1 flbB5301 deoC1 ptsF25 rbsR | 2 |
| MC4100ΔacrB | Derivative of MC4100 that lacks acrB | 5 |
| MC4100ΔacrBΔmdtEF | Derivative of MC4100ΔacrB that lacks mdtEF | 5 |
| MC4100ΔevgSA | Derivative of MC4100 that lacks evgSA | 5 |
| MC4100ΔgadX | Derivative of MC4100 that lacks gadX | 5 |
| MC4100Δhfq | Derivative of MC4100 that lacks hfq | This study |
| MC4100ΔmdtEF | Derivative of MC4100 that lacks mdtEF | This study |
| MC4100ΔrpoS | Derivative of MC4100 that lacks rpoS | This study |
| MC4100ΔtnaAB | Derivative of MC4100 that lacks tnaAB | 5 |
| Plasmids | ||
| pNN387 | Single-copy vector, Cpr,a NotI-HindIII cloning site upstream of promoterless lacZ | 3 |
| pNNacrA | pNN387 (acrAB gene promoter-lacZ) | This study |
| pNNacrD | pNN387 (acrD gene promoter-lacZ) | 5 |
| pNNacrE | pNN387 (acrEF gene promoter-lacZ) | This study |
| pNNbcr | pNN387 (bcr gene promoter-lacZ) | This study |
| pNNcusC | pNN387 (cusCFBA gene promoter-lacZ) | This study |
| pNNemrA | pNN387 (emrRAB gene promoter-lacZ) | This study |
| pNNemrD | pNN387 (emrD gene promoter-lacZ) | This study |
| pNNemrE | pNN387 (emrE gene promoter-lacZ) | This study |
| pNNemrK | pNN387 (emrKY gene promoter-lacZ) | 5 |
| pNNfsr | pNN387 (fsr gene promoter-lacZ) | This study |
| pNNgadE | pNN387 (gadE mdtEF gene promoter-lacZ) | 5 |
| pNNgadX | pNN387 (gadX gene promoter-lacZ) | This study |
| pNNgadY | pNN387 (gadY gene promoter-lacZ) | This study |
| pNNhfq | pNN387 (hfq gene promoter-lacZ) | This study |
| pNNmacA | pNN387 (macAB gene promoter-lacZ) | This study |
| pNNmdfA | pNN387 (mdfA gene promoter-lacZ) | This study |
| pNNmdtA | pNN387 (mdtABC gene promoter-lacZ) | 5 |
| pNNrpoS | pNN387 (rpoS gene promoter-lacZ) | This study |
| pNNyceE | pNN387 (yceE gene promoter-lacZ) | This study |
| pNNyceL | pNN387 (yceL gene promoter-lacZ) | This study |
| pNNydgF | pNN387 (ydgFE gene promoter-lacZ) | This study |
| pNNydhE | pNN387 (ydhE gene promoter-lacZ) | This study |
| pNNyidY | pNN387 (yidY gene promoter-lacZ) | This study |
| pNNyjiO | pNN387 (yjiO gene promoter-lacZ) | This study |
Cpr is a chloramphenicol resistance marker.
Construction of reporter plasmids.
The reporter plasmids were constructed as follows. DNA fragments including the putative promoter region were amplified by PCR using the primers listed in Table 2. Chromosomal DNA of E. coli MG1655 (1) was used as a PCR template. The DNA fragments were cloned in front of the lacZ reporter gene in a single-copy pNN387 vector, which carries chloramphenicol resistance as a marker (3). Since the emrAB, cusBA, and mdtEF genes are transcribed in the emrRAB (10), cusCFBA (4), and gadE-mdtEF (14; our unpublished data) operons, respectively, emrR, cusC, and gadE promoter-fused lacZ are reporters for the respective exporter expression. The resulting plasmids were introduced into host cells for β-galactosidase activity measurements.
TABLE 2.
