Abstract
Activator-dependent recruitment of TFIID initiates formation of the transcriptional preinitiation complex. TFIID binds core promoter DNA elements and directs the assembly of other general transcription factors, leading to binding of RNA polymerase II and activation of RNA synthesis. How TATA box-binding protein (TBP) and the TBP-associated factors (TAFs) are assembled into a functional TFIID complex with promoter recognition and coactivator activities in vivo remains unknown. Here, we use RNAi to knock down specific TFIID subunits in Drosophila tissue culture cells to determine which subunits are most critical for maintaining stability of TFIID in vivo. Contrary to expectations, we find that TAF4 rather than TBP or TAF1 plays the most critical role in maintaining stability of the complex. Our analysis also indicates that TAF5, TAF6, TAF9, and TAF12 play key roles in stability of the complex, whereas TBP, TAF1, TAF2, and TAF11 contribute very little to complex stability. Based on our results, we propose that holo-TFIID comprises a stable core subcomplex containing TAF4, TAF5, TAF6, TAF9, and TAF12 decorated with peripheral subunits TAF1, TAF2, TAF11, and TBP. Our initial functional studies indicate a specific and significant role for TAF1 and TAF4 in mediating transcription from a TATA-less, downstream core promoter element (DPE)-containing promoter, whereas a TATA-containing, DPE-less promoter was far less dependent on these subunits. In contrast to both TAF1 and TAF4, RNAi knockdown of TAF5 had little effect on transcription from either class of promoter. These studies significantly alter previous models for the assembly, structure, and function of TFIID.
Keywords: RNA interference, TATA box-binding protein, S2 cells
Regulated initiation of transcription to produce mRNA in eukaryotes requires the stepwise assembly of an elaborate multiprotein preinitiation complex consisting of the general transcription factors, various coactivators, and RNA polymerase II (for review, see ref. 1). The core promoter-recognition complex, TFIID, consists of the TATA box-binding protein (TBP) and 8–12 TBP-associated factors (TAFs). In addition to binding core promoter elements and initiating formation of the preinitiation complex, this TBP·TAF multisubunit transcription factor also serves as a coactivator by transmitting signals from sequence-specific activators to other components of the basal machinery (for review, see ref. 2).
Critical to dissecting the diverse functions of TFIID in both promoter recognition and coactivation is an understanding of how the complex is assembled and maintained in cells. Initial in vitro assembly reactions suggested that TBP and the largest TAF subunit (TAF1) may form a scaffold on which the other TAFs bind to form holo-TFIID (3). Subsequent studies have proposed that TAF5 may dimerize and also help coordinate complex assembly (4). Recent low-resolution electron microscopy/single-particle reconstruction models of TFIID have revealed a trilobed architecture containing a large central cavity that has been conserved from yeast to humans, with TBP situated at the junction of the three lobes (5, 6). The TAFs, some of which are present in multiple copies per TFIID complex (7), interact to form the separate lobes. However, with neither an atomic resolution structure nor a clear idea of the interactions responsible for coordinating the assembly and maintaining the stability of the complex in vivo, the mechanism of action and the structure–function relationship of the different subunits of the TBP·TAF complex have remained obscure.
Here, we exploit the robust RNAi response in Drosophila tissue culture cells to address the stability and potential assembly path of TFIID in vivo. We have also begun to characterize functionally the role of individual TAFs at an inducible promoter. In contrast to the previously reported central roles of TBP and TAF1 in nucleating the assembly of holo-TFIID, our studies revealed a stable core subcomplex that is nucleated by the C terminus of TAF4 and the N terminus of TAF6 and that includes TAF5, TAF9, and TAF12, which then becomes decorated with peripheral subunits TBP, TAF1, TAF2, and TAF11 to form holo-TFIID. We also found that both TAF1 and TAF4 play a critical role in mediating activated transcription from a TATA-less, downstream core promoter element (DPE)-containing promoter but not from a TATA-containing, DPE-less promoter, whereas knockdown of TAF5 has little effect on transcription from either promoter. Our results shed light on the in vivo assembly of TFIID and identify key roles for TAF1 and TAF4 in mediating transcription from a specific core promoter architecture.
Results
TFIID Integrity Is Differentially Dependent on TBP·TAFs.
