Abstract
We review here a new approach to mapping the human cerebral cortex into distinct subdivisions. Unlike cytoarchitecture or traditional functional imaging, it does not rely on specific anatomical markers or functional hypotheses. Instead, we propose that the unique activity time course (ATC) of each cortical subdivision, elicited during natural conditions, acts as a temporal fingerprint that can be used to segregate cortical subdivisions, map their spatial extent, and reveal their functional and potentially anatomical connectivity. We argue that since the modular organisation of the brain and its connectivity evolved and developed in natural conditions, these are optimal for revealing its organisation. We review the concepts, methodology and first results of this approach, relying on data obtained with functional magnetic resonance imaging (fMRI) when volunteers viewed traditional stimuli or a James Bond movie. Independent component analysis (ICA) was used to identify voxels belonging to distinct functional subdivisions, based on their differential spatio-temporal fingerprints. Many more regions could be segregated during natural viewing, demonstrating that the complexity of natural stimuli leads to more differential responses in more functional modules. We demonstrate that, in a single experiment, a multitude of distinct regions can be identified across the whole brain, even within the visual cortex, including areas V1, V4 and V5. This differentiation is based entirely on the differential ATCs of different areas during natural viewing. Distinct areas can therefore be identified without any a priori hypothesis about their function or spatial location. The areas we identified corresponded anatomically across subjects, and their ATCs showed highly area-specific inter-subject correlations. Furthermore, natural conditions led to a significant de-correlation of interregional ATCs compared to rest, indicating an increase in regional specificity during natural conditions. In contrast, the correlation between ATCs of distant regions of known substantial anatomical connections increased and reflected their known anatomical connectivity pattern. We demonstrate this using the example of the language network involving Broca's and Wernicke's area and homologous areas in the two hemispheres. In conclusion, this new approach to brain mapping may not only serve to identify novel functional subdivisions, but to reveal their connectivity as well.
Keywords: fMRI, independent component analysis, cytoarchitecture, functional neuroconnectivity, movie, natural vision
1. Chronoarchitecture: the principle of functional independence and natural conditions
One hundred years after their derivation, the cytoarchitectonic and myeloarchitectonic maps of the cerebral cortex derived by Brodmann (1905, 1909), Campbell (1905), Vogt & Vogt (1919), von Economo & Koskinas (1925) and others are, remarkably, still in use. This may seem surprising given that there are many subdivisions that they have not revealed and given the advent of new imaging techniques of infinitely greater sophistication. Perhaps one important clue to their success lies in the fact that they do not hypothesize about functions. Instead, their only hypothesis is that anatomical subdivisions will reflect functional subdivisions. By contrast, activations obtained in neuroimaging experiments depend heavily on the exact nature of the stimuli used, and their interpretation is heavily hypothesis-driven (Friston et al. 1995; Frackowiak et al. 1997). Moreover, the same cortical region (e.g. parietal cortex) may be activated in a variety of different ways, including more or less of its functional subdivisions, depending upon the stimulation paradigm used. While such a picture may well reveal the functional complexity of the parietal cortex, it also makes it difficult to subdivide it in a compelling way. The challenge of mapping cortical subdivisions thus lies in finding the right anatomical or functional markers. Unfortunately, there are relatively few functional maps provided by modern neuroimaging experiments in the human that can be reproduced reliably, such as that of area V5 or of retinotopically mapped visual regions, while extent and functional interpretation of activations in large parts of the cortex, such as parietal, temporal or frontal regions, vary with the stimuli and with changing insight. Equally, despite their reliability, cytoarchitectonic maps (Brodmann 1909) or other anatomical mapping techniques like recent attempts of metabolic or molecular maps (Livingstone & Hubel 1984; Zilles & Clarke 1997) have provided relatively selective cerebral maps that do not nearly reveal all the functional and structural subdivisions of the cortex or that apply only to subregions. This is perhaps best illustrated in the case of cytoarchitectonic area 18, which has been shown in subsequent physiological and anatomical studies to contain at least four further areas that differ significantly in their functions. Among these are areas V2, V3, V3A and V4 in the monkey (Zeki 1978). Modern multi modal databases that combine maps derived from several methods are more powerful as they combine the strengths of individual methods, and together with large databases and new approaches, such as computer-aided high-resolution anatomical mapping, will without doubt provide more powerful tools in the future (Mazziotta et al. 2001; Roland et al. 2001; Van Essen 2002).
(a) Chronoarchitecture
Here, we review a new way of mapping the functional subdivisions of the brain introduced recently (Bartels & Zeki 2004a,b, 2005). It is based on time and we therefore term the maps derived from it as chronoarchitectonic maps. Just as the sole working hypothesis of cytoarchitectonic and myeloarchitectonic methods is that functional differences will be reflected in anatomical differences, so too is the hypothesis of our approach that each specialized cortical area or even subarea of the brain will have its own specific, and characteristic, ATC during natural conditions. Chronoarchitectonic maps are thus derived from the analysis of ATCs of part or the whole brain, ideally at a high temporal and spatial resolution. We hypothesize that the dynamic complexity of more natural stimuli that include a variety of attributes that the brain originally evolved to deal with is most likely to activate a maximal number of areas differentially. Therefore, our approach does not rely on specific anatomical markers that are limited to revealing only a subset of the cortical subdivisions, such as differences in cellular compositions (cytoarchitecture), the degree of myelination (myeloarchitecture) or metabolic activity of cytochrome oxidase. Nor does it depend on functional hypotheses, such as traditional neuroimaging experiments, which can only reveal particular regions that are ‘targeted’ by a priori hypotheses and particular stimuli. Instead, our approach exploits the fact that distinct cortical and subcortical subdivisions differ in their ATC in natural conditions, by virtue of a complex of factors. This includes differences in their functional feature preference and thus in processing loads, differences in their structure, such as myelination and hence speed of conduction, as well as other synaptic, cellular and molecular properties. We argue that this provides a ‘temporal fingerprint’ that is unique to each area, allowing one to map distinct areas of the cortex, just as Brodmann, Campbell and others used ‘cellular fingerprints’ for their maps. This is what constitutes the chronoarchitecture of the cortex. As it is a consequence of the functional and structural organization of the cortex, it does not reveal a cortical map that is distinct from that derived from anatomical tools or through classical functional mapping techniques. However, it is based on a marker that is omnipresent and inherently as specific to each subdivision as its functional and structural distinctness. Moreover, because it does not rely on a single functional hypothesis or on a single anatomical property, it may provide maps of the cerebral cortex that are more extensive than those provided by the more traditional methods. In the present paper, we introduce the conceptual framework for this new approach, and demonstrate its power on the example of imaging data collected while subjects viewed a James Bond film, resulting in a successful segregation of known regions in the visual and auditory/language systems. Naturally, chronoarchitectonic maps are not limited to fMRI or the specific analysis used (ICA in this case). Different, future methods of data collection with better spatial or temporal resolution combined with improved computational segregation tools may yield chronoarchitectonic maps of more detail. In addition, our stimulation only covered audio-visual aspects of brain activity occurring in natural conditions and improved or alternative experimental settings may reveal subdivisions in higher cognitive, motor or emotional systems in more detail. Combined with modern multi modal databases and a solid quantification of spatial extent and reliability, these maps can then be combined and compared with existing ones derived from other methods (Mazziotta et al. 2001; Roland et al. 2001; Van Essen 2002). It also is important to note that, in principle, every functional imaging experiment reveals a time-based map, no matter whether a hypothesis-driven or a data-driven analysis is used, since every experiment uses some ‘temporal fingerprint’ in the BOLD signal to identify specialized regions. However, in conventional experiments, the temporal marker is imposed by the experimenter, using a controlled and highly constrained set of stimuli specifically designed to activate only a specific subset of regions at predefined times. In addition, in the case of an epoch-based/blockwise stimulus presentation, an artificially high temporal correlation may be induced among regions even though they are involved in processing different aspects of the stimulus. Conventional experiments are thus ideal to reveal a subset of ‘targeted’ regions, in isolation or as a group, by relating them to a particular stimulus or task, and are thus captured well by the term ‘functional mapping’. In contrast to conventional experiments, the approach of chronoarchitecture seeks to activate as many regions as possible, as differentially as possible, with as few artificial constraints as possible, without any need of a priori hypotheses with regard to ATCs, all of which is diametrically opposed to the conventional approach. Hence, we use the term chronoarchitecture or time-based mapping for this approach that exploits inherent spatio-temporal fingerprints of distinct regions to identify them. The maps obtained with this ‘blind’ time-based anatomy should not differ from those obtained in traditional functional (or anatomical) experiments, but may reveal many more regions, including some with unknown functions, together with information about their potential connectivity, and can be obtained in a single experimental session.