Oligonucleotides used for plasmid construction
| Oligonucleotide | Oligonucleotide sequence (5′ to 3′) | Length (bp) |
|---|---|---|
| acrA_primerPF | CGCGCGGCCGCAGAGTGGATCGCCAGGGAA | 400 |
| acrA_primerPR | CGCAAGCTTATGTAAACCTCGAGTGTCCGA | |
| acrD_primerPF | GCGGCGGCCGCAACGCGCGGAACGGCTAGG | 276 |
| acrD_primerPR | GCGAAGCTTTAAAAGAGGACCTCGTGTTTC | |
| acrE_primerPF | CGCGCGGCCGCGATTAATTATTCAGGAAAT | 400 |
| acrE_primerPR | CGCAAGCTTTACTATTCCTCAAAAAACCAA | |
| bcr_primerPF | CGCGCGGCCGCGGTGCTGATGACTGATGAT | 400 |
| bcr_primerPR | CGCAAGCTTAACGGGCTCCTGAAAGTCATT | |
| cusC_primerPF | CGCGCGGCCGCCGGCAACCTGAAACTGACT | 550 |
| cusC_primerPR | CGCAAGCTTAGGCTCATAATTTCTGGTGAT | |
| emrD_primerPF | CGCGCGGCCGCTTCTATAATATCACTGTAC | 600 |
| emrD_primerPR | CGCAAGCTTTATCACGGATGCTTTTATAAA | |
| emrE_primerPF | CGCGCGGCCGCGCTGAAAGTGGAATGTATC | 450 |
| emrE_primerPR | CGCAAGCTTAGCATATTCTTTCCTGTTCAA | |
| emrK_primerPF | CGCGCGGCCGCTCCCTTTGCAATGAAGCAT | 408 |
| emrK_primerPR | CGCAAGCTTTATTATCTCTCATTTCTCATA | |
| emrR_primerPF | CGCGCGGCCGCGTTACTAGTTGGCGTGGCG | 400 |
| emrR_primerPR | CGCAAGCTTTTGGGTATGACCTCATTAATT | |
| fsr_primerPF | CGCGCGGCCGCCGTTTTTTGCGCCGCCAGA | 400 |
| fsr_primerPR | CGCAAGCTTAGGAAAGTCACTTTTTCAGGG | |
| gadE_primerPF | CGCGCGGCCGCTTACCCCGGTTGTCACCCG | 798 |
| gadE_primerPR | CGCAAGCTTAACTTGCTCCTTAGCCGTTAT | |
| gadX_primerPF | GCGGCGGCCGCATTGCCCAGCAGAACAGC | 403 |
| gadX_primerPR | GCGAAGCTTTAGTTGACTTAATATTACATA | |
| gadY_primerPF | GCGCGGCCGCGATTATCCCTTATATTTCATAC | 1,389 |
| gadY_primerPR | GCAAGCTTAAAAACCCGGCATAGGGGAC | |
| hfq_primerPF | GCGGCGGCCGCCGCATTGAGCAGCGTTTTC | 400 |
| hfq_primerPR | GCGAAGCTTTCTCTCTTTTCCTTATATGC | |
| macA_primerPF | CGCGCGGCCGCCTCCAGCAATTGCTTACGG | 450 |
| macA_primerPR | CGCAAGCTTAAATTTTCTGAATACTCCA | |
| mdfA_primerPF | CGCGCGGCCGCGTCCCATCGTCCAGGACAC | 550 |
| mdfA_primerPR | CGCAAGCTTGCAATTTCTTCGCCAATAATAATCGCGC | |
| mdtA_primerPF | GCGGCGGCCGCAGCTTATGACTAAGAGCAC | 321 |
| mdtA_primerPR | GCGAAGCTTCGTTAAGAGTTTCTCTTCCTG | |
| rpoS_primerPF | GCGGCGGCCGCGATACCGTGGAGGCCGTG | 1,395 |
| rpoS_primerPR | GCGAAGCTTAAGGTGGCTCCTACCCGTG | |
| yceE_primerPF | CGCGCGGCCGCCACCGGATCATGATTACGG | 500 |
| yceE_primerPR | CGCAAGCTTAGCAATCCGCTGTTGGTGCGC | |
| yceL_primerPF | CGCGCGGCCGCATAACAGTGGCTTTCGTCG | 400 |
| yceL_primerPR | CGCAAGCTTTTCCCCTCCCGGGAAATAAAA | |
| ydgF_primerPF | CGCGCGGCCGCGCTTGTCCCCGTTTTTTCT | 550 |
| ydgF_primerPR | CGCGGATCCATATATACGACAGAGAAATCA | |
| ydhE_primerPF | CGCGCGGCCGCGTCAAAACTGACATGGTTG | 400 |
| ydhE_primerPR | CGCAAGCTTGTGAACACCTTTTATTTGTAG | |
| yidY_primerPF | CGCGCGGCCGCTGCTGTTAACCTTCCTGCC | 400 |
| yidY_primerPR | CGCAAGCTTGGGCTAAAGCGTCCTGATAGT | |
| yjiO_primerPF | CGCGCGGCCGCTGCTGGTCGTGAAGTTTGT | 500 |
| yjiO_primerPR | CGCAAGCTTAACAAACAACTCCTTGTCCGG |
Reporter gene assay.
To investigate the growth phase-dependent transcription of various reporter constructs, each bacterial strain was grown at 37°C in LB broth containing 15 mg/liter chloramphenicol until the optical density at 600 nm (OD600) reached 0.4 (early logarithmic phase), 0.8 (late logarithmic phase), 3.0 (early stationary phase), or 6.5 (late stationary phase). When we determined the effect of indole on the transcription of mdtEF, cells were grown at 37°C in LB broth until the OD600 reached 0.8 with 15 mg/liter chloramphenicol and 1 mM indole or only with 15 mg/liter chloramphenicol and the solvent (dimethyl sulfoxide) as a control. β-Galactosidase activity in cell lysates was assayed using o-nitrophenyl-β-d-galactopyranoside (ONPG) as a substrate as described by Miller (16), with a slight modification.
Quantitative real-time RT-PCR.
Quantitative real-time reverse transcription-PCR (RT-PCR) was performed as follows. Cells were grown at 37°C in LB broth until the absorbance at 600 nm reached 0.8 (logarithmic phase), 3.0 (early stationary phase), or 6.0 (late stationary phase). The purification of total RNAs and the synthesis of cDNAs were performed by the methods described previously (6). The specific primer pairs are listed in Table 3. Real-time PCR was performed with each specific primer pair, using SYBR green PCR master mix (PE Applied Biosystems). The E. coli rrsA gene was chosen as a control for normalization of the cDNA loading in each PCR. The reactions were performed with an ABI PRISM 7000 sequence detection system (PE Applied Biosystems).
TABLE 3.