Current models of metazoan TFIID suggest that TAF1 and TBP serve as central scaffold subunits on which the other subunits assemble. However, these early studies were largely based on in vitro assembly reactions using tagged recombinant proteins. To address the contribution of individual subunits to the integrity of TFIID in vivo and perhaps gain some insight into the order of assembly in cells, we treated Drosophila Schneider Line 2 (S2) cells with dsRNA directed against specific TAF subunits or TBP, and we immunoblotted whole-cell lysates to monitor the disappearance of TBP·TAFs. As shown in Fig. 1A, the targeted reduction of certain TAFs results in a dramatic depletion/degradation of other TAFs and to a lesser extent TBP, whereas a reduction of other individual TAFs has minimal or no effect on the nontargeted subunits. RNA analysis confirmed that the mRNA of the RNAi-targeted TAF subunit was degraded. In contrast, mRNA corresponding to each of the nontargeted TAFs continued to be expressed, suggesting that the observed depletion of nontargeted subunits is most likely due to a loss of protein stability (8). Strikingly, RNAi-mediated depletion of TAF4 had the most dramatic global effects on overall Drosophila TFIID integrity and stability. When TAF4 (formerly dTAFII110) is lost, all of the other TAF subunits tested except TAF2 (formerly dTAFII150) become severely depleted, presumably because of destabilization of the TFIID complex followed by degradation. This finding suggests that TAF4 likely acts as a keystone subunit, nucleating and maintaining TFIID stability. Furthermore, although nearly every subunit is degraded when directly targeting TAF4 for RNAi knockdown, the levels of this core component become depleted only when its histone-fold partner, TAF12 (formerly dTAFII30α), is targeted for RNAi knockdown. As might be expected, RNAi depletion of TAF12 has a drastic effect on the other TFIID subunits, reducing the levels of TBP and all of the TAFs except TAF2 and TAF6 (formerly dTAFII60). These results suggest that TAF12 may also be a key component of a core subcomplex. However, it is also possible that TAF12 is required primarily to maintain the stability of TAF4 and that the depletion of the other subunits is an indirect result of depleting TAF4.
Fig. 1.
Analysis of TFIID stability in vivo. (A) S2 cells were either left untreated (NT) or treated with dsRNA targeting the TFIID subunit indicated at the top of the panel for 3 days. Whole-cell lysates were then subjected to Western blot analysis with antibodies directed against the subunits indicated at the left of the panel. β-Tubulin served as a loading control. (B) A TAF4 monoclonal antibody was used to immunoprecipitate TAF4-containing complexes from nuclear extracts prepared from either untreated S2 cells (NT) or cells treated with TAF1 dsRNA. The precipitated proteins were eluted and subjected to Western blot analysis with antibodies against the proteins indicated at the left of the panel. (Left) Input. (Right) Eluted coprecipitating (IP) proteins. Protein G–Sepharose beads were used as a nonspecific control.
Another potential core component is TAF5 (formerly dTAFII80) because reduction of this subunit results in the degradation of most other subunits except TAF2, with TAF4 affected only moderately. This finding suggests that TAF5 may also play a key role in TFIID assembly or stability, which was unexpected because a partial complex containing recombinant Drosophila TAF4, TAF6, and TBP can be assembled on an immobilized, truncated TAF1 (formerly dTAFII230) in vitro (3). Thus, the in vitro-assembled complexes may not always accurately reflect the stability and/or assembly of endogenous complexes in vivo. On the other hand, these data are consistent with the model proposing that TAF5 can dimerize and act as a scaffold to coordinate the two separate lobes of the TFIID structure (see the Introduction).
Targeting TAF6 by RNAi results in the depletion of all but TAF2 and TAF4, indicating that it too may play a role in complex stability. By contrast, RNAi knockdown directed against TAF9 (formerly dTAFII40), a proposed histone-fold partner to TAF6, results mainly in the loss of TBP, TAF1, and TAF5. Interestingly, the levels of its putative partner, TAF6, remain unchanged, suggesting that TAF6 is either not an obligate partner of TAF9 or it can make contacts with subunits other than TAF9 and that these interactions may be sufficient to hold TAF6 as a stable component of TFIID even in the absence of TAF9. However, as described above, the reciprocal relationship between TAF6 and TAF9 (i.e., loss of TAF6 but preservation of TAF9) does not hold true.
Treating S2 cells with TAF2 dsRNA has minimal impact on the stability of the core complex, reducing primarily the levels of TAF2 itself and to some extent TAF6. Likewise, TAF2 is the lone subunit that remains largely intact when the other TAF subunits are targeted for RNAi knockdown. Depleting TAF11 (formerly dTAFII30β) by RNAi modestly reduces the levels of TBP while leaving all of the other TAFs unaffected. Surprisingly, depleting TBP has little effect on the stability of the core TAF subcomplex. Also unexpected was the only modest effect on overall TAF levels seen when TAF1 levels were decreased, indicating that this largest of the subunits does not serve as a critical scaffold. By contrast, TAF1 itself appears to be one of the most labile subunits, becoming significantly depleted when nearly any of the other TAFs is targeted for depletion. Similarly, a significant amount of TBP becomes destabilized and degraded when any TAF is targeted, with the exception of TAF2. The fraction of TBP that remains is likely a combination of “free” TBP released from the destabilized TAFs and TBP present in other complexes such as the RNA polymerase I complex SL1, but not TFIIIB, because TRF1 functionally replaces TBP in the formation of an RNA polymerase III preinitiation complex in Drosophila (9, 10). Taken together, these results suggest that TBP, TAF1, TAF2, and TAF11 are likely peripheral subunits of TFIID, whereas TAF4, TAF5, TAF6, TAF9, and TAF12 comprise a core subcomplex that can retain its integrity in the absence of the peripheral components.