(b) The principle of functional independence
The principle of functional independence accounts for our hypothesis that distinct subdivisions have distinct ATCs during natural conditions. Functional specialization entails that distinct regions have a preference to process distinct features, such as a preference for colour, motion or objects, or in the non visual domain, emotions, actions or reward (Zeki 1978). There are several reasons why functional specialization may have been an evolutionarily advantageous principle for the brain, even for a single modality such as vision. However, a critical reason from the present viewpoint is that distinct features can, and often do, vary independently of each other over time. We hypothesize that features that vary independently in natural conditions are processed in separate subdivisions. If two features in the environment covaried entirely, then, from a sensory and behavioural perspective, they would constitute a single feature, and there would have been no evolutionary need to process them in separate specialized subdivisions. Thus, we assume that every functional subdivision processes a feature that can vary independently of other features processed in other subdivisions. From this, it follows that since the brain evolved to cope with natural conditions, the most suitable way to activate its specialized regions, and each in its most specific way, is to expose it to the dynamic complexity of natural conditions. Accordingly, we have shown psychometrically for a small set of features that they vary with a surprisingly low degree of temporal correlation during natural conditions, and that the intensity with which each of these features is perceived correlates linearly with the intensity of activity in the regions specialized for this feature (Bartels & Zeki 2003). This is in accord with a later study showing that the peak activity of areas with known specialization correlated with the presence of the corresponding feature, consistently across subjects (Hasson et al. 2004). Our results further showed that the temporal correlation of activity between the distinct regions processing these distinct features was low, even within the visual system. Conversely, there were relatively high, significant and highly area-specific temporal correlations between anatomically corresponding regions across subjects who viewed the same film (see figure 10 and Bartels & Zeki 2003, 2004a). In addition, we showed that natural viewing activated manifold more regions differentially than traditional epoch-based studies (see figure 11 and Bartels & Zeki 2004a). These initial findings thus support our more general notion that distinct regions do in fact have distinct time courses of activity during more natural conditions, thus supporting the principle of functional independence. Note that the word ‘independence’ is not used in its strict statistical sense here, but it refers to the notion that distinct features may at times vary independently over time, leading to characteristic (even though not necessarily statistically independent) ATCs in the regions specialized for these features. For a more detailed discussion see Bartels & Zeki (2004b).
Figure 10.
Between-subject correlations of ATCs were specific to anatomically corresponding areas. For each of the 10 areas shown in figure 8, the mean inter subject correlation coefficients (r±s.e.m.) and the median inter subject ranks (rank±quartiles, normalized to 100) are shown. When, instead of anatomically corresponding areas, random areas out of the 10 (control 1: c1) or any random IC (control 2: c2) were chosen, correlations were no longer different from zero, and ranks not skewed towards zero. **p < 0.0001 (t-test on correlation coefficients.); p < 10−13 (Kolmogorov–Smirnov test on ranks). See figure 8 for abbreviations. (n): number of subjects with that area (from Bartels & Zeki 2004a).
Figure 11.
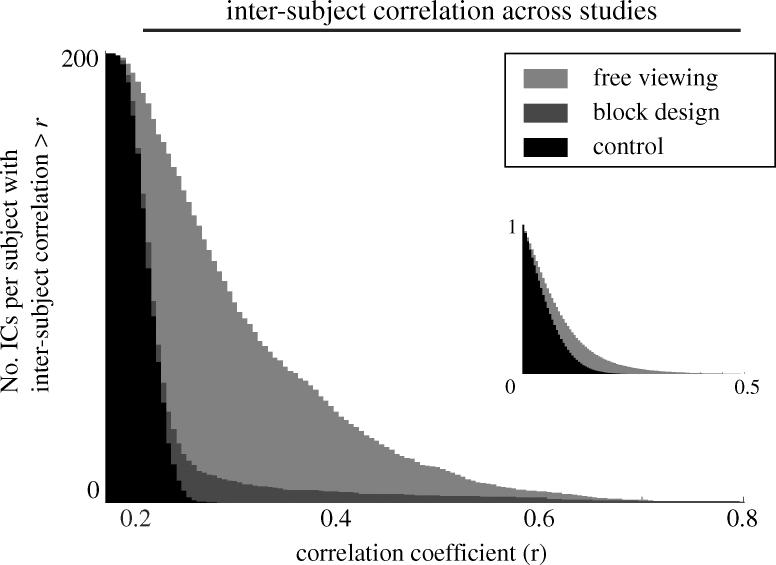
More areas were differentially activated during free viewing than during conventional epoch stimulation. Shown are the cumulative distributions of maximal inter subject correlations among all IC-ATCs and the normalized cumulative distributions of inter subject correlations (inset) (see Bartels & Zeki 2004a). Both indicate how many ICs have ATCs that correlate across subjects and therefore provide a measure for the number of stimulus-driven regions that were differentially activated in each study. Free viewing data are shown in red and epoch data in blue. Shown in black is the expected distribution for simulated random ATCs as a baseline (white noise convolved with the haemodynamic response function; from Bartels & Zeki 2004a).