Oligonucleotides used for quantitative real-time PCR
| Gene | Sequence of primer (5′ to 3′)
|
|
|---|---|---|
| Forward | Reverse | |
| gadX | TTTATACCGCTGCTTCTGAACGT | GTGTCCACTCATGGGCGATATTA |
| gadY | GCAAGCTTAAAAACCCGGCATAGGGGAC | AGAGCACAAAGTTTCCCGTG |
| hfq | CAAGCACGCGATTTCTACTGTT | CACCGGCGTTGTTACTGTGA |
| mdtE | CCCCCGGTTCGGTCAA | GGACGTATCTCGGCAACTTCAT |
| rpoS | TCGCAGGGAGCCACACA | TGAATAACCAATCTCACCAAGGTAAA |
| rrsA | CGGTGGAGCATGTGGTTTAA | GAAAACTTCCGTGGATGTCAAGA |
Drug tolerance assay.
Each bacterial strain, grown at 37°C in LB broth until OD600 reached 0.8 (logarithmic phase) or 6.5 (stationary phase), was diluted to an OD600 of 0.1. Then, growth was measured in the presence or absence of crystal violet or kanamycin.
Survival assay.
Each bacterial strain was grown at 37°C in LB broth until the OD600 reached 0.6 (logarithmic phase) or 6.5 (stationary phase). The stationary-phase cells were diluted to an OD600 of 0.6 with fresh medium, and then crystal violet was added to each bacterial cell culture (final concentration, 50 or 200 mg/liter). After incubation for 30 min at 37°C, aliquots of the cell cultures were spread on YT agar plates. After overnight incubation, the numbers of colonies were determined and percent survival was calculated in comparison with that of untreated cells.
Indole production assay.
The extracellular indole concentration was determined by high-performance liquid chromatography (HPLC). The E. coli strain was cultured at 37°C and then pelleted by centrifugation at 20,000 × g. The resulting supernatant was extracted twice with ethyl acetate. The ethyl acetate phase was loaded onto a Symmetry C18 column (5 μm, 4.6 by 150 mm; Waters Corp.) attached to an L2130 HPLC system (HITACHI). The loaded samples were eluted with acetonitrile-H2O (1:1) at the flow rate of 0.8 ml/min. Then the indole peak was detected relative to the absorbance at the wavelength of 276 nm and was identified by the corresponding peak of the purified indole (Sigma). The indole concentration was calculated from the ratio of the detection peak area to the standard peak one.
RESULTS
The growth phase-dependent expression of the intrinsic drug exporter genes in Escherichia coli.
In order to profile the growth phase-dependent expression levels of intrinsic drug exporter genes, we examined their reporter enzyme activities at different growth phases using single-copy plasmids containing lacZ-fused gene promoters. Because the emrAB, cusBA, and mdtEF genes are transcribed as a part of the operons of emrRAB (10), cusCFBA (4), and gadE-mdtEF (14 and our unpublished data), respectively, emrR, cusC, and gadE promoter-fused lacZ constructs were used as reporters for these three genes. Each bacterial strain was grown until the OD600 reached 0.4 (early logarithmic phase), 0.8 (late logarithmic phase), 3.0 (early stationary phase), or 6.5 (late stationary phase), and then β-galactosidase activities were measured. Among the 20 exporter genes, only expression of the mdtE gene greatly increased with growth cessation (Fig. 1). Compared with the late logarithmic phase, 14- and 41-fold increases in the mdtE expression were detected at the early and late stationary phases, respectively. No other exporter genes showed such increases in expression at the stationary phase.
FIG. 1.
Expression of the 20 drug exporter genes at different growth phases. The expression of the drug exporter genes was determined by the β-galactosidase reporter enzyme assay. Single-copy reporter plasmids were transformed into E. coli strain MC4100. E. coli cells were cultured until the OD600 reached 0.4 (early logarithmic phase), 0.8 (late logarithmic phase), 3.0 (early stationary phase), or 6.5 (late stationary phase), and then β-galactosidase activity was measured. pNN387 indicates the vector control.
At the logarithmic phase, the gene that showed the highest expression level was ydgF (109 Miller units); however, YdgF confers low-level drug resistance only to deoxycholate and sodium dodecyl sulfate (SDS), even when overproduced from a multicopy plasmid (19). The ydgF-knockout strain did not show hypersensitivity to drugs, including deoxycholate and SDS. The emrE, mdfA, and acrA genes showed relatively high expression levels at the logarithmic phase, next to that of ydgF. The deletion of these genes is known to increase drug susceptibility (29). The expression level of mdtE at the logarithmic phase was lower than those of emrE, mdfA, and acrA. In addition, RND (resistance nodulation cell division)-type multidrug exporter genes acrE, acrD, and mdtABC, which cause high multidrug resistance when they are overexpressed (19), showed very low expression levels throughout the cell growth. Therefore, it seems that the drug tolerance of E. coli cells under laboratory conditions mainly reflects the expression levels of these drug exporters at the logarithmic phase except for ydgF.
On the other hand, at the late stationary phase, the expression level of mdtE (260 Miller units) was the highest out of those of the 20 drug exporter genes. The second highest was that of emrE, but the level of its activity (50 Miller units) was far lower than that of mdtE. Such a growth phase-dependent increase in mdtE gene expression was also confirmed by determination of transcripts by quantitative RT-PCR analysis, although the maximum level was observed at the early stationary phase with respect to the mRNA level. The mdtE gene transcripts showed 380- and 76-fold increases at the early and late stationary phases, respectively, compared to the logarithmic phase. This indicates that the promoter activity of mdtEF is highest at the early logarithmic phase. Although the reporter enzyme was accumulated during the stationary phase, the amount of mRNA of mdtEF was gradually decreased, probably due to its high turnover rate. In summary, MdtEF is greatly induced at the stationary phase and contributes the intrinsic drug tolerance.
