Abstract
The differential expression and the regulatory roles of microRNAs (miRNAs) are being studied intensively these years. Their minute size of only 19–24 nucleotides and strong sequence similarity among related species call for enhanced methods for reliable detection and quantification. Moreover, miRNA expression is generally restricted to a limited number of specific cells within an organism and therefore requires highly sensitive detection methods. Here we present a simple and reliable miRNA detection protocol based on padlock probes and rolling circle amplification. It can be performed without specialized equipment and is capable of measuring the content of specific miRNAs in a few nanograms of total RNA.
Keywords: microRNA, padlock probes, rolling circle
INTRODUCTION
MicroRNAs constitute a class of small 19–24-nt long noncoding RNAs predicted to include more than 800 species in primates (Du and Zamore 2005; Zamore and Haley 2005). The miRNAs are processed from primary transcripts with partially double-stranded inverted repeat regions in a process mediated by the sequential action of the two RNase III proteins Drosha and Dicer in animals (Carmell and Hannon 2004). The processed RNA, in the form of a short RNA imperfect duplex, is then recruited to the RISC complex where only one strand is retained. Nearly all animal miRNAs investigated to date regulate gene expression by imperfect base pairing to the 3′-untranslated region (3′-UTR) of target mRNAs leading to translational inhibition (Wightman et al. 1993; Olsen and Ambros 1999) and in some cases also degradation by the RNA interference (RNAi) mechanism (Hutvagner and Zamore 2002; Ambros 2004; Yekta et al. 2004). In addition, some miRNAs may act to silence gene expression by inducing DNA methylation although the significance of this mechanism in vertebrates remains to be investigated (Volpe et al. 2002; Verdel et al. 2004). Biological processes regulated by miRNAs in metazoans include cell differentiation and proliferation, developmental timing, neuronal asymmetry, apoptosis, insulin secretion, and different metabolic reactions (Poy et al. 2004; Hornstein et al. 2005; Lim et al. 2005; Chen et al. 2006; Esau et al. 2006; Schratt et al. 2006). Apart from controlling the normal life of a cell, miRNAs have also been associated with malfunction of cells. Significantly, changes in miRNA levels are observed in many human diseases, including cancer, suggesting that miRNAs are legitimate targets for anti-cancer therapy (Caldas and Brenton 2005).
In order to investigate the functions of miRNAs, a good system for detecting their expression patterns is needed. Since the discovery of the first miRNA in 1993 (Lee et al. 1993), Northern blots have often been used to validate and quantify miRNA expression. Different variants of the Northern blot methodology have been developed (Valoczi et al. 2004; Ramkissoon et al. 2006), but they all require a relatively large amount of RNA material. For this reason different very sensitive methods have been developed based on either real-time PCR (Schmittgen et al. 2004; Chen et al. 2005; Raymond et al. 2005) or confocal laser-induced fluorescence detection (Neely et al. 2006). Also, oligo-array-based technologies to investigate the expression of hundreds of miRNAs at the same time have been developed (see, for example, Babak et al. 2004; Nelson et al. 2004; Thomson et al. 2004). A common trait of these techniques is that they all rely on an advanced read-out system and their level of specificity is not always addressed. Here we describe a novel miRNA detection principle using the padlock probe technology.
Padlock probes are linear DNA probes where the terminal sequences are designed to hybridize to two adjacent target sequences (Fig. 1; Nilsson et al. 1994). Provided with the right conditions, DNA ligase will ligate the termini of the padlock probe on a perfectly matching RNA template, thereby accurately distinguishing matched and mismatched substrates (Nilsson et al. 2000). The miRNA that is used as a template can subsequently be used as primer for rolling circle amplification, thereby linearly amplifying the target sequence in a process that has been shown to be highly quantitative (Fig. 1; Nilsson et al. 2002). In this study we demonstrate that the padlock probe technology provides a simple low-tech method for analysis of miRNAs quantitatively in a few nanograms of total RNA.
FIGURE 1.