TFIID Comprises a Decorated TAF4-Containing Core Complex.
To establish further the possibility of a stable core subcomplex in the absence of TAF1 and TBP, we prepared nuclear extracts from either untreated cells or cells treated with TAF1 dsRNA. Using a monoclonal antibody against TAF4, we immunoprecipitated this core subunit from each nuclear extract and probed the coprecipitating proteins by Western blot. As shown in Fig. 1B, a full TFIID complex is precipitated by the TAF4 antibody in untreated control extracts, whereas a subcomplex containing just the core subunits, devoid of TBP and TAF1, is efficiently precipitated from nuclear extracts derived from S2 cells treated with TAF1 dsRNA. These data, taken with our findings presented above, provide evidence that Drosophila holo-TFIID consists of a TAF4-containing core complex, which is decorated on the “outside” with TBP, TAF1, TAF2, and TAF11.
TAF4 C-Terminal Region (CTR) Rescues TFIID Stability.
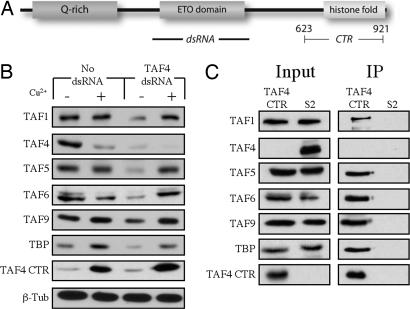
Given the importance of TAF4 in forming and maintaining a stable TFIID complex, we decided to investigate the functional domains of this critical subunit. TAF4 consists of a C-terminal histone fold, a middle region called the ETO domain found in metazoans, and an N-terminal metazoan-specific glutamine-rich coactivation domain (Fig. 2A) (11, 12). The C-terminal region of human TAF4 has previously been shown to be sufficient to incorporate into TFIID (13). We sought to address whether the corresponding portion of Drosophila TAF4 could not only incorporate into TFIID but also be sufficient to nucleate the assembly of the core subcomplex and rescue the stability of the ensemble. To test this hypothesis, we generated a copper-inducible stable S2 cell line expressing an epitope-tagged C-terminal region of TAF4. These cells were either left untreated or treated with TAF4 dsRNA against the 5′ region of TAF4, which is not present in the transgenic TAF4 CTR construct (Fig. 2A). The untreated and TAF4 RNAi cells were then either left untreated or treated with copper to induce expression of the TAF4 CTR. This strategy allows us to largely deplete TAF4 and replace it with the TAF4 CTR. As shown in Fig. 2B, when expressed in S2 cells the TAF4 CTR efficiently rescues the TAF4 RNAi-induced degradation of TAF1, TAF5, TAF6, TAF9, and TBP, suggesting that this C-terminal region is sufficient to assemble and stabilize most, if not all, holo-TFIID. The TAF4 CTR also appears to have a dominant-negative effect, in that full-length TAF4 becomes depleted when the CTR is overexpressed, most likely because of competition for recognition sites in stable TFIID complexes. This finding suggests that the core TAFs may be limiting for the assembly of the complex because overexpression of the TAF4 CTR does not result in an increase in the amount of holo-TFIID. Next, we asked whether it is possible to actually isolate a holo-TFIID complex lacking full-length TAF4. To test this possibility, we immunoprecipitated the TAF4 CTR from nuclear extracts prepared from untreated S2 cells and from the TAF4 CTR cell line under conditions in which the TAF4 CTR is expressed and the endogenous TAF4 is depleted. The antibody against the TAF4 CTR efficiently precipitated TFIID lacking full-length TAF4, whereas TFIID failed to be precipitated from the control S2 nuclear extracts (Fig. 2C). These data indicate that the TAF4 CTR is necessary and sufficient for nucleating a holo-TFIID complex, suggesting that the N-terminal two-thirds of full-length TAF4, which is present in metazoan organisms, very likely evolved to fulfill a nonstructural function within TFIID, possibly serving as a coactivation domain suitable for contacting activators or other components of the basal machinery, as has been postulated (11).
Fig. 2.
TAF4 CTR rescues TFIID stability. (A) Schematic of the Drosophila TAF4 protein indicating the glutamine-rich region, the ETO domain, the histone fold, and the CTR construct. Also noted is the region of TAF4 targeted by RNAi. (B) A stable cell line expressing the TAF4 CTR under the control of the copper-inducible MtnA promoter was either left untreated or treated with TAF4 dsRNA. These cells were then either left untreated or treated with copper, as indicated. Whole-cell lysates were then immunoblotted for the TFIID subunits indicated on the left. β-Tubulin was included as a loading control. (C) The M2 anti-FLAG monoclonal antibody was used to immunoprecipitate (IP) 3× FLAG-tagged TAF4 CTR-containing complexes from nuclear extracts prepared from either WT control S2 cells or the TAF4 CTR cell line treated with TAF4 dsRNA and copper. The input and immunoprecipitated proteins were probed with antibodies directed against the subunits indicated on the left. (Left) Input. (Right) Immunoprecipitated eluates.