Drug tolerance mediated by up-regulation of mdtEF at the stationary phase.
In order to determine whether the growth phase-dependent induction of mdtEF contributes to drug tolerance, cell growth was compared in the presence of drugs after the stationary phase and in logarithmic-phase cells being diluted to the same density with fresh medium. If cells at two different phases have different susceptibilities to the drug, the growth rate must reflect their initial viability. At first, the growth rate was compared in the ΔacrB background because the high-performance housekeeping drug exporter AcrAB may mask the contribution of MdtEF. MC4100ΔacrB and MC4100ΔacrBΔmdtEF cells were first grown to the logarithmic phase (OD600 of 0.8) or to the stationary phase (OD600 of 6.5) in the absence of drugs, and then the cells were diluted to the same density with fresh medium. The growth was monitored in the absence or presence of several drugs, dyes, detergents, and antiseptics. Our previous studies revealed that MdtEF confers resistance to erythromycin, doxorubicin, crystal violet, ethidium bromide, rhodamine 6G, tetraphenylphosphonium bromide (TPP), benzalkonium, SDS, and deoxycholate when overexpressed (19). We used these compounds for the drug tolerance assay. In addition to these compounds, kanamycin, nalidixic acid, and norfloxacin, which are not substrates of MdtEF, were used as negative controls (19). In Fig. 2A and B, the growth curves in the presence or absence of crystal violet and kanamycin are shown as examples for MdtEF substrates and negative controls, respectively. All logarithmic-phase and stationary-phase cells grew at about the same rate in the absence of drugs, while the growth of logarithmic-phase cells was greatly retarded with 1.56 mg/liter crystal violet or 6.25 mg/ liter kanamycin (Fig. 2A and B). Although the growth of MC4100ΔacrBΔmdtEF stationary-phase cells was also greatly retarded in the presence of crystal violet, the growth of MC4100ΔacrB stationary-phase cells was significantly recovered in the presence of crystal violet (Fig. 2A). Because MC4100ΔacrB stationary-phase cells did not exhibit growth recovery in the presence of kanamycin (Fig. 2B), which is not a substrate of MdtEF, the drug tolerance of the stationary-phase cells to crystal violet was certainly due to MdtEF in the ΔacrB background. Similarly, we observed mdtEF-dependent drug tolerance of the stationary-phase cells to erythromycin, doxorubicin, rhodamine 6G, ethidium bromide, TPP, benzalkonium, SDS, and deoxycholate, but not to nalidixic acid or norfloxacin (data not shown).
FIG. 2.
Growth phase dependence of drug tolerance. Each strain (MC4100ΔmdtEF, MC4100ΔacrB, and MC4100ΔacrBΔmdtEF) was grown until the OD600 reached 0.8 or 6.5 and then diluted to an OD600 of 0.1 with fresh medium. Cell growth was monitored in the absence or presence of drugs. (A) MC4100ΔacrB and MC4100ΔacrBΔmdtEF with or without crystal violet. (B) MC4100ΔacrB and MC4100ΔacrBΔmdtEF with or without kanamycin. (C) MC4100 and MC4100ΔmdtEF with or without crystal violet. (D) MC4100 and MC4100ΔmdtEF with or without kanamycin.
Then, in order to determine whether MdtEF contributes to drug tolerance even in the presence of the acrB gene, we measured the growth of wild-type MC4100 and MC4100ΔmdtEF. In the presence of 1.56 mg/liter crystal violet, both logarithmic- and stationary-phase cells showed full growth independent of mdtEF. However, in the presence of high concentration (12.5 mg/liter) of crystal violet, an increase in the mdtEF-dependent drug tolerance of the stationary-phase cells of the wild-type strain was observed (Fig. 2C), although the degree of the relative increase in drug tolerance (MC4100/MC4100ΔmdtEF) was lower than that in the ΔacrB background. In contrast, wild-type stationary-phase cells did not exhibit tolerance to kanamycin (Fig. 2D). These observations indicate that MdtEF actually contributes to the multidrug tolerance of E. coli at the stationary phase.
Subsequently, in order to confirm the increase in drug tolerance at the stationary phase, the viability of the cells was measured after short exposure to bactericidal compounds. MC4100ΔacrB and MC4100ΔacrBΔmdtEF cells were first grown to the logarithmic phase (OD600 of 0.6) or to the stationary phase (OD600 of 6.5). Then the stationary-phase cells were diluted to the same density as the logarithmic-phase cells (OD600 of 0.6) with fresh medium. Both types of cells were exposed to 50 mg/liter crystal violet. After incubation for 30 min at 37°C, the survival rate was calculated as described in Materials and Methods (Fig. 3A). The logarithmic-phase cells of MC4100ΔacrB and MC4100ΔacrBΔmdtEF showed very low survival rates (0.7% and 1.6%, respectively). On the other hand, the MC4100ΔacrB stationary-phase cells showed very high viability (101%), whereas MC4100ΔacrBΔmdtEF cells still exhibited low viability (2.2%) at the stationary phase. In the acrB+ background, both logarithmic- and stationary-phase cells were fully viable in the presence of 50 mg/liter of crystal violet independent on the presence or absence of mdtEF. However, in the presence of 200 mg/liter of crystal violet, the viability of the stationary-phase cells (57%) was significantly higher than that of the logarithmic-phase cells (4%) and the viability of the stationary-phase ΔmdtEF cells (25%) was significantly lower than the wild-type cells. These results indicated that the induction of mdtEF gene expression at the stationary phase contributes to the drug tolerance, while in the high drug concentration, the drug resistance mechanisms other than MdtEF also partly contribute to the stationary-phase drug tolerance.