Schematic drawing showing the principal of padlock probe recognition of miRNAs and the rolling circle amplification. (A) Padlock probes are designed to specifically recognize a particular miRNA. (B) The padlock probes annealing to the miRNA are circularized upon addition of DNA ligase. (C) After ligation the annealed miRNA serves as a primer for extension by a phi29 DNA polymerase. (D) The phi29 DNA polymerase facilitates rolling circle amplification, thereby producing a DNA product containing multiple copies of the miRNA sequence.
RESULTS
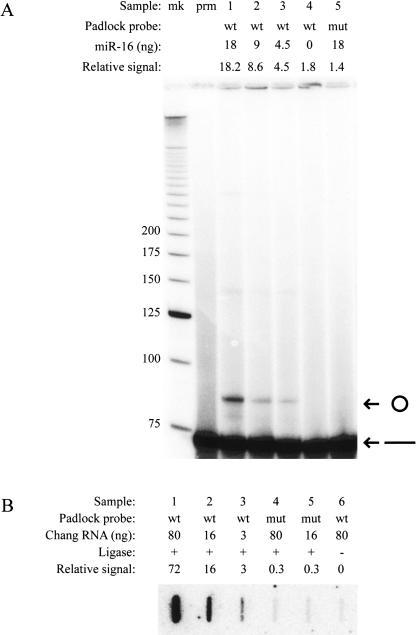
A DNA padlock probe was designed to specifically recognize miR-16 (pad-miR-16), and, as a control, the same padlock probe with a mutation in each of the two arms was also constructed (pad-miR-16mut). The ability of the probes to be ligated on an in vitro transcribed version of miR-16 was tested. As predicted, the pad-miR-16 was ligated into a circular form only in the presence of the miR-16 transcript and in a quantitative fashion (Fig. 2A, lanes 1–3). In contrast, no ligated species of pad-miR-16mut were observed, indicating that the ligation is highly specific for the perfectly matched duplex (Fig. 2A, lane 5); neither did pad-miR-16 self-ligate in the absence of miR-16 (Fig. 2A, lane 4). The faint band seen in the gel just below the 150-nt marker presumably corresponds to two padlock probes ligated together. The pad-miR-16mut was fully functional since this probe was readily circularized when ligated on an in vitro transcribed version of miR-16mut (data not shown).
FIGURE 2.
miR-16 detection. (A) Detection of circularized padlock probes ligated on an in vitro transcribed miR-16. The amount of miR-16 present and whether pad-miR-16 (wt) or pad-miR-16mut (mut) was used as the padlock probe is denoted above the autoradiogram. Sample “mk” is a size marker, and sample “prm” is radio-labeled pad-miR-16. By quantifying and normalizing the background signal in sample prm to 0 and sample 3 as 4.5, the “relative signal” shows the linearity in the experiment. The bands corresponding to the nonligated and ligated circular padlock probes are observed around 72 nt and 85 nt, respectively. (B) Detection of rolling circle amplification of padlock probes ligated on the 20-nt pool of total RNA purified from Chang liver cells. The amount of total input RNA, type of padlock probe, and presence of ligase are indicated above the autoradiogram. By quantifying and normalizing the signal in sample 6 to 0 and sample 3 to 3, the “relative signal” shows the linearity in the experiment.
To amplify the signal, we used the miRNA strand situated on the circular padlock probe after ligation as a primer for rolling circle amplification, and the resulting concatamer was quantified by slot-blot analysis using the padlock DNA as a probe. This procedure was used for the detection of miR-16 in total RNA originating from Chang liver cells (Fig. 2B). To ensure that the signals obtained originated from mature miRNAs, we used the 20-nt pool of the total RNA (see Materials and Methods; all numbers refer to the amount of total cellular RNA). We found that the signal after the amplification step remained linear and with a clear discrimination between pad-miR-16 and pad-miR-16mut, using 3-ng total RNA.