TAF6 N-Terminal Region (NTR) Is Sufficient for TFIID Stability.
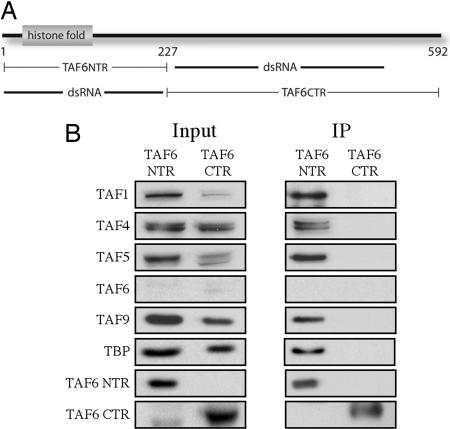
Unlike TAF4, TAF6 is conserved from yeast to man over its entire length and bears its histone fold in the N terminus. The domain of TAF6 required for integration into TFIID has not yet been determined. To map which region of TAF6 is required for TFIID stability, this subunit was divided into a histone-fold-containing NTR and a CTR. These constructs were epitope-tagged, placed under the control of a copper-inducible promoter, and transfected into S2 cells to generate stable cell lines expressing these truncated versions. These cells were each induced with copper and treated with TAF6 dsRNA directed against a region of TAF6 that allows the truncated form to be expressed (see Fig. 3A) while depleting the endogenous protein; after 3 days, the cells were harvested and nuclear extracts were prepared. Antibodies against the epitope tag were subsequently used to immunoprecipitate the TAF6 truncations, and the coprecipitating proteins were detected by Western blotting. As shown in Fig. 3B, the TAF6 NTR is able to coprecipitate the other subunits of TFIID, whereas the CTR failed to do so. No full-length TAF6 was detectable in these immunoprecipitations, indicating that the TAF6 NTR is necessary and sufficient to stabilize TFIID, leaving the CTR to perform other functions, such as binding to core promoter elements and possibly serving as a coactivator target.
Fig. 3.
The TAF6 NTR is sufficient for TFIID stability. (A) Schematic of the Drosophila TAF6 protein showing the histone fold, the NTR and CTR constructs, and the regions of TAF6 targeted by RNAi. (B) Copper-inducible stable S2 cell lines were generated expressing either 3× FLAG-tagged TAF6 NTR or 3× FLAG-tagged TAF6 CTR. These cells were treated with dsRNA against TAF6, and copper and nuclear extracts were prepared. The M2 anti-FLAG antibody was used to precipitate truncated TAF6-containing complexes, and Western blotting with antibodies against the proteins indicated at the left of the panel was used to detect the coprecipitating proteins. (Left) Input. (Right) Immunoprecipitated (IP) proteins.
A TATA-less, DPE-Containing Promoter Is Dependent on TAF1 and TAF4.
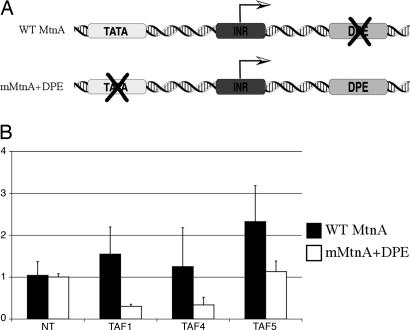
Having analyzed the effects of depleting individual TAFs or TBP on the integrity of holo-TFIID in Drosophila cells, we next investigated the functional consequences of some of these RNAi-mediated alterations in TFIID composition. We previously showed that although TFIID plays a role at the metallothionein A (MtnA) gene to potentiate an appropriately tuned response to heavy-metal stimuli, some of the TAFs actually appeared to be negatively regulating transcription, whereas other TAFs contributed very little to MtnA expression (8). In previous studies, a correlation was found between TFIID dependence and TATA-less core promoters (14). Also, the DPE is thought to be recognized by TFIID (15). The WT Drosophila MtnA promoter contains a strong TATA box and initiator element but no DPE, perhaps partly explaining the observed weak TFIID dependence. We therefore asked whether mutating the TATA box and adding a consensus DPE to the MtnA core promoter would reveal a more central role for the TAFs in transcription activation of the MtnA gene. To test this possibility, we used either a construct containing the WT MtnA promoter driving the expression of the firefly luciferase gene (MtnA-luc) or a construct in which the WT MtnA TATA box was mutated and a consensus DPE sequence was inserted downstream from the transcription start site (mMtnA+DPE-luc) to transfect S2 cells that were treated with dsRNA directed against individual TAFs. After 3 days, the cells were treated with copper to induce transcription. Luciferase expression was analyzed and normalized to a cotransfected control vector containing the actin 5C promoter driving expression of Renilla luciferase. As expected, targeted depletion of TAF1, TAF4, or TAF5 had either no effect or slightly increased