FIG. 3.
Growth phase-dependent increase in cell viability with the bactericidal drugs. (A) Each bacterial strain (MC4100ΔacrB and MC4100ΔacrBΔmdtEF) was grown at 37°C in LB broth until the OD600 reached 0.6 (logarithmic phase) or 6.5 (stationary phase). The stationary-phase cells were diluted to an OD600 of 0.6. Then crystal violet was added to each bacterial cell culture (final concentration, 50 mg/liter). After incubation for 30 min at 37°C, viability was measured as described in Materials and Methods. (B) Each bacterial strain (MC4100 and MC4100ΔmdtEF) was grown at 37°C in LB broth until an OD600 of 0.6 (logarithmic phase) or 6.5 (stationary phase). Then the bacterial cell viability with medium containing crystal violet (200 mg/liter) was measured as described above.
The effect of the evgSA and tnaAB gene deletions on the growth phase-dependent induction of mdtEF gene expression.
Previously, we found that the EvgSA two-component system positively controls mdtEF expression (20). In order to determine whether the growth phase-dependent mdtEF induction is controlled by the EvgSA system, the expression level of mdtEF was measured in the ΔevgSA background. It was found that the level of induction of mdtEF at the stationary phase was not affected by evgSA deletion (Fig. 4A), indicating that the growth phase-dependent regulation of mdtEF was not mediated by the EvgSA system.
FIG. 4.
Effects of deletion of mdtEF regulatory genes on the expression of the gadE-mdtEF genes. The expression of gadE-mdtEF in the wild type (MC4100) or each gene deletion mutant (MC4100ΔevgSA, MC4100ΔtnaAB, MC4100ΔgadX, MC4100Δhfq, and MC4100ΔrpoS) was determined by means of the β-galactosidase reporter enzyme assay. E. coli cells cultured for 2, 3, 4, 12, and 24 h were collected, followed by β-galactosidase activity measurement. (A) Effect of evgSA deletion. (B) Effect of tnaAB deletion and addition of indole (500 μM). (C) Effect of deletion of the gadX, hfq, and rpoS genes. At least three independent experiments were performed in each case.
We also previously reported that indole, which is a toxic metabolite synthesized from tryptophan by a tryptophanase, TnaA (26), up-regulates mdtEF expression (5). The extracellular indole concentration in wild-type cells increased with cell growth and reached about 500 μM after 24 h of culture, whereas there was no detectable level of indole in the culture medium of ΔtnaAB cells even after 24 h of culture (Fig. 5). In order to determine the contribution of indole to the expression level of mdtEF, we measured the growth phase-dependent mdtEF induction in the ΔtnaAB background. The tnaAB deletion significantly decreased the level of induction of mdtEF at the stationary phase to about 65% of the wild-type level (Fig. 4B). When 500 μM indole was added in the medium, the expression level of mdtEF in the ΔtnaAB strain was restored to almost the same level as the stationary-phase tnaAB+ strain (Fig. 4B). Thus, indole plays some role in the growth phase-dependent induction of mdtEF.
FIG. 5.
Indole accumulation with cell growth. The extracellular indole concentrations of the wild-type and ΔtnaAB strains were measured by HPLC analysis as described in Materials and Methods. Black squares, MC4100 (wild type); white squares, MC4100ΔtnaAB.
The effect of the gadX, hfq, and rpoS genes on growth phase-dependent induction of mdtEF gene expression.
Since indole induces the expression of mdtEF via transcriptional regulator GadX (5), we examined whether the growth phase-dependent induction of mdtEF is mediated by gadX. In the gadX deletion mutant (MC4100ΔgadX), growth phase-dependent induction of mdtEF was completely abolished (Fig. 4C). Recently, a small-RNA regulator, GadY, which binds to Hfq protein, was found to be induced at the stationary phase in a sigma factor RpoS-dependent manner (22). The overexpression of GadY enhances the mRNA level of gadX (22). Therefore, we then deleted the hfq and rpoS genes. In the resultant strains, the growth phase-dependent induction of mdtEF was completely abolished, like on the gadX deletion. Thus, the growth phase-dependent mdtEF induction is mediated by the RpoS-GadY(Hfq)-GadX signaling pathway.
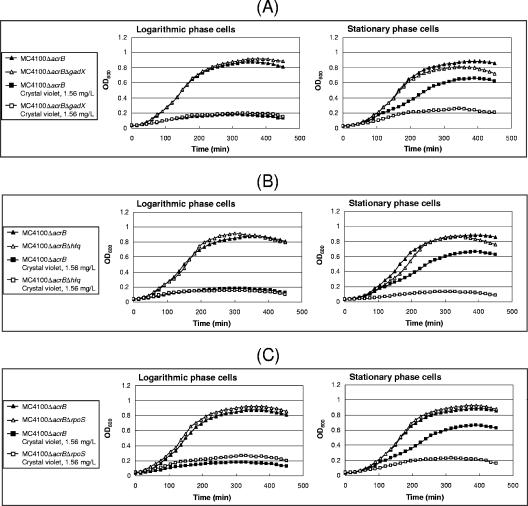
Then we investigated the effect of rpoS, hfq, and gadX deletion on drug tolerance of E. coli. As shown in Fig. 6, the deletion of these genes greatly reduced drug tolerance of the stationary-phase cells to crystal violet in the ΔacrB background. The deletion of these genes did not affect the crystal violet sensitivity of the logarithmic-phase cells.