To optimize the incubation time for the polymerization step, we performed the rolling circle amplification on the 20-nt pool of variable amounts of total RNA from Chang liver cells for 30 min, 2 h, or 8 h. The results from three independent experiments are summarized in Table 1. A nearly linear relationship was observed between incubation time and signal, indicating that a strong gain in signal can be obtained after 8 h without compromising linearity.
TABLE 1.
Optimization of rolling circle amplification time using miR-16 as template
It has previously been shown that padlock probes are very sensitive to mismatches when placed at the ligated junction (Nilsson et al. 2000). To further test the ability of our system to discriminate among closely related miRNAs, we designed padlock probes to detect miR-17–5p (pad-miR-17–5p) and miR-20a (pad-miR-20a). These two miRNAs differ only in their 5′ and 3′ terminus and by a single C to U change in the middle of the mature miRNAs (Griffiths-Jones 2004; Griffiths-Jones et al. 2006). Using in vitro transcribed versions of the two miRNAs, we varied the ratio between miR-17–5p and miR-20a, keeping the total amount of RNA constant at 20 pg. Both pad-miR-17–5p and pad-miR-20a were able to discriminate between miR-17–5p and miR-20a, respectively, illustrating that padlock probes can be designed for highly specific detection of miRNAs (Fig. 3).
FIGURE 3.
Discrimination between in vitro transcribed miR17–5p and miR-20a using padlock probes. The samples contained the indicated ratio of miR-17–5p and miR-20a using the same total amount of RNA (20 pg). The theoretically predicted signal is shown as “pred.,” while the observed signal is shown as “obs.” The predicted and the observed values were set to zero in the absence of the probed miRNA and the observed values were normalized to 20 at 20 pg of the probed miRNA.
Next, we tested the ability of the padlock probes to detect changes in miRNA levels between different cell types. We constructed a padlock probe against miR-27a (pad-miR-27a), which is known from previous studies to be expressed in Chang liver cells but not in HEK293T cells (data not shown). When using the 20-nt pool from 500 or 125 ng total RNA, a clear miR-27a specific signal was observed for Chang liver cells, whereas no, or only a very faint signal, was observed for HEK293T cells, in agreement with the results from Northern blots (Fig. 4A).
FIGURE 4.
Quantification of miR-27a, miR-92, and miR-21 expression in different cell lines. A representative slot-blot autoradiogram is shown for each of the miRNA, and gray bars in a column diagram above indicate the average signal from three repeated experiments. In B and C the relative signal is compared with the result obtained when using Northern blot (black columns). (A) Detection of miR-27a in HEK293T cells (HEK) and Chang liver cells (Chang). Detection was done on the 20-nt pool of RNA purified from either 500 ng (4×) or 125 ng (1×) of total RNA. In the column diagram the signal from Chang 1× was normalized to 1. The background signal obtained from the sample containing H2O instead of RNA was set to 0. (B) Detection of miR-92 in HEK, Chang, and murine neuroblastoma cells (N2a). Detection was done on the 20-nt pool of RNA purified from 10 ng of total RNA. The background signal obtained from the sample containing yeast RNA was normalized to 0 and the signal obtained from the N2a cells was set arbitrarily to 1 in the column diagram. To compare with data from the Northern blot, the signal obtained from the N2a cells in the Northern blot was normalized to the signal obtained from N2a cells, using rolling circle amplification. (C) Detection of miR-21 in HEK, Chang, N2a, and HeLa cells. Five nanograms of miRNA-enriched RNA were used for each sample. The background signal obtained from the tRNA control sample was normalized to 0 and the signal obtained from the N2a cells was set arbitrarily to 1 in the column diagram. In order to compare with data from the Northern blot, the signal obtained from the Chang cells in the Northern blot was normalized to the signal obtained from Chang cells, using rolling circle amplification.
Similarly, a padlock probe directed against miR-92 (pad-miR-92) was tested using the 20-nt pool from 10 ng total RNA derived from three different cell types. Again, the results obtained using the padlock probes closely correspond to the results obtained from the Northern blots (Fig. 4B).