expression of the WT MtnA-luc promoter (Fig. 4). By contrast, depletion of TAF1 resulted in a severe decrease (five times) in luciferase expression from the mMtnA+DPE-luc construct, indicating that the TATA-less but DPE-containing core promoter architecture reveals a significant role for TFIID at this altered promoter compared with the WT MtnA promoter. Not surprisingly, targeting TAF4 by RNAi also resulted in a substantial decrease in transcription from the mutant MtnA promoter, although the effect was not as severe as the decrease observed when TAF1 is depleted. This result was somewhat surprising because TAF4 RNAi results in the degradation of most of the TAFs, including TAF1, as well as a significant amount of TBP. We suspect that the “TFIID” activity detected in the TAF4 RNAi cells is likely due to a residual amount of TAF1 remaining in these cells (see Fig. 1A). In contrast to the depletion of TAF4 by RNAi, the targeted reduction of TAF5 levels had no detectable effect on the mMtnA+DPE-luc promoter, which was certainly unexpected because of the previously observed similarities between the effects of knocking down TAF4 and TAF5 on overall TFIID stability (Fig. 1A). This intriguing result suggests that a reduction of TAF1 levels alone during TAF4 depletion is not likely to be solely responsible for the observed reduction in mMtnA+DPE-luc reporter activity. Instead, these findings suggest that TAF4 may be specifically required to potentiate activation of the altered promoter. Alternatively, it is possible that a TFIID component that we have not specifically monitored becomes differentially depleted between the TAF4 and TAF5 knockdown cells. These data also suggest that the stable core subcomplex may not be required for full activation of a TFIID-dependent promoter. Our analysis of TFIID integrity coupled with the functional characterization of the MtnA promoter reveals a specific role for both TAF1 and TAF4 in transcription activation from a TATA-less, DPE-containing promoter that is not observed with a TATA-containing, DPE-less promoter. These results, taken together, suggest highly gene-specific and TAF subunit-selective transactions that contribute to the formation of an “activated” preinitiation complex at endogenous promoters.
Fig. 4.
Transcription from a TATA-less, DPE-containing promoter requires TAF1 and TAF4. (A) Diagrams of the reporter constructs used to transfect S2 cells. (Upper) WT MtnA promoter driving luciferase expression. It contains a TATA box (TATAAAA) and an initiator (TCAGTT), but no DPE (AATCATC starting at position +28). (Lower) WT MtnA promoter with a mutated TATA box (GCGCCCC) and a DPE (AGACGTG) inserted at position +28 from the transcription start site. (B) S2 cells were either left untreated (NT) or treated with dsRNA directed against TAF1, TAF4, or TAF5 and transfected with either the WT MtnA-luc reporter or the mMtnA+DPE-luc reporter and actin-Renilla to control for transfection efficiency. After 3 days, the transfected cells were treated with copper to induce transcription. Six hours later, luciferase expression was determined, normalized to Renilla expression, and plotted as fraction of untreated luminescence.
Discussion
Model of Holo-TFIID Assembly.
The findings described above allow us to build a model of TFIID assembly wherein the TAF4 CTR participates in forming a stable core subcomplex along with the TAF6 NTR. Because the depletion of TAF5, TAF9, or TAF12 by RNAi results in the depletion of many of the other TFIID subunits, they are also likely to be part of the stable core subcomplex. We postulate that after formation of this stable core subcomplex, holo-TFIID is subsequently generated by the addition of TBP, TAF1, TAF2, and TAF11 as peripheral subunits (Fig. 5).
Fig. 5.
Model of TFIID assembly in vivo. TFIID consists of a stable core subcomplex made up of TAF4, TAF5, TAF6, TAF9, and TAF12, which becomes decorated with TBP, TAF1, TAF2, and TAF11. Subunit stoichiometry is adapted from Sanders et al. (7).
Assembly of Multiple Distinct TAF-Containing Complexes.