FIG. 6.
Effect of deletion of the RpoS-dependent signaling pathway on drug tolerance of the stationary-phase cells. Each strain (MC4100, MC4100ΔacrB, MC4100ΔacrBΔgadX, MC4100ΔacrBΔhfq, and MC4100ΔacrBΔrpoS) was grown until an OD600 of 0.8 or 6.5 and then diluted to an OD600 of 0.1 with fresh medium. Cell growth was monitored in the absence or presence of crystal violet. (A) MC4100ΔacrB and MC4100ΔacrBΔgadX. (B) MC4100ΔacrB and MC4100ΔacrBΔhfq. (C) MC4100ΔacrB and MC4100ΔacrBΔrpoS.
Growth phase-dependent expression of rpoS, hfq, and gadX.
In order to characterize the RpoS-GadY(Hfq)-GadX signaling pathway, we measured the growth phase-dependent changes in the expression of the rpoS, gadY, hfq, and gadX genes by means of the β-galactosidase reporter assay. As shown in Fig. 7A, the expression of rpoS, gadY, and gadX showed growth phase dependence and the maximum expression was observed at the late stationary phase, except in the case of gadX, of which the expression levels at the early and late stationary phases were almost equal to each other within experimental error. On the other hand, the expression level of hfq did not show such significant growth phase dependence. Compared with the expression levels at the early logarithmic phase (OD600 of 0.4), the expression levels of rpoS, gadY, and gadX at the late stationary phase were increased by factors of 8.8, 2.5, and 3.6, respectively. That the degree of increase in the expression levels of regulator genes was relatively lower than that of mdtEF (41-fold) may be due to the fact that GadX acts as a dimer in transcriptional regulation. In summary, the growth phase-dependent control of mdtEF was mediated through modification of the amounts of RpoS-dependent small-RNA GadY and transcriptional regulator GadX. One exception is that, although rpoS, gadY, and gadX expression was increased from early to late log phase (Fig. 7A), the mdtEF expression was slightly decreased during this period (Fig. 1). The slight decrease of mdtE expression might be within the experimental deviation; however, there may be a possibility of the postexponential activation of RpoS.
FIG. 7.
Expression levels of rpoS, hfq, gadY, and gadX and effect of their deletion on induction of mdtEF genes by indole. (A) The growth phase-dependent expression of the rpoS, hfq, gadY, and gadX genes was determined by means of the β-galactosidase reporter enzyme assay. E. coli cells were cultured until the OD600 reached 0.4 (early logarithmic phase), 0.8 (late logarithmic phase), 3.0 (early stationary phase), or 6.5 (late stationary phase), and then β-galactosidase activity was measured. (B) Effect of deletion of mdtEF regulatory genes on the induction of mdtEF by indole. The wild-type and mutant strains (ΔevgSA, ΔgadX, Δhfq, and ΔrpoS) were grown until the OD600 reached 0.8 in LB broth with (black bars) or without (white bars) 1 mM indole. The β-galactosidase activity of the lacZ fusion of the gadE-mdtEF promoter was measured. (C) Effect of indole on expression of rpoS, hfq, gadY, and gadX. The expression of the rpoS, hfq, gadY, and gadX genes was determined by means of the β-galactosidase reporter enzyme assay. E. coli cells were grown until the OD600 reached 0.8 in LB broth with (black bars) or without (white bars) 1 mM indole, and then the β-galactosidase activity of the lacZ fused to each promoter was measured.
The growth phase-dependent expression of rpoS, gadY, and gadX was confirmed on quantitative PCR analysis. At the early stationary phase, the mRNA of mdtE was drastically increased (380-fold), and those of rpoS, gadY, and gadX were also moderately increased (2.5-, 18-, and 23-fold, respectively), whereas a significant change in the hfq mRNA level was not observed (Table 4).
TABLE 4.
Induction of mdtE, rpoS, hfq, gadY, and gadX gene transcripts attributed to the growth phase, as determined by amplification of cDNA samples
| Gene | Fold change from stationary phase vs logarithmic phasea |
|---|---|
| mdtE | 380 |
| rpoS | 2.5 |
| hfq | 0.66 |
| gadY | 18 |
| gadX | 23 |
Values indicate the fold change in the transcript level of cells cultured up to an OD600 of 3.0 (early stationary phase) compared to that of cells cultured up to an OD600 of 0.8 (logarithmic phase).
GadX, Hfq, and RpoS are essential for indole-induced mdtEF expression, and the expression is induced via rpoS, gadY, and gadX up-regulation.
We investigated the relationship between the induction of mdtEF expression by indole and the RpoS-GadY(Hfq)-GadX signaling pathway. At first, indole production was measured in wild-type and tnaAB, evgSA, gadX, hfq, and rpoS deletion mutant cells. After a 24-h culture of these cells, the indole concentration in the culture medium of the respective deletion mutants was the same as that of the wild type, except in the case of the ΔtnaAB mutant (data not shown), in which indole production was not observed (Fig. 5), indicating that RpoS-GadY(Hfq)-GadX signaling does not affect indole production.