Finally, we tested the ability of a miR-21 specific padlock (pad-miR-21) to quantify miR-21 in 5 ng of miRNA-enriched RNA. We detected miR-21 in three different cell lines using pad-miR-21 and the amount correlated closely with data obtained by Northern blotting (Fig. 4C).
DISCUSSION
The recent explosion in small RNA research has called for more sensitive and specific methods for quantification of short RNA oligonucleotides in a complex mixture of RNA. The padlock probe-based detection system presented in this paper was successfully used to detect miRNAs in a few nanograms of total RNA. Assuming that each cell contains ∼10 pg of RNA, this corresponds to RNA from a few hundred cells. This is well below the RNA level used in Northern blots where the limit is ∼1 μg of total RNA (Valoczi et al. 2004; Ramkissoon et al. 2006). Other sensitive systems for miRNA detection have also been developed. A system based on confocal laser-induced fluorescence detection was recently published having a detection level around the same level as our padlock system (Neely et al. 2006). Also, real-time PCR methods have been designed for miRNA detection (Schmittgen et al. 2004; Chen et al. 2005; Raymond et al. 2005), some capable of detecting miRNAs present in only picograms of total RNA. Whereas all these methods require advanced laboratory equipment and chemically modified oligonucleotides, our method requires inexpensive DNA oligonucleotides and only equipment no more advanced than for Northern blotting.
It has previously been shown that the ligation of a padlock probe is very sensitive to mismatches between the RNA template and the padlock probe (Nilsson et al. 2000). Therefore, our method should be optimal to distinguish between closely related miRNAs, especially if the miRNAs differ at a position near the middle region of the miRNA. In accordance with this we showed that the padlock approach could distinguish two very closely related miRNAs such as miR-17–5p and miR-20a and also miR-16 and miR-16mut. The amplification strategy ensures yet another level of specificity. Since the miRNA is used as a primer, only duplexes that are perfectly matched at the 3′ end of the miRNA will be a substrate for phi29 DNA polymerase. This should also prevent any signal from genomic DNA or pri-miRNA contaminations. However, if one wants strictly to measure mature miRNA, we recommend that a gel purification of miRNA-sized RNAs is performed first, especially if the mature miRNA is situated in the 3′ arm of the pre-miRNA. The method is probably not useful for quantification of plant miRNA, since this RNA contains a 2′-OMe at the 3′ end, from which Phi29 will likely not extend.
Importantly, the rolling circle product contains repeated miRNA sense sequences, which can be detected using miRNA specific oligonucleotide arrays. Hence, our method holds the possibility for being developed into an array assay combining several different padlock probes in the same reaction.
When quantifying RNA, it is generally useful to include a ubiquitously expressed gene as an internal control for RNA recovery. However, since the RNA undergoes size fractionation in our assay, and there is no known miRNA that are expressed to the same level in all cells, we suggest that an artificial miRNA is added at a fixed concentration immediately after cell lysis and subsequently measured using an artificial matching padlock probe.
Padlock probes have been used for in situ hybridizations (Lizardi et al. 1998; Landegren et al. 2004; Larsson et al. 2004). Our technique could possibly be developed into an in situ hybridization assay to visualize miRNA localization in cells. No additional primer needs to be added since the miRNA can function as a primer itself. Such a method has the potential of being more sensitive than the conventional methods already developed for in situ miRNA detection (Wienholds et al. 2005; Kloosterman et al. 2006). However, it is unclear whether the cross linking of small RNAs would affect the performance in this assay.
In summary, we have developed a new sensitive padlock-based method to detect and quantify miRNA expression. The method can discriminate between closely related miRNAs and measure miRNA expression in a few nanograms of total RNA.