In addition to representing key subunits of TFIID, a subset of the TAFs are also found in other multiprotein complexes, such as Spt-Ada-Gin5-acetyltransferase (SAGA) and TBP-free TAF-containing complex (TFTC) (16–18). It is interesting to note that the stable core subcomplex described here, although not found in yeast SAGA, is found in the human TFTC complex. Indeed, the characteristic trilobed architecture of holo-TFIID can also be seen in human TFTC (6). Furthermore, a subcomplex of human TAF4, TAF5, TAF6, TAF8, TAF9, TAF10, and TAF12, which contains our stable core TAF subunits, plus TAF8 and TAF10, also can form a trilobed structure when coexpressed in insect cells (4). This finding suggests that the TFIID-specific peripheral subunits (i.e., TAF1, TAF11, and TBP) may not be necessary to form a trilobed architecture. The Drosophila SAGA- or TFTC-like complex has not been thoroughly characterized, so it is not known whether all of the TAF components of the core subcomplex are also found in these other complexes. However, TAF5, TAF9, and TAF10 were indeed found in a SAGA-like complex, together with other proteins associated with the human TFTC complex, such as GCN5, Spt3, and Tra1 (19). We therefore speculate that the stable core subcomplex we have identified in this study may serve as a common precursor to both TFIID and TFTC-like complexes. For the generation of TFIID, TBP, TAF1, TAF2, and TAF11 would be representative peripheral subunits decorating the stable core subcomplex, as described above. To generate a TFTC-like complex, dGCN5, dSpt3, dTra1, and dAda2b would serve as alternative peripheral subunits associated with the same stable core subcomplex that is nucleated by TAF4 and TAF5.
Testis-Specific Partial Core Subcomplex.
In addition to the 8–12 ubiquitously expressed TAFs, recent reports have identified tissue-specific TAF homologs in both Drosophila and mammals. Although only one tissue-specific TAF has been characterized in mammals [TAF4b (20)], five tissue-specific TAF homologs have been described in Drosophila (21, 22). These tissue-specific TAFs are expressed in the developing spermatocytes within the testis and are termed no hitter (nht), cannonball (can), meiosis I arrest (mia), spermatocyte arrest (sa), and ryan express (rye), which are cell-type-specific homologs of TAF4, TAF5, TAF6, TAF8, and TAF12, respectively. Remarkably, with the exception of TAF9, there is a testis-specific homolog corresponding to each of the subunits of the stable core subcomplex described here, which suggests that a distinct tissue-specific core subcomplex may be formed in the testis. Because the testis TAFs have not been characterized biochemically, it is not known whether these tissue-specific TAFs associate with the ubiquitously expressed TAFs or TBP. It will be interesting to discover whether the testis TAFs form a stable core subcomplex similar to their ubiquitously expressed counterparts that serves as a platform for the generation of a tissue-specific holo-TFIID or TFTC, or whether they have evolved to scaffold an entirely different set of proteins.
Core Promoter Architecture-Specific Activity of TAF1 and TAF4.
Using two reporter constructs, one containing the WT TATA-containing, DPE-less MtnA promoter and the other containing the same promoter with the TATA box mutated and a DPE inserted downstream, coupled with RNAi knockdown of specific TAFs, we found that transcription from the MtnA promoter becomes significantly more dependent on TFIID when the TATA box is mutated and a DPE is inserted. Transcription from this altered promoter depended heavily on TAF1 and TAF4, but not on TAF5, TAF6, or TAF9. Previous studies identified a conserved C-terminal region of yeast TAF1 that was required for association of TFIID with endogenous promoters (23). Perhaps this same region of Drosophila TAF1 is required for TFIID to bind efficiently to TATA-less, DPE-containing promoters but is dispensable at promoters containing a TATA box. In strong TATA-containing promoters, the TBP–TATA interaction may be sufficient for stable binding of the complex to promoter DNA. Another possibility is that one or more of the multiple enzymatic activities of TAF1 become necessary for full activation of the MtnA promoter when the TATA box is mutated and a DPE is inserted.
Although TAF4 has long been known to have coactivator activity, our results suggest that it may also influence, directly or indirectly, core promoter recognition because a change in core promoter elements at the MtnA promoter led to an increased dependence on TAF4 for transcription activation. An alternative possibility is that the change from a TATA-containing, DPE-less core promoter to a TATA-less, DPE-containing promoter may unmask alternative coactivator usage, causing the activator, MTF-1, to rely more heavily on TAF4 as a coactivator. Indeed, activators have been known to target multiple coactivators and other components of the transcription machinery (24). Our in vivo functional analysis suggests an intriguing role for both TAF1 and TAF4 in mediating transcription from a TATA-less, DPE-containing promoter that is not apparent when using a TATA-containing, DPE-less promoter.
Materials and Methods
Cell Culture and RNAi.
S2 cells were grown at 25°C in M3+BPYE medium (Drosophila Genomics Resource Center, Bloomington, IN). RNAi was performed as described by using 20 μg of dsRNA per 106 cells (25). Oligonucleotide sequences used to generate RNAi constructs are available on request.
Extract Preparation and Immunoprecipitations.