Then the mdtEF reporter gene expression by these strains was measured in the presence or absence of externally added indole. In the wild-type and evgSA deletion mutant, the expression was similarly increased by the addition of 1 mM indole, whereas when the gadX, hfq, or rpoS gene was deleted, the expression of mdtEF was no longer increased by indole at all (Fig. 7B); therefore, the induction of mdtEF by indole is also mediated by the RpoS-GadY(Hfq)-GadX signaling pathway.
The effects of indole on the expression levels of rpoS, hfq, gadY, and gadX were examined by means of the reporter gene assay. The expression of these genes was also increased by 1 mM indole, except in the case of hfq (Fig. 7C). These results indicate that indole controls mdtEF gene expression via increasing the amounts of RpoS, GadY, and GadX. Quantitative PCR analysis of rpoS, hfq, gadY, and gadX gave similar results (data not shown).
DISCUSSION
In this study, we comprehensively investigated the growth phase-dependent expression of drug exporter genes in E. coli. We found that out of the 20 drug exporter genes, only the expression of mdtEF greatly increased with cell growth (Fig. 1 and Table 4). The induction of mdtEF expression actually conferred drug tolerance to E. coli at the stationary phase (Fig. 2 and 3). The growth phase-dependent activation of the mdtEF promoter was mediated by the RpoS-GadY(Hfq)-GadX signaling pathway and enhanced by indole (Fig. 4 and 7). As expected, the deletion of rpoS, hfq, and gadX caused the lack of the MdtEF-dependent drug tolerance of the stationary-phase cells.
Schellhorn et al. (25) reported that yhiUV, which is an old name for mdtEF, is one of the RpoS-dependent genes that are induced at the stationary phase. Our previous study revealed that YhiUV is a multidrug exporter system (20), and thus it was renamed MdtEF (21). In this study, we revealed that the growth phase-dependent expression of mdtEF confers drug tolerance at the stationary phase. In addition, we revealed that the growth phase regulation of mdtEF is mediated by RpoS-dependent small-RNA GadY and transcriptional regulator GadX, which is the same signaling pathway as indole signaling.
Sulavik et al. reported that most drug exporter genes did not contribute to drug tolerance under laboratory conditions, except for acrAB, emrE, and mdfA, in an exporter gene knockout experiment (29). The results of this study indicate that the drug hypersensitization of exporter gene deletion mutants reflects the expression levels of drug exporter genes at the logarithmic phase, except in the case of ydgF, which is a weak exporter having a very narrow substrate range. Since the expression level of mdtEF is very low at the logarithmic phase, on deletion of the mdtEF gene, no hypersensitization might be seen on MIC measurement. On the other hand, at the stationary phase, MdtEF is a major drug exporter and certainly confers drug tolerance, although the contribution of AcrAB to the stationary-phase drug tolerance is still significant.
As for the possibility that MdtEF confers tolerance against indole, experimental detection was difficult because the indole toxicity is very low. However, the fact that the ΔmdtEF mutant showed a somewhat reduced indole concentration in the stationary-phase medium and increased accumulation of indole in the cells when indole was externally added (data not shown) suggests the possibility that MdtEF plays some role in indole export.
The mdtEF genes are cotranscribed with acid response regulator gadE, which is encoded upstream of mdtEF in the same operon. The gadE expression is controlled as a response to acid stress (22, 30, 31, 32). However, in our experiments, the pH of the medium at the stationary phase was moderately alkaline (about pH 8.5). Therefore, the signal causing growth phase-dependent induction must be different from acid stress. We examined the effect of alkaline pH on mdtE expression by means of quantitative PCR analysis. The expression level of mdtE was not altered when MC4100 was cultured until the logarithmic phase (OD600 of 0.8) in the LB broth at pH 7.0 or in the LB broth that had been prepared to pH 8.5 (data not shown).
In our previous study, we reported that 2 mM indole added to the culture medium significantly induced the expression of mdtE, acrD, acrE, emrK, yceL, and cusB at the logarithmic phase, whereas when the indole concentration was 1 mM, only mdtE induction was significant (5). The concentration of indole in the MC4100 culture medium at the stationary phase was around 500 μM with the complex laboratory medium. That is the reason why the induction of the drug exporters other than mdtE by intrinsic indole at the stationary phase was not observed in this study. Of course, this fact does not exclude the possibility that indole-dependent drug exporter genes other than mdtEF may be actually induced and play some roles at infection sites due to the high local concentration of indole produced by other bacteria.
In P. aeruginosa, N-(3-oxododecanoyl)-l-homoserine lactone and N-(butyryl)-l-homoserine lactone are known as quorum-sensing signal molecules at the stationary phase, while E. coli does not produce acylhomoserine lactones. On the other hand, the indole concentration increases with cell growth as a by-product of pyruvate production in E. coli cells (33) (Fig. 5). Besides, it has been reported that indole regulates biofilm formation by E. coli and is associated with the virulence of Haemophilus influenzae (12, 13). Our results confirmed the finding of Wang et al. (33) that indole acts as a stationary-phase signal molecule.
It is well known that the drug resistance of pathogens at the sites of infection is generally higher than that under laboratory conditions (8). We believe that elucidation of the mechanisms underlying the growth phase-dependent induction of multidrug exporter genes is important for understanding such acquired drug resistance mechanisms at the site of infection.
Acknowledgments
We wish to thank George M. Church for plasmid pKO3 and Ronald W. Davis for plasmid pNN387.
H. Hirakawa was supported by a research fellowship from the Japan Society for the Promotion of Science for Young Scientists. This work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Zoonosis Control Project of the Ministry of Agriculture, Forestry and Fisheries of Japan.