MATERIALS AND METHODS
RNA and padlock probe preparation
All padlock probes were chemically synthesized with a phosphate at the 5′end. The sequences are:
pad-miR-16: pTACGTGCTGCTATTTATTTCCTCAATGCTGCTGCTGTACTACTAGTGATTTACTTGGATGTCTCGCCAATATT;
pad-miR-16mut: pTACCTGCTGCTATTTATTTCCTCAATGCTGCTGCTGTACTACTAGTGATTTACTTGGATGTCTCGCCAGTATT;
pad-miR-17–5p: pTAAGCACTTTGTTTATTTCCTCAATGCTGCTGCTGTACTACTAGTGATTTACTTGGATGTCTACTACCTGCACTG;
pad-miR-20a: pTAAGCACTTTATTTATTTCCTCAATGCTGCTGCTGTACTACTAGTGATTTACTTGGATGTCTCTACCTGCACTA;
pad-miR-21: pCTGATAAGCTATTTATTTCCTCAATGCTGCTGCTGTACTACTAGTGATTTACTTGGATGTCTTCAACATCAGT;
pad-miR-27a: pTAGCCACTGTGAATTTATTTCCTCAATGCTGCTGCTGTACTACTAGTGATTTACTTGGATGTCTGCGGAACT;
pad-miR-92: pCGGGACAAGTGCAATATTTATTTCCTCAATGCTGCTGCTGTACTACTAGTGATTTACTTGGATGTCTCAGGC.
Total RNA from cultured cells was purified with Trizol reagent (Invitrogen) and either enriched for small RNAs using a mirVana miRNA isolation kit (Ambion; miRNA-enriched RNA) or purified on a 8% polyacrylamide gel (100 mM Tris Borate, pH 7.5, 1 mM EDTA) selectively for RNAs in the range of 15–30 nt (20-nt pool). In vitro transcribed RNA was transcribed using a mirVana miRNA probe construction kit (Ambion).
Ligation assay
Ligations were performed in 4.5 μL ligation buffer (10 mM Tris-HCl pH 7.5, 10 mM MgCl2, 10 μM ATP) in the presence of varying amounts of in vitro transcribed miR-16 or miR-16mut RNA, and 2.5 fmol of P32 5′ end-labeled of the corresponding padlock probe. The samples were heated at 65°C for 3 min, cooled slowly to room temperature (RT) over 10 min. Two hundred units of T4-DNA ligase (New England Biolabs) were then added in a total reaction volume of 5 μL. The reactions were incubated at 37°C for 2 h. Two volumes of RNA load buffer containing 90% formamide were added to stop the reactions. The ligation products were analyzed on a 8% polyacrylamide gel (100 mM Tris Borate, pH 7.5, 1 mM EDTA) alongside a P32 5′-endlabeled 25-bp DNA marker (Invitrogen) and 2.5 fmol of untreated P32 5′-end-labeled pad-miR-16.
Ligation followed by rolling circle assay
The ligation was performed as described above with the following modifications. Either in vitro transcribed RNA, miRNA-enriched RNA, or gel-purified 15–30-nt fractions from total RNA were used depending on the experiment. Padlock probes containing a nonradioactive 5′ phosphate were present in the sample instead of the radioactively labeled probes. After the ligation the samples were subjected to rolling circle amplification in 100 μL in the presence of 50 mM Tris-HCl pH 7.5, 10 mM MgCl2, 10 mM (NH4)2SO4, 4 mM dithiothreitol, 200 μg/mL BSA, 10 U of phi29 DNA polymerase (New England Biolabs), and 0.2 mM dNTPs. The reactions were then incubated 8 h at 30°C followed by 10 min at 65°C to inactivate the polymerase. The samples were transferred to a 2× SSC soaked hybond N+ filter (Amersham) using a slot-blot apparatus. The DNA was UV-X-linked to the filter with 1200 uJ. Hybridization reactions were carried out as described with the Northern blots. A P32 5′-end-labeled DNA-probe anti-sense to the miRNA being detected was used to visualize the rolling circle amplification.
Northern blots
The Northern protocol used was adapted from Lau et al. (2001). In short, RNA (20 μg total RNA or 1 μg miRNA-enriched RNA) was loaded on a 15% polyacrylamide gel. After running the gels, RNA was blotted onto a Hybond N+ membrane (Amersham) using a semidry blotter. DNA probes complementary to the cloned miRNAs were used in the hybridizations.