(c) Natural conditions and ICA
Because the brain's subdivisions and its connectivity have evolved in natural conditions, we argue that these conditions will also be optimal to expose the brain's organization. Ideally, one would record brain activity for a very long period of time while the subject is engaged in various aspects of natural behaviour and stimulation. Given the constraints of the experimental setting, we had to limit ourselves in this study to the practically achievable. To optimize the distinctness of the ‘temporal fingerprints’ of distinct subdivisions, at least in the visual-auditory systems, we asked participants to freely watch a film. This is not exactly a natural condition, but one that approximates it given the constraints of the experimental setup. Certainly, it is more natural than most of the traditional imaging experiments, which use repeated, highly specific and often quite artificial stimuli to selectively activate a particular pathway. We expected film viewing to provide natural conditions at least for the visual and auditory system, and thus to lead to characteristic ATCs in distinct subdivisions within these systems. To achieve a segregation of subdivisions, for example, in motor, higher cognitive or emotional systems, one may have to use other types of complex natural stimuli that are better suited to involve these systems more extensively. Our fMRI recordings allowed us to measure activity throughout the brain with a spatial resolution of 3 × 3 × 3 mm, leading to about 60 000 voxels per whole-brain image every approximately 4 s. If distinct functional subdivisions were activated with distinct ATCs, then it would be a major challenge to identify and segregate voxels belonging to a given subdivision from the remaining voxels of the brain, given that we had no a priori hypothesis about their time courses. There are a variety of methods that could be applied to achieve such a data-driven dissection of the brain, each with its own benefits and pitfalls. These range from simple correlation analyses to more sophisticated clustering techniques. One of the most powerful methods to meet this challenge is ICA (Bell & Sejnowski 1995). ICA has the power to unmix a linear mixture of signals, based on measures of mutual independence. In the case of fMRI data, it segregates spatially independent clusters of voxels into separate ‘independent components’ (ICs), each constituting a spatial brain map, with an associated ATC. We provide a brief review of the sense and no-sense of applying data-driven methods such as ICA in this paper. For our approach, we expect distinct functional subdivisions to be isolated in distinct, spatially independent ICs.
In the following, we first provide a short historical overview of anatomical mapping methods that are as blind with regard to function as is our new approach proposed here. Then we provide a short review of the method of ICA applied to fMRI data also employed here, with examples of its application to traditional datasets. We then demonstrate how the brain can be ‘blindly’ dissected into its functionally distinct cortical subdivisions during natural conditions, based entirely on their unique temporal fingerprints (Bartels & Zeki 2004a). We conclude by extending our approach to mapping connectivity between distinct regions, which we propose is related to interregional correlations during natural vision (Bartels & Zeki 2005).
2. Anatomical maps and their relation to function
The concept underlying any anatomical, structural or architectural method is simple and enduring. First formally enunciated by Oskar & Cecile Vogt (Vogt & Vogt 1919), it merely states that architectural differences are indicative of functional differences and, conversely, that functional differences demand differences in architecture—the dominant and most successful theme in cortical studies. The enduring success of this approach is reflected in the continued use of the resulting brain maps today. Indeed, the approach has seen something of a spectacular comeback following the introduction of imaging studies and attests to the approach's conceptual soundness. Speaking in modern terms, brain function is implemented in the form of neuronal hardware. Thus, by definition, differences in function require differences in hardware, visible in terms of cell types, connectivity, synaptic and molecular structures. The principal methods used by the early cartographers of the cerebral cortex were those of cytoarchitectonics and myeloarchitectonics, the former revealing the manner in which cells are stacked upon one another in layers and the latter the pattern of myelination in different zones of the cerebral cortex (Flechsig 1901; Brodmann 1909). Irrespective of the shortcomings of these methods, they had two over-riding advantages. First, whatever architectural boundaries they delineated could be assumed to delineate functional differences. Second, the very nature of the anatomical approach, which is ‘blind’ with respect to the function, ensured a certain objectivity and longevity of the findings. This is especially apparent when compared with current, hypothesis-led imaging experiments, the results of which depend heavily on the particular paradigms used and rarely lead to an unchallenged attribution of function to a given area. With this supposition at its basis, cartographers such as Brodmann (1905), Campbell (1905), Vogt & Vogt (1919) and von Economo & Koskinas (1925) did not have to overly concern themselves with attaching a function to every subdivision that they managed to delineate. Instead, they hoped that future functional and clinical studies would validate their subdivisions. This has turned out to be true to some extent. Apart from the well-worn examples of the striate cortex and the motor cortex, there are several other architectural subdivisions that have proven to reflect, perhaps to an unexpected degree, a functional differentiation. One example is provided by the subdivisions of the parietal cortex. Although authorities may still disagree on whether the subdivisions of this cortex provided by Brodmann accurately reflect the functional picture that modern physiological evidence is providing, its separation from the rest of the prestriate cortex is reflected in its functional uniqueness. Equally, Brodmann's delineation of area 19 from area 18, at least in the upper reaches of the superior temporal sulcus, fairly accurately reflects the transition into area V5 or, to put it more accurately, into the V5 complex of areas.
Of the two techniques used by the early cartographers, the more successful and interesting has been the myeloarchitectonic method. This reveals the pattern of myelination in different areas. Among its successes are the delineation of an area in what used to be considered as ‘associational’ cortex that is myelinated at birth and coincides almost exactly with area V5 (Flechsig 1901; Watson et al. 1993), the sharp delineation of a boundary between areas 18 and 19, representing the transition to the cortex of area V5 as one moves rostrally across the cortex, and the delineation of several subdivisions in the fusiform gyrus (Lungwitz 1937), an early indication of a now well-established fact that the fusiform gyrus consists of several distinct areas that are apparently engaged in different tasks (Bartels & Zeki 2000a; Malach et al. 2002; Spiridon & Kanwisher 2002). However, there is another feature of myeloarchitectonic maps that has not been commented on to date. That is, that myeloarchitectonic maps, in theory, may give an indirect indication of the speed at which signals travel within or to a given region, based on the basic physiological knowledge that myelin enhances the speed of action potential propagation within the axon. Recent evidence confirmed this directly, suggesting that differential regional myelination yields constant latency irrespective of axonal distance, in this case between thalamus and cortex (Salami et al. 2003). The evolutionary reason for distinct myelination in distinct cortical regions is unclear—whether it may speed up signals of particular behavioural importance, or whether it is an attempt to synchronize percepts arising from regions with distinct processing delays is not known (see the hypothesis of perceptual synchronization; Bartels & Zeki 2004b). Whichever interpretation is true, myeloarchitectonic maps could be regarded, in a sense, as the anatomical reflection of chronoarchitectonic maps for the millisecond range and are probably related in part to the distinct signal arrival times observed across distinct regions, as well as the distinct, psychophysically determined, perceptual delays of different features (Moutoussis & Zeki 1997; Zeki & Bartels 1998a; Schmolesky et al. 1998).