Whole-cell lysates were prepared by harvesting cells, washing once with 1× PBS, and lysing cells in 1× SDS sample buffer. Nuclear extracts were prepared as follows. Approximately 108 cells were harvested and washed once with 1× PBS. All subsequent steps were performed at 4°C or on ice. The cells were resuspended in 2 packed-cell pellet vol of buffer I (15 mM Hepes, pH 7.6/10 mM KCl/2 mM MgCl2/0.5 mM EDTA/0.5 mM EGTA/350 mM sucrose/1 mM DTT/0.2 mM PMSF). Triton X-100 was added to 0.1%, and cells were processed with a Dounce homogenizer. Nuclei were then pelleted at 7,650 × g and resuspended in 4 packed-cell pellet vol of buffer AB [15 mM Hepes, pH 7.6/110 mM KCl/2 mM MgCl2/0.5 mM EDTA/0.5 mM EGTA/1 mM DTT/0.2 mM PMSF/1× Complete protease inhibitor (Roche, Indianapolis, IN)]. Nuclei were lysed by adding 1/10th vol of 4 M (NH4)2SO4 and then mixing for 30 min. The lysate was cleared by centrifugation at 37,000 rpm in a TL-100 ultracentrifuge (Beckman, Mountain View, CA). Next, the extract was precipitated with 1 vol of saturated (NH4)2SO4, centrifuged at 16,000 × g, and dissolved in 0.2 packed-cell pellet vol of buffer C [25 mM Hepes, pH 7.6/150 mM KCl/0.1 mM EDTA/10% (vol/vol) glycerol/1 mM DTT/0.2 mM PMSF/1× Complete protease inhibitor]. The nuclear extract was desalted by using a 0.5-ml Sephadex G-25 (GE Healthcare, Uppsala, Sweden) spin column equilibrated with buffer C. Immunoprecipitations were performed by adding 25 μl of antibody-saturated protein G–Sepharose beads (GE Healthcare) or M2–agarose (Sigma, St. Louis, MO) to ≈150 μg of nuclear extract diluted to 250 μl in 1× PBS and mixing overnight at 4°C. The beads were then washed five times with 1× PBS plus 0.5 M NaCl and 0.1% Triton X-100 and eluted with 2 bead vol of 1× PBS plus 0.5% N-lauroylsarcosine.
TAF Truncation Constructs and Cell Lines.
The TAF4 CTR construct was made by PCR-amplifying a DNA fragment corresponding to residues 623–921 and ligating the fragment in frame into a vector containing the MtnA promoter, an N-terminal 3× FLAG tag, and the blasticidin-resistance gene. This vector was used to transfect S2 cells, and resistant cells were selected with blasticidin. The TAF6 NTR and CTR were made as described above. The TAF6 NTR construct encodes residues 1–227, and the TAF6 CTR construct encodes residues 230–592. Expression of the TAF truncations was induced with 0.5 mM CuSO4. Oligonucleotide sequences used are available on request.
Luciferase Assays.
The MtnA-luc reporter was described in ref. 8. The mMtnA+DPE reporter was made by mutating the TATA box of the MtnA-luc construct by PCR-based mutagenesis. A DPE was then inserted downstream from the transcription start site by using the same technique. The TATA mutation and DPE sequence are described in ref. 26. Cells were transfected in triplicate by using Effectene reagent (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Transcription from the reporters was induced by adding 0.5 mM copper sulfate for 6 h. Luciferase assays were performed by using the Dual-Luciferase assay system (Promega, Madison, WI) according to the manufacturer’s recommendations. Data presented in Fig. 4B represent the average of three independent experiments.
Antibodies.
The anti-TAF1 30H9 (3), anti-TAF2 (27), anti-TAF4 3E12 (8), anti-TAF5 3D10 (28), and anti-TAF9 4H6 (29) antibodies were described previously. The anti-TAF6 25B4 antibody and the anti-TBP 3C3 antibody were screened from previously described hybridomas (11). The M2 anti-FLAG antibody (Sigma) was used to detect and immunoprecipitate the TAF4 CTR, TAF6 NTR, and TAF6 CTR.
Acknowledgments
We thank M. Deato and Y. Fong for the critical review of the manuscript, M. Haggart for technical assistance, and members of the Tjian laboratory for helpful suggestions.