ACKNOWLEDGMENTS
The work was supported in part by grants from the Sixth Research Framework Programme of the European Union, Project RIGHT (LSHB-CT-2004-005276), Vilhelm Pedersen og Hustrus legat, Danish Cancer Society, and the Danish Technical Research Council. S.P.J was supported by Aarhus University.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.110706.
REFERENCES
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Babak T., Zhang W., Morris Q., Blencowe B.J., Hughes T.R. Probing microRNAs with microarrays: Tissue specificity and functional inference. RNA. 2004;10:1813–1819. doi: 10.1261/rna.7119904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldas C., Brenton J.D. Sizing up miRNAs as cancer genes. Nat. Med. 2005;11:712–714. doi: 10.1038/nm0705-712. [DOI] [PubMed] [Google Scholar]
- Carmell M.A., Hannon G.J. RNase III enzymes and the initiation of gene silencing. Nat. Struct. Mol. Biol. 2004;11:214–218. doi: 10.1038/nsmb729. [DOI] [PubMed] [Google Scholar]
- Chen C., Ridzon D.A., Broomer A.J., Zhou Z., Lee D.H., Nguyen J.T., Barbisin M., Xu N.L., Mahuvakar V.R., Andersen M.R., et al. Real-time quantification of microRNAs by stem–loop RT-PCR. Nucleic Acids Res. 2005;33 doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.F., Mandel E.M., Thomson J.M., Wu Q., Callis T.E., Hammond S.M., Conlon F.L., Wang D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T., Zamore P.D. microPrimer:The biogenesis and function of microRNA. Development. 2005;132:4645–4652. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- Esau C., Davis S., Murray S.F., Yu X.X., Pandey S.K., Pear M., Watts L., Booten S.L., Graham M., McKay R., et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S. The microRNA registry. Nucleic Acids Res. 2004;32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S., Grocock R.J., van Dongen S., Bateman A., Enright A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein E., Mansfield J.H., Yekta S., Hu J.K., Harfe B.D., McManus M.T., Baskerville S., Bartel D.P., Tabin C.J. The microRNA miR-196 acts upstream of Hoxb8 and Shh in limb development. Nature. 2005;438:671–674. doi: 10.1038/nature04138. [DOI] [PubMed] [Google Scholar]
- Hutvagner G., Zamore P.D. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- Kloosterman W.P., Wienholds E., de Bruijn E., Kauppinen S., Plasterk R.H. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat. Methods. 2006;3:27–29. doi: 10.1038/nmeth843. [DOI] [PubMed] [Google Scholar]
- Landegren U., Nilsson M., Gullberg M., Soderberg O., Jarvius M., Larsson C., Jarvius J. Prospects for in situ analyses of individual and complexes of DNA, RNA, and protein molecules with padlock and proximity probes. Methods Cell Biol. 2004;75:787–797. doi: 10.1016/s0091-679x(04)75034-7. [DOI] [PubMed] [Google Scholar]
- Larsson C., Koch J., Nygren A., Janssen G., Raap A.K., Landegren U., Nilsson M. In situ genotyping individual DNA molecules by target-primed rolling-circle amplification of padlock probes. Nat. Methods. 2004;1:227–232. doi: 10.1038/nmeth723. [DOI] [PubMed] [Google Scholar]
- Lau N.C., Lim L.P., Weinstein E.G., Bartel D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans . Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Lim L.P., Lau N.C., Garrett-Engele P., Grimson A., Schelter J.M., Castle J., Bartel D.P., Linsley P.S., Johnson J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Lizardi P.M., Huang X., Zhu Z., Bray-Ward P., Thomas D.C., Ward D.C. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat. Genet. 1998;19:225–232. doi: 10.1038/898. [DOI] [PubMed] [Google Scholar]