However, modern studies have also shown the limitations of this anatomical approach. Perhaps one of the best-known examples is to be found in area 18 of Brodmann. While the cytoarchitectonic and myeloarchitectonic methods have not been able to show the subdivisions within area 18 that we now recognize, the fact remains that other techniques, for example, the technique of metabolic architecture (Wong-Riley 1979), have revealed a distinctive architecture for area V2—consisting of the well-known cycle of metabolically distinctive stripes (Livingstone & Hubel 1984)—and for area V3, consisting of a band of heavy metabolic activity (Jen & Zeki 1984). Moreover, functional studies have succeeded in associating distinct architectural subdivisions within individual areas such as V1 and V2—revealed by the technique of cytochrome oxidase architecture—with distinct functional groupings of cells (Livingstone & Hubel 1984; DeYoe & Van Essen 1985; Shipp & Zeki 1985; Xiao et al. 2003).
Thus, the statement that functional differences are reflected in architectural differences remains true today. We propose that the structural and functional borders can be inferred by the temporal marker of ATCs observed during natural conditions.
3. ICA applied to fMRI
(a) The method: review and intuition
Traditional fMRI data-analysis methods require knowledge of stimulus timing or of the location of a region of interest in order to identify and characterize cortical responses to the stimuli given (Friston et al. 1995a, Frith et al. 1995). In contrast, in our new mapping approach we want to rely on the differences of activity in distinct functional subdivisions, without any a priori knowledge about their activity wave forms or locations or indeed about their functions (even though the opposite is still possible, despite uncontrolled stimuli; Bartels & Zeki 2003). Thus, to obtain a more objective dissection of the brain without the biases associated with human hypotheses, we need to rely on a data-driven analysis method. This method should be able to identify voxels belonging to one functional subdivision and segregate them from the remaining voxels that belong to other functional subdivisions. We hypothesize that voxels belonging to a given functional subdivision will have higher temporal correlations (or more general temporal dependencies) among themselves compared with voxels belonging to other subdivisions. Of course, separate functional subdivisions may also have temporal dependencies as a consequence of anatomical connections or through common modulation through third regions, but we assume these to be smaller than those between voxels belonging to the same subdivision. An ideal segregation method would thus provide some sort of a hierarchical clustering result, revealing at one end of the hierarchy fine subdivisions of the brain such as visual areas V1, V4 or V5, and at the other end, their interconnections into networks of functionally connected regions (see also figure 13). For this first experimental approach of a ‘blind’ subdivision of the cortex, we used ICA, as this method seemed optimally suited to fulfil our needs. Based on information theory, ICA is a powerful method for unmixing or decomposing linear mixtures of independent sources, which need not be known a priori (Bell & Sejnowski 1995). The (mixed) data are iteratively fed through an unmixing matrix, which is adjusted such that the information in its output channels is maximized. This minimizes the mutual information among them, thus rendering them independent of each other. ICA can be applied to retrieve either spatially or temporally independent sources or optimize independence in both dimensions. In the case of EEG, it is usually applied to retrieve temporally independent sources. Each electrode can be considered to ‘listen’ to a linear mixture of various electrical sources active somewhere in the brain. Given n electrodes (or n mixed signals), ICA can then recover n temporally independent sources, each having an associated map of its spatial distribution. So far, this was probably the most successful application of ICA in neuroscience as it separated artefacts and various neural sources that contributed to the highly mixed EEG signal (Makeig et al. 1997, 2002). With fMRI, ICA has been mainly applied to retrieve spatially independent maps. One can consider various voxels to be active in various combinations, superimposed by artefactual signals (e.g. induced by head-movement). ICA can then separate n spatial mixtures (each one whole brain image) into n spatially independent maps, each of which has an associated time course. Other techniques such as PCA are less powerful for the identification of distinct brain activation patterns. The key weakness of PCA stems from its strength in other applications; namely, from its constraint to impose orthogonality (only with regard to second-order correlation) upon its data projection for both the temporal and the spatial aspects and to align it such that the first component accounts for the maximal amount of variance in the data. Principal components thus have a pronounced hierarchy in terms of the amount of variance they explain, and are entirely spatially and temporally uncorrelated. This is not suitable to reflect the brain's organization. First, there is no reason to assume that one task (or in our case, one functional subdivision) will account for more variance in the data than others. Second, many intersecting sets of brain regions have some temporal correlations of varying strengths with each other due to their rich yet specific connectivity, and even artefacts (e.g. those derived from movement) may be correlated with task-related activity. Thus, PCA is less likely to yield physiologically very meaningful results. Any algorithm trying to segregate the brain's areas, artefacts or task-related networks should therefore allow each component to account for similar amounts of variance, and allow for partial correlation in time, which spatial ICA does. Accordingly, McKeown et al. (1998b) demonstrated in the first application of ICA to fMRI data that ICA outperformed other data-driven methods for fMRI, and showed that ICA could isolate voxels whose ATCs correlated with the task condition or with artefactual signals into separate ICs. It should be noted, however, that if spatial ICA is used to detect functionally connected (i.e. temporally correlated) networks of areas, then it should be complemented by alternative methods (such as correlation analysis). The reason for this is that spatial ICA applies the independence criterion only to the spatial, not to the temporal, mode of its components. This means that while voxels co isolated into a single IC will have considerable temporal dependencies, it does not exclude the possibility that voxels of another IC may also be temporally related to them, even though at a reduced level (see also figure 12 and Bartels & Zeki 2005). Nevertheless, the original version of ICA has several shortcomings. Among them are some physiologically non plausible assumptions, for example, complete independence in either space or time, the absence of noise and the unknown number of sources underlying the original signal (Calhoun et al. 2001b; Esposito et al. 2002; Stone et al. 2002). Furthermore, ICA per se does not provide statistical measures that would allow inference at a single-subject or group level. Several groups have thus improved different aspects of the method since, and we will consider some of these in our future analyses (McKeown 2000; Nakada et al. 2000; Calhoun et al. 2001a,b,c; Gu et al. 2001; Nybakken et al. 2002; Stone et al. 2002; Suzuki et al. 2002; Beckmann & Smith 2004; Meyer-Baese et al. 2004a,b; Lange et al. 2004). A powerful improvement has been proposed by Beckmann & Smith (2004), whose probabilistic ICA allows for the presence of Gaussian noise in the mixing process and also for an estimation of the number of sources in the mixed signal. Meyer-Baese et al. (2004a,b) combine the strengths of Kohonen maps with ICA and also show that some clustering algorithms may outperform original versions of ICA. Another improvement that clearly outperforms spatial or temporal ICA has been proposed by Stone et al. (2002) who optimized the algorithm such that its assumptions better fit the physiological signal recorded using fMRI. Specifically, their skew spatio-temporal ICA extracts components that are maximized both in their spatial and temporal independence, while at the same time allowing for non independence, which is physiologically more plausible. At the same time, it allows for non symmetric skewness of the IC's probability density functions, which is also physiologically more plausible (Stone et al. 2002). In the studies reviewed here, we used the infomax spatial ICA proposed by Bell & Sejnowski (1995), as applied by McKeown, Jung et al. (1998). Despite its limitations, the comparison of the ICA's results with those obtained using traditional statistical tools in the epoch-based studies reviewed here, as well as the results obtained from natural viewing data, demonstrate its impressive power as a data-driven method and reveal the first time-based dissection of the visual and non visual brain into its functional subdivisions during natural conditions. Future analyses will without doubt benefit from the recent improved implementations of the method discussed above and from alternative methods.