Glossary
Abbreviations
- CTR
C-terminal region
- DPE
downstream core promoter element
- luc
luciferase
- MtnA
metallothionein A
- NTR
N-terminal region
- S2 cells
Drosophila Schneider 2 cells
- SAGA
Spt-Ada-Gin5-acetyltransferase
- TAF
TBP-associated factor
- TBP
TATA box-binding protein
- TFTC
TBP-free TAF-containing complex.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Lemon B., Tjian R. Genes Dev. 2000;14:2551–2569. doi: 10.1101/gad.831000. [DOI] [PubMed] [Google Scholar]
- 2.Albright S. R., Tjian R. Gene. 2000;242:1–13. doi: 10.1016/s0378-1119(99)00495-3. [DOI] [PubMed] [Google Scholar]
- 3.Weinzierl R. O., Dynlacht B. D., Tjian R. Nature. 1993;362:511–517. doi: 10.1038/362511a0. [DOI] [PubMed] [Google Scholar]
- 4.Leurent C., Sanders S. L., Demeny M. A., Garbett K. A., Ruhlmann C., Weil P. A., Tora L., Schultz P. EMBO J. 2004;23:719–727. doi: 10.1038/sj.emboj.7600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andel F., III, Ladurner A. G., Inouye C., Tjian R., Nogales E. Science. 1999;286:2153–2156. doi: 10.1126/science.286.5447.2153. [DOI] [PubMed] [Google Scholar]
- 6.Brand M., Leurent C., Mallouh V., Tora L., Schultz P. Science. 1999;286:2151–2153. doi: 10.1126/science.286.5447.2151. [DOI] [PubMed] [Google Scholar]
- 7.Sanders S. L., Garbett K. A., Weil P. A. Mol. Cell. Biol. 2002;22:6000–6013. doi: 10.1128/MCB.22.16.6000-6013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marr M. T., II, Isogai Y., Wright K. J., Tjian R. Genes Dev. 2006;20:1458–1469. doi: 10.1101/gad.1418806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takada S., Lis J. T., Zhou S., Tjian R. Cell. 2000;101:459–469. doi: 10.1016/s0092-8674(00)80857-0. [DOI] [PubMed] [Google Scholar]
- 10.Comai L., Tanese N., Tjian R. Cell. 1992;68:965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- 11.Hoey T., Weinzierl R. O., Gill G., Chen J. L., Dynlacht B. D., Tjian R. Cell. 1993;72:247–260. doi: 10.1016/0092-8674(93)90664-c. [DOI] [PubMed] [Google Scholar]
- 12.Tanese N., Saluja D., Vassallo M. F., Chen J. L., Admon A. Proc. Natl. Acad. Sci. USA. 1996;93:13611–13616. doi: 10.1073/pnas.93.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furukawa T., Tanese N. J. Biol. Chem. 2000;275:29847–29856. doi: 10.1074/jbc.M002989200. [DOI] [PubMed] [Google Scholar]
- 14.Basehoar A. D., Zanton S. J., Pugh B. F. Cell. 2004;116:699–709. doi: 10.1016/s0092-8674(04)00205-3. [DOI] [PubMed] [Google Scholar]
- 15.Burke T. W., Kadonaga J. T. Genes Dev. 1997;11:3020–3031. doi: 10.1101/gad.11.22.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant P. A., Schieltz D., Pray-Grant M. G., Steger D. J., Reese J. C., Yates J. R., III, Workman J. L. Cell. 1998;94:45–53. doi: 10.1016/s0092-8674(00)81220-9. [DOI] [PubMed] [Google Scholar]
- 17.Ogryzko V. V., Kotani T., Zhang X., Schiltz R. L., Howard T., Yang X. J., Howard B. H., Qin J., Nakatani Y. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 18.Wieczorek E., Brand M., Jacq X., Tora L. Nature. 1998;393:187–191. doi: 10.1038/30283. [DOI] [PubMed] [Google Scholar]
- 19.Kusch T., Guelman S., Abmayr S. M., Workman J. L. Mol. Cell. Biol. 2003;23:3305–3319. doi: 10.1128/MCB.23.9.3305-3319.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dikstein R., Zhou S., Tjian R. Cell. 1996;87:137–146. doi: 10.1016/s0092-8674(00)81330-6. [DOI] [PubMed] [Google Scholar]
- 21.Hiller M. A., Lin T. Y., Wood C., Fuller M. T. Genes Dev. 2001;15:1021–1030. doi: 10.1101/gad.869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiller M., Chen X., Pringle M. J., Suchorolski M., Sancak Y., Viswanathan S., Bolival B., Lin T. Y., Marino S., Fuller M. T. Development (Cambridge, U.K.) 2004;131:5297–5308. doi: 10.1242/dev.01314. [DOI] [PubMed] [Google Scholar]
- 23.Mencia M., Struhl K. Mol. Cell. Biol. 2001;21:1145–1154. doi: 10.1128/MCB.21.4.1145-1154.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triezenberg S. J. Curr. Opin. Genet. Dev. 1995;5:190–196. doi: 10.1016/0959-437x(95)80007-7. [DOI] [PubMed] [Google Scholar]
- 25.Worby C. A., Simonson-Leff N., Dixon J. E. Sci. STKE. 2001;2001:PL1. doi: 10.1126/stke.2001.95.pl1. [DOI] [PubMed] [Google Scholar]
- 26.Butler J. E., Kadonaga J. T. Genes Dev. 2001;15:2515–2519. doi: 10.1101/gad.924301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verrijzer C. P., Yokomori K., Chen J. L., Tjian R. Science. 1994;264:933–941. doi: 10.1126/science.8178153. [DOI] [PubMed] [Google Scholar]
- 28.Dynlacht B. D., Weinzierl R. O., Admon A., Tjian R. Nature. 1993;363:176–179. doi: 10.1038/363176a0. [DOI] [PubMed] [Google Scholar]
- 29.Goodrich J. A., Hoey T., Thut C. J., Admon A., Tjian R. Cell. 1993;75:519–530. doi: 10.1016/0092-8674(93)90386-5. [DOI] [PubMed] [Google Scholar]