- Neely L.A., Patel S., Garver J., Gallo M., Hackett M., McLaughlin S., Nadel M., Harris J., Gullans S., Rooke J. A single-molecule method for the quantitation of microRNA gene expression. Nat. Methods. 2006;3:41–46. doi: 10.1038/nmeth825. [DOI] [PubMed] [Google Scholar]
- Nelson P.T., Baldwin D.A., Scearce L.M., Oberholtzer J.C., Tobias J.W., Mourelatos Z. Microarray-based, high-throughput gene expression profiling of microRNAs. Nat. Methods. 2004;1:155–161. doi: 10.1038/nmeth717. [DOI] [PubMed] [Google Scholar]
- Nilsson M., Malmgren H., Samiotaki M., Kwiatkowski M., Chowdhary B.P., Landegren U. Padlock probes: Circularizing oligonucleotides for localized DNA detection. Science. 1994;265:2085–2088. doi: 10.1126/science.7522346. [DOI] [PubMed] [Google Scholar]
- Nilsson M., Barbany G., Antson D.O., Gertow K., Landegren U. Enhanced detection and distinction of RNA by enzymatic probe ligation. Nat. Biotechnol. 2000;18:791–793. doi: 10.1038/77367. [DOI] [PubMed] [Google Scholar]
- Nilsson M., Baner J., Mendel-Hartvig M., Dahl F., Antson D.O., Gullberg M., Landegren U. Making ends meet in genetic analysis using padlock probes. Hum. Mutat. 2002;19:410–415. doi: 10.1002/humu.10073. [DOI] [PubMed] [Google Scholar]
- Olsen P.H., Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev. Biol. 1999;216:671–680. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- Poy M.N., Eliasson L., Krutzfeldt J., Kuwajima S., Ma X., Macdonald P.E., Pfeffer S., Tuschl T., Rajewsky N., Rorsman P., et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- Ramkissoon S.H., Mainwaring L.A., Sloand E.M., Young N.S., Kajigaya S. Nonisotopic detection of microRNA using digoxigenin labeled RNA probes. Mol. Cell. Probes. 2006;20:1–4. doi: 10.1016/j.mcp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Raymond C.K., Roberts B.S., Garrett-Engele P., Lim L.P., Johnson J.M. Simple, quantitative primer-extension PCR assay for direct monitoring of microRNAs and short-interfering RNAs. RNA. 2005;11:1737–1744. doi: 10.1261/rna.2148705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T.D., Jiang J., Liu Q., Yang L. A high-throughput method to monitor the expression of microRNA precursors. Nucleic Acids Res. 2004;32 doi: 10.1093/nar/gnh040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt G.M., Tuebing F., Nigh E.A., Kane C.G., Sabatini M.E., Kiebler M., Greenberg M.E. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Thomson J.M., Parker J., Perou C.M., Hammond S.M. A custom microarray platform for analysis of microRNA gene expression. Nat. Methods. 2004;1:47–53. doi: 10.1038/nmeth704. [DOI] [PubMed] [Google Scholar]
- Valoczi A., Hornyik C., Varga N., Burgyan J., Kauppinen S., Havelda Z. Sensitive and specific detection of microRNAs by Northern blot analysis using LNA-modified oligonucleotide probes. Nucleic Acids Res. 2004;32 doi: 10.1093/nar/gnh171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdel A., Jia S., Gerber S., Sugiyama T., Gygi S., Grewal S.I., Moazed D. RNAi-mediated targeting of heterochromatin by the RITS complex. Science. 2004;303:672–676. doi: 10.1126/science.1093686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe T.A., Kidner C., Hall I.M., Teng G., Grewal S.I., Martienssen R.A. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- Wienholds E., Kloosterman W.P., Miska E., Alvarez-Saavedra E., Berezikov E., de Bruijn E., Horvitz H.R., Kauppinen S., Plasterk R.H. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- Wightman B., Ha I., Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans . Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Yekta S., Shih I.H., Bartel D.P. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Zamore P.D., Haley B. Ribo-gnome: The big world of small RNAs. Science. 2005;309:1519–1524. doi: 10.1126/science.1111444. [DOI] [PubMed] [Google Scholar]