Figure 13.
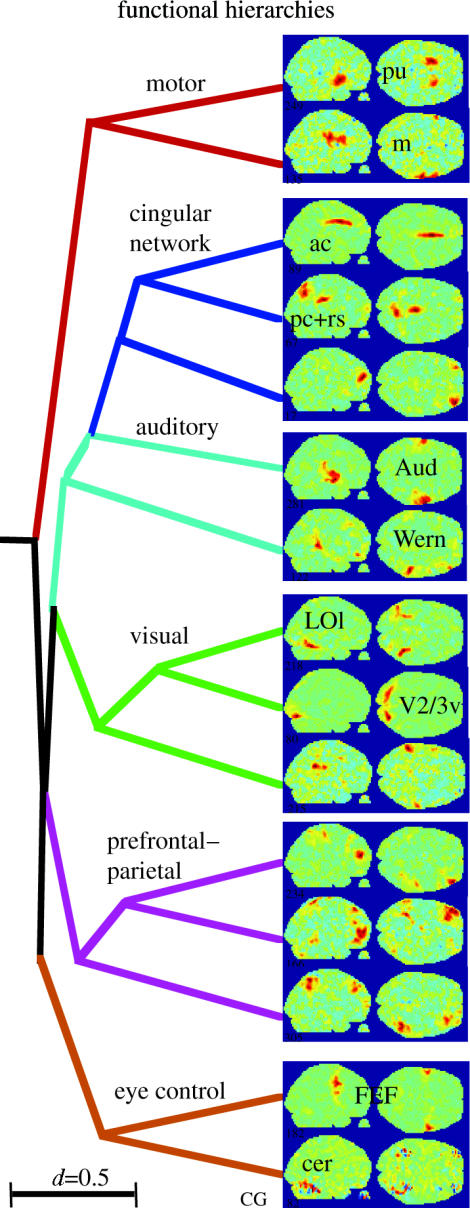
Hierarchical functional relationships between cortical areas revealed by the correlation of their ATCs. A clustering algorithm was used to group 15 representative ICs of one subject based on the correlation coefficients matrix of their ATCs (distance measure, d=1−|r|). Branches were coloured subsequently for graphical clarity. ac, anterior cingulate; cer, cerebellum; FEF, frontal eye fields; m, motor cortex; pu, putamen (from Bartels & Zeki 2004a).
Figure 12.
ICs and functional CMs of Wernicke's speech area and the primary auditory cortex on the example of four subjects (1–4). (a) ICs are shown colour-coded and superimposed on the subjects’ structural renderings (green: IC containing auditory cortex, red: IC containing Wernicke's area (BA22)). ICs were thresholded at 30% activity. (b,c) CMs derived from seeds taken from the hottest voxels in either BA22 or auditory cortex reveal their functional connections, which correspond to anatomical ones (modified from Bartels & Zeki 2004b, 2005).
(b) ICA and fMRI: a solution in search of a problem?
Despite this power to ‘blindly’ identify task-correlated or artefactual activity, ICA applied to fMRI has been seen by many as a solution in search of a problem. Both artefact removal and detection of task-related activity are predominantly (and very successfully) performed using traditional methods, which are often considerably easier to apply and more transparent, even though ICA has also been shown to reliably detect artefactual components (Friston et al. 1995, 1996; McKeown et al. 2003; Bartels & Zeki 2004a). Data-driven analysis methods are therefore only truly needed when there is uncertainty about the position and timing of activity, making their range of applications very limited. Truly uncontrolled fMRI experiments are extremely rare. It is sufficient to know just the onset of brain activity for powerful traditional statistical methods in order to characterize its waveform, to localize it and to compare it to different conditions (Friston et al. 1995a, Frith et al. 1995; Buchel et al. 1998; Henson et al. 2002). The timing of stimulation, or at least the onset of activity, is usually accessible to the experimenter even in experiments that do not fix such events a priori, for example, in the case of hallucinations, the reversal of visual illusions or other perceptually bistable stimuli, thus allowing for an analysis using traditional statistical methods (ffytche et al. 1998; Kleinschmidt et al. 1998; Lumer et al. 1998; Castelo-Branco et al. 2002). Thus, most ICA–fMRI studies have concentrated on improvements in the method, without demonstrating the need for its application. There are very few exceptions where the lack of a priori knowledge of the activation necessitated the use of a data-driven method, for example, during simulated car-driving or spontaneous signals during anaesthesia and rest (Calhoun et al. 2002; Kiviniemi et al. 2003; Van De Ven et al. 2004). Perhaps a combination of ICA with reliable statistical inference and software packages that more easily allow a combination of complementary methods will render ICA–fMRI applications more useful and widespread in the neuroimaging community (McKeown 2000; Calhoun et al. 2001a). However, our application of ICA to fMRI data collected during natural viewing is a model scenario for its use. We assume that the brain's exposure to more natural conditions will lead to distinct activity in each of the activated functional subdivisions. We aim to exploit this without applying any a priori knowledge about spatial location or activity time courses. This requires us to use a data-driven method to segregate groups of voxels from each other whose ATCs differ.
4. Experimental comparison of ICA and SPM in controlled datasets
Before we used ICA to segregate functional subdivisions based on their ‘temporal fingerprints’, which we hypothesized to be optimally present during natural conditions, we had to convince ourselves that ICA was capable of doing so. In this section, we review the application of ICA to four fMRI datasets from controlled experiments which we also analysed using conventional SPM (Friston et al. 1995). This allows a direct comparison of the ICA results with those of SPM. These experiments include studies on colour vision, romantic love, spatial attention and object recognition (Zeki & Bartels 1999; Bartels & Zeki 2000a,b, 2004a). Figures 1–4 provide a comparison of the results obtained with ICA with those obtained using SPM. The equivalence of the results is evident. Figure 1 shows activity evoked by coloured abstract ‘Mondrian’ stimuli in the fusiform gyrus in the ventral cortex, which contains two separate colour-selective regions, V4 and V4α. The SPM analysis revealed adjacent representations of upper and lower visual fields in V4, which was not evident in V4α, while ICA simply isolated both colour-selective regions together in one IC (Bartels & Zeki 2000a). Figure 2 shows two of the regions that were specifically active when volunteers viewed pictures of their partner/loved one (in comparison to gender-, age- and familiarity-matched friends), namely a subdivision of the anterior cingulate cortex and the medial insula (Bartels & Zeki 2000b). The same regions were also activated when mothers viewed their own children as opposed to other acquainted children or their best friend (Bartels & Zeki 2004c). ICA isolated these regions in two separate components, indicating a differential involvement and the associated time courses reveal their specific activation in the attachment condition (Bartels & Zeki 2000b). Figure 3 shows activation related to spatial attention to either colour or motion in each of four visual quadrants. While the stimuli remained identical, spatial attention modulated activity in visual areas in a retinotopic fashion. SPM and ICA revealed identical results (Zeki & Bartels 1999). It should be noted that after one region has been isolated in one IC, the same region is unlikely to appear in other ICs again, mainly because of the ICA's constraint of spatial independence. Thus, only the SPM analysis of this dataset revealed that the colour-selective regions V4 and V4α, and the motion-selective region V5 were modulated not only by the spatial aspect of attention, but also by the feature attended to within the attended quadrant (motion or colour), which was not apparent in ICA (Bartels & Zeki 2000a). Thus, while ICA proved to deliver reliable results, classical statistical analyses allowed for additional statistical comparisons. Finally, figure 4 shows results from an object recognition study in which objects and their scrambled counterparts were either moving or stationary and were alternated with blank stimuli. Both ICA and SPM revealed indistinguishable results and the results obtained using ICA were highly reproducible across subjects (Bartels & Zeki 2004a). We found that the reason for the variability in the spatial extent of early visual areas in the ICs across the five subjects lay in oversplitting of this region into several ICs in some of the subjects. This could be easily detected using CMs that revealed early visual cortex as a whole, and would presumably be less likely to happen with recent optimized versions of ICA. More importantly, early visual areas (V1/V2) had consistently different activity time courses compared with those of the motion-selective area V5, even within a single condition: V1 showed adaptation while V5 did not, which was consistent across subjects. This provides a hint that apart from their distinct feature selectivity, distinct regions exhibit distinct dynamics in their response, which will work to our advantage in the ‘blind’ dissection of the cortex during natural conditions. We thus conclude that spatial ICA applied to fMRI data is a powerful and reliable way of segregating temporally related voxels. In fact, it is so successful that its results are qualitatively equivalent to those obtained using statistical mapping and may at times supersede them, as they demonstrate specificity of activity in separate areas or temporal correlations in networks of areas rather than correlation with experimental tasks. For the analysis of traditional, controlled datasets, however, SPM offers the advantage of explicit statistical testing of hypotheses, making it preferable over data-driven approaches. Indeed, several others share this view (Friston 1998; Calhoun et al. 2001a; Nybakken et al. 2002; Quigley et al. 2002). It is therefore questionable whether data-driven approaches such as ICA will remain widely used unless they are improved to enable more sound statistical inference (McKeown 2000; Calhoun et al. 2001b). Another difficulty lies in the selection of ICs because various non-neuronal signal fluctuations such as movement, breathing and heart-rate changes may potentially lead to ICs that are difficult to discern from ones relating to neuronally induced signal changes. In our view, hypothesis-driven analyses, such as SPM, are thus currently preferable for most imaging experiments. We see the key application of data-driven approaches, such as ICA, in the determination of spatial activity patterns based on their differential activity time courses in completely uncontrolled experiments. Irrespective of the precise experimental settings, we refer to them as chronoarchitectonic mapping, as they rely entirely on temporal markers.
Figure 1.
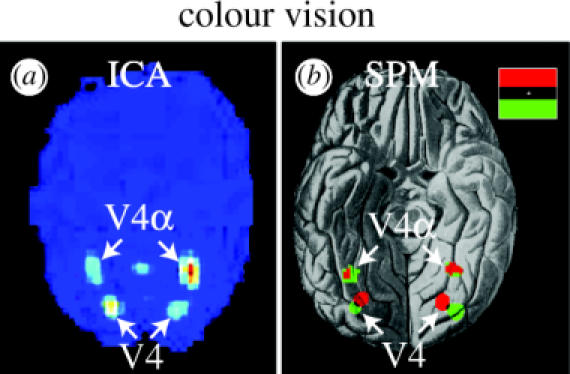
The colour-processing V4-complex in the human brain, revealed when subjects viewed coloured versus isochromatic stimuli in the lower or upper hemifield. (a) An IC obtained by ICA (glass brain view) containing the V4-complex in a single subject. (b) A composite image of SPM maps projected onto the ventral view of a human brain, showing colour-selective activity related to upper and lower hemifield stimulation (see inset for colour coding) (from Bartels & Zeki 2000a).
Figure 2.

Activity related to the subjective experience of romantic love. The SPM group analysis shown in (a) revealed, among other areas, activity in a region within the anterior cingulate cortex (ac) and the middle insula (I). (b,c) ICA applied to single subjects isolated these areas in separate ICs, indicating specific ATCs in each. ATCs are shown averaged across conditions, with the blue stripe indicating the ‘love condition’. Error bars: s.e.m. See figure 4 for colour code of ICs (from Bartels & Zeki 2000b).
Figure 3.
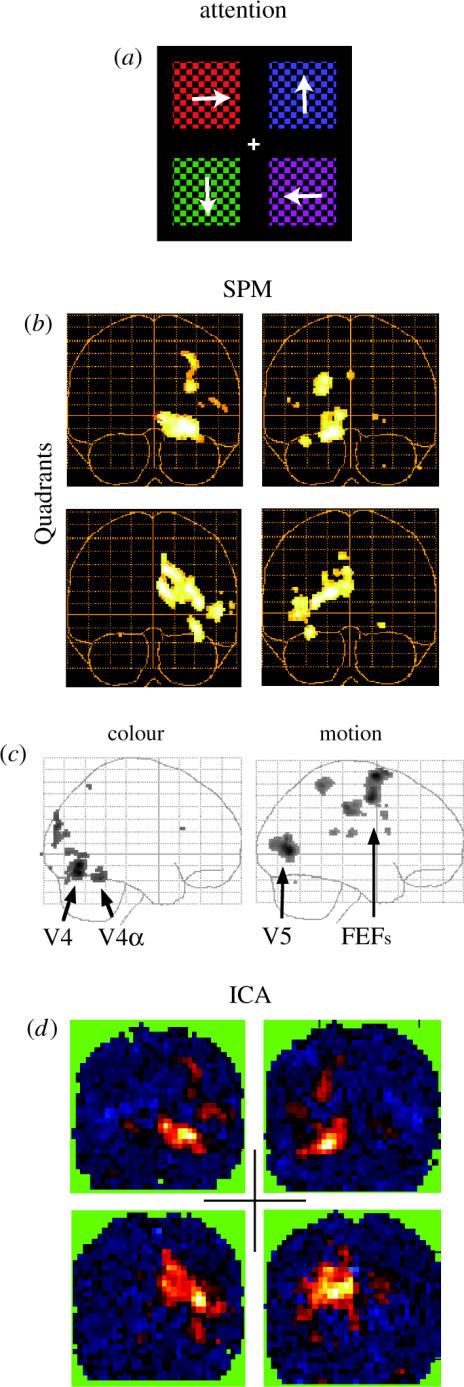
Attentional modulation of activity in the visual cortex. (a) Subjects fixated the middle of a screen while attending to either colour or motion in one of the four quadrants. (b,c) SPM analysis: (b) attention to different quadrants activated visual areas in a retinotopic fashion, shown as coronal glass brain views of a single subject arranged such that the one depicting attention to the top right is located in the top right, etc. (contrasts: attention to colour and motion in one quadrant versus attention to both attributes in the remaining three quadrants; p<0.05, corrected) (c) attention to colour or to motion reveals activity in V4 or in V5 (SPM contrasts: colour versus motion and vice versa). (d) ICA analysis (same subject as above): the ICs whose ATCs correlated most with the task conditions corresponded to the retinotopic attention maps shown in (b) (from Zeki & Bartels 1999; Bartels & Zeki 2000a).
Figure 4.
Comparison of SPM with ICA and reproducibility of ICA across subjects. Upper panel: analysis of single subject data from a traditional epoch design fMRI study on motion and object recognition. (a–c) The three ICs obtained by ICA whose ATCs correlated most with the stimulus conditions. They revealed differential involvement of early visual (V1/V2; a), visual motion selective (V5; b) and object selective (LOC) areas (c) in different stimulus conditions, as is apparent in the ATCs shown to their right. (d–f) Statistical parametric maps (SPMs; p<0.001, uncorrected) show the same cortical areas as revealed by ICA. BOLD signals taken from the most significant voxel are shown to the right of each SPM (averaged over condition repeats). Lower panel: consistent ICA results from additional four subjects of ICs containing early visual and motion selective regions. mScr, moving scramble; sObj, static object; mObj, moving object; sScr, static scramble; [all–Rest], all stimuli versus rest; [m–s], motion versus static; [Obj–Scr], objects versus scramble. Intensity maps of ICs have arbitrary units, green being neutral (i.e. no contribution to the variance). ATCs were averaged over the eight repeats of the conditions. Error bars: s.e.m. (from Bartels & Zeki 2004a).
5. The chronoarchitecture of the cerebral cortex
The perceptual richness and complexity of natural conditions that the brain evolved to deal with is likely to reveal the functional specialization of cortical regions optimally, as we explained above with the principle of functional independence. We approximated natural conditions by showing eight volunteers part of the James Bond film Tomorrow Never Dies (Bartels & Zeki 2004a). Note that this approach is diametrically opposed to that used in traditional fMRI experiments, which aim to differentially activate as few regions as possible by using as well-controlled stimuli as possible. Thus, the ‘blind’ nature of our approach required the development of some new techniques to obtain physiologically meaningful results, which we described in detail in our original publication (Bartels & Zeki 2004a). While the application of ICA to the free viewing fMRI data per se was identical to that in the traditional datasets, this time we could not use the onsets and timings of distinct conditions to select our ICs of interest. The selection of meaningful ICs is a major task in every ICA analysis, as there are as many ICs as original whole-brain scans, in our case up to 368 ICs per subject. Other groups have used PCA to reduce the amount of data before application of ICA, but we refrained from doing so because weak but physiologically important signals may get lost during this process. Firstly, variance of the fMRI signal due to neuronal processing can be smaller than that induced by artefacts such as breathing, head movement and so on. Low variance PCs may thus contain the data we are interested in keeping. Secondly, since some artefacts may be partially correlated with signal of neuronal origin, discarding PCs containing artefacts may also remove signal we are interested in due to PCA-imposed orthogonality. Thus, many of the ICs contain clusters of voxels that are not of primary interest to us in this context, such as ventricles, blood vessels and regions affected by head movements. Examples of such artefactual ICs are shown in figure 5. We used a conservative two-stage process to select ICs, thus ensuring that only ICs functionally involved in processing the stimulus were selected as determined by the significance of the intersubject correlations of their ATCs. In general, one can assume that the less stringent the selection criteria, the higher the probability of confusing some artefact with a functional subdivision. First, anatomical criteria such as location, extent and bilaterality were used to manually select candidate ICs, which reduced the original number to a few dozen per subject. In the second stage, we exploited anatomical and temporal correspondence of ICs between subjects. Functionally corresponding ICs of different subjects not only corresponded anatomically, but also showed significant inter subject correlations over time (see inter subject correlation and inter subject ranking in Bartels & Zeki 2004a). In addition, for each IC, a CM was calculated based on its most active voxel to confirm that the full spatial extent of the functional cluster was captured in the IC. This is a good method to check whether ICA super-fractionated a given area into small units (Bartels & Zeki 2004a, 2005).
Figure 5.
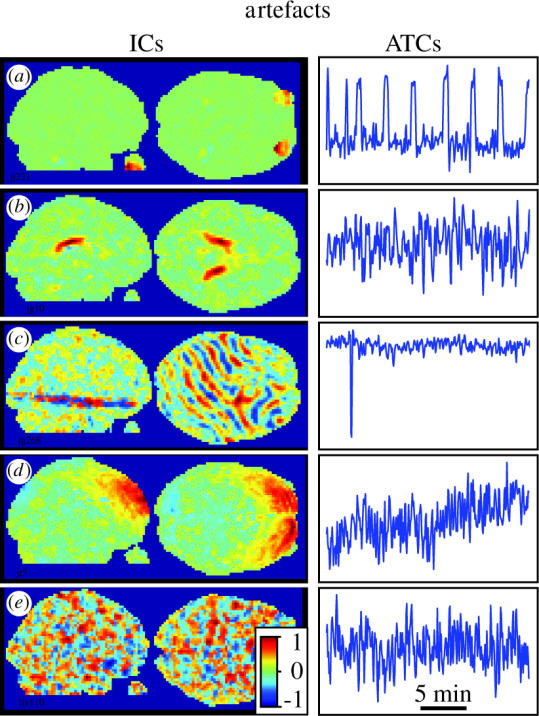
Representative artefacts isolated by ICA from natural viewing data, together with their ATCs. (a) Eyes. ATCs reflect eye movements, which are reduced during the eight blank periods that interrupted the film. (b) Part of the ventricles. In each subject the complete ventricles were isolated, but fractionated into separate ICs, probably due to fluid flow. (c) Scanner-induced ‘spike’ affecting only one slice at one point in time. (d) Movement artefact affecting a large region on the brain's surface, with a steadily increasing signal. (e) ‘Noise’ artefact. About 30% of ICs in our analyses contained this type of noise (from Bartels & Zeki 2004a).
(a) Results
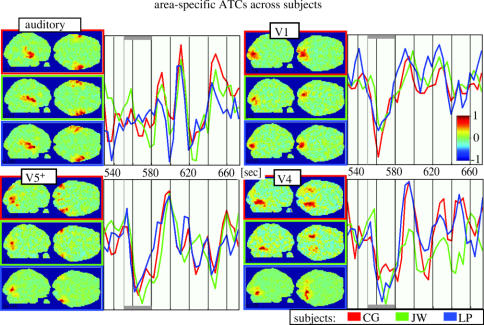
The results of this first attempt to obtain a time-based dissection of the brain are fascinating. In every single subject, ICA identified up to a dozen distinct regions, even within the visual cortex. Many of these regions corresponded not only anatomically but also temporally across subjects in a very area-specific way. Only anatomically corresponding regions achieved significant temporal inter subject correlation. This was not the case for anatomically non corresponding regions. This showed that anatomically corresponding regions were activated in a similar way in different subjects, thus proving that these ICs contained regions that were stimulus-driven and functionally specialized. Thus, in conjunction with natural viewing, ICA allowed us to identify and segregate distinct visual regions based on their activity time course alone. Figure 6 shows ICs of a single subject, each containing a functionally distinct bilateral region in the occipital (visual) cortex. The first 3 min of the ATCs associated to each of these ICs are shown next to them to illustrate how distinctly these visual regions responded during the same stimulus period. Each of the ICs shown for this subject was also present in the other subjects, with significant and area-specific intersubject correlations (see also figures 9 and 10; Bartels & Zeki 2004a). A single experiment of natural viewing thus allowed us to identify many of the visual areas known in the human, based on time alone. Figure 7 illustrates the key reason why this approach works. The BOLD signal correlations are plotted between the most active voxels of each cluster identified in the distinct ICs of figure 6. It is apparent that the temporal correlations between distinct cortical regions are very low during free viewing. In fact, the true correlation of neuronally induced BOLD signal may have been even lower since most artefacts in fMRI would, if anything, have contributed to global positive correlations (for detailed analyses see Bartels & Zeki 2004a, 2005). This is why ICA was able to isolate the voxels belonging to different regions in distinct ICs. Mainly the left/right counterparts of the same regions have high temporal correlations and were also grouped together in the same IC. Figure 8 shows the same (and some additional) ICs that were significantly inter subject correlated, superimposed on a structural brain, where each IC is displayed in a distinct colour. To illustrate the area specificity of the ATCs and their preservation across subjects who were exposed to the same film, we superimposed ATCs of corresponding ICs from three subjects in figure 9. The ATC of putative area V1, for example, is markedly distinct from that of putative area V5, while ATCs of the same area are very similar across subjects. This is quantified and statistically verified in figure 10 for the 10 cortical ICs/areas (71 across all subjects; see figure 8) identified most consistently in each subject. Each of these 10 ICs had significantly correlated ATCs with their anatomically corresponding counterparts across subjects, indicating that these areas were (i) stimulus-driven and (ii) processing corresponding features of the film. However, as a control for each subject, when one of the above 10 areas was randomly selected instead of grouping them as anatomically corresponding, this significant inter subject correlation was abolished completely. By proving that only anatomically corresponding regions had significant inter subject correlations, these results also show that each area has a time course that is specific to it, and that these findings cannot be accounted for by unspecific correlation of ATCs. Similar results were obtained when the ATC of one subject's IC was used to rank-order all ICs of the remaining subjects: the first ranks were occupied by ICs which corresponded anatomically to this initial IC (Bartels & Zeki 2004a, 2005). This time-based dissection was not limited to the visual cortex, but revealed many more cortical and sub cortical regions, some of which showed inter subject correlations, while others did not. Because the principle of functional independence (and our approach more generally) rests on the assumption that natural viewing conditions lead to more differential activity in more functional subdivisions in the brain, we wanted to compare the efficiency of natural conditions in eliciting differential activations with that of an epoch study. To obtain a more general and objective measure for the number of ICs with a significant inter subject correlation, we counted the average number of ICs per subject-pair that had an inter subject correlation of a given value, for example, r > 0.5 (which is a measure of the number of differentially activated, stimulus-driven regions). Conducted for all possible subject-pairs and correlation values, one obtains a distribution of inter subject correlations. This measure lends itself to a comparison of technically equivalent studies that used differential stimulation paradigms and provides a measure of the number of differentially activated, stimulus-driven regions. Figure 11 shows the distributions obtained for the natural viewing data, the epoch study of figure 4, and for a control of simulated random ATCs. The results were dramatic. For example, the average number of ICs per subject-pair which had an inter subject correlation of r>0.4 during natural viewing was more than eightfold higher compared with that during the epoch study. This demonstrates that more natural conditions (approximated here by watching an action film) lead to a dramatic increase not only in the number of regions activated, but also in the specificity in which they become active. As an example of non visual regions activated with specific and significant inter subject correlations, figure 12a shows the putative primary auditory cortex (BA 41/42) and putative Wernicke's area (BA 22), each isolated in a distinct IC. Thus far, we have used the differential ATCs only as a marker to identify distinct cortical subdivisions. However, another advantage inherent to using ATCs compared with other markers is that they may contain information about the mutual interaction between distinct regions as well. For example, one would expect functionally related regions to have more correlated ATCs than functionally completely distinct regions. Figure 13 shows an attempt to group different ICs according to the correlation between their ATCs. It displays ICs selected from a single subject on a hierarchical tree, using the same methodology used to display the relatedness of distinct species, based on the similarity of their DNA sequence. However, this time, the correlation coefficient matrix of the ATCs of these ICs is used as a measure for relatedness. Some ICs (e.g. from the visual cortex) appear clustered within the same sub-branch of the tree, indicating a high functional relatedness. In sum, however, this approach turned out to be only of limited use because distinct ICs, even within the visual cortex, had often very low mutual correlations, which is indicative of their high functional independence. It is this very temporal independence of distinct subdivisions which makes their ‘temporal fingerprints’ so unique and our approach of mapping of these areas so successful.
Figure 6.

ICs containing visual areas obtained from a subject who freely viewed the James Bond film Tomorrow Never Dies