REFERENCES
- 1.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrik, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 2.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541-555. [DOI] [PubMed] [Google Scholar]
- 3.Ellege, S. J., and R. W. Davis. 1989. Position and density effects on repression by stationary and mobile DNA-binding proteins. Genes Dev. 3:185-197. [DOI] [PubMed] [Google Scholar]
- 4.Franke, S., G. Grass, and D. H. Nies. 2001. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology 147:965-972. [DOI] [PubMed] [Google Scholar]
- 5.Hirakawa, H., Y. Inazumi, T. Masaki, T. Hirata, and A. Yamaguchi. 2004. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol. Microbiol. 55:1113-1126. [DOI] [PubMed] [Google Scholar]
- 6.Hirakawa, H., K. Nishino, T. Hirata, and A. Yamaguchi. 2003. Comprehensive studies of drug resistance mediated by overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Bacteriol. 185:1851-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange, R., and R. Hengge-Aronis. 1991. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol. 5:49-59. [DOI] [PubMed] [Google Scholar]
- 8.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomovskaya, O., K. Lewis, and A. Matin. 1995. EmrR is a negative regulator of the Escherichia coli multidrug resistance pump EmrAB. J. Bacteriol. 177:2328-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma, D., M. Alberti, C. Lynch, H. Nikaido, and J. E. Hearst. 1996. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol. Microbiol. 19:101-112. [DOI] [PubMed] [Google Scholar]
- 12.Martin, K., G. Morlin, A. Smith, A. Nordyke, A. Eisenstark, and M. Golomb. 1998. The tryptophanase gene cluster of Haemophilus influenzae type b: evidence for horizontal gene transfer. J. Bacteriol. 180:107-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martino, P. D., R. Fursy, L. Bret, B. Sundararaju, and R. S. Phillips. 2003. Indole can act as an extracellular signal to regulate biofilm formation of Escherichia coli and other indole-producing bacteria. Can. J. Microbiol. 49:443-449. [DOI] [PubMed] [Google Scholar]
- 14.Masuda, N., and G. M. Church. 2003. Regulatory network of acid resistance genes in Escherichia coli. Mol. Microbiol. 48:699-712. [DOI] [PubMed] [Google Scholar]
- 15.Mazzariol, A., Y. Tokue, T. M. Kanegawa, G. Cornaglia, and H. Nikaido. 2000. High-level fluoroquinolone-resistant clinical isolates of Escherichia coli overproduce multidrug efflux protein AcrA. Antimicrob. Agents Chemother. 44:3441-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller, J. H. 1992. A short course in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikaido, H. 1998. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin. Infect. Dis. 27:S32-S41. [DOI] [PubMed] [Google Scholar]
- 19.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishino, K., and A. Yamaguchi. 2002. EvgA of the two-component signal transduction system modulates production of the YhiUV multidrug transporter in Escherichia coli. J. Bacteriol. 184:2319-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino, K., J. Yamada, H. Hirakawa, T. Hirata, and A. Yamaguchi. 2003. Roles of TolC-dependent multidrug transporters of Escherichia coli in resistance to β-lactams. Antimicrob. Agents Chemother. 47:3030-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Opdyke, J. A., J.-G. Kang, and G. Storz. 2004. GadY, a small-RNA regulator of acid response genes in Escherichia coli. J. Bacteriol. 186:6698-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poole, K. 2001. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 3:255-264. [PubMed] [Google Scholar]
- 24.Rosenberg, E. Y., D. Bertenthal, M. L. Nilles, K. P. Bertrand, and H. Nikaido. 2003. Bile salts and fatty acids induce the expression of the Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol. Microbiol. 48:1609-1619. [DOI] [PubMed] [Google Scholar]
- 25.Schellhorn, H. E., J. P. Audia, L. I. C. Wei, and L. Chang. 1998. Identification of conserved, RpoS-dependent stationary-phase genes of Escherichia coli. J. Bacteriol. 180:6283-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snell, E. E. 1975. Tryptophanase: structure, catalytic activities, and mechanism of action. Adv. Enzymol. Relat. Areas Mol. Biol. 42:287-333. [DOI] [PubMed] [Google Scholar]
- 27.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperandio, V., A. G. Torres, and J. B. Kaper. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 43:809-821. [DOI] [PubMed] [Google Scholar]
- 29.Sulavik, M. C., C. Houseweart, C. Cramer, N. Jiwani, N. Murgolo, J. Greene, B. DiDomenico, K. J. Shaw, G. H. Miller, R. Hare, and G. Shimer. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tramonti, A., P. Visca, M. De Canio, M. Falconi, and D. De Biase. 2002. Functional characterization and regulation of gadX, a gene encoding an AraC/XylS-like transcriptional activator of the Escherichia coli glutamic acid decarboxylase system. J. Bacteriol. 184:2603-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tucker, D. L., N. Tucker, and T. Conway. 2002. Gene expression profiling of the pH response in Escherichia coli. J. Bacteriol. 184:6551-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tucker, D. L., N. Tucker, Z. Ma, J. W. Foster, R. L. Miranda, P. S. Cohen, and T. Conway. 2003. Genes of the GadX-GadW regulon in Escherichia coli. J. Bacteriol. 185:3190-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, D., X. Ding, and P. N. Rather. 2001. Indole can act as an extracellular signal in Escherichia coli. J. Bacteriol. 183:4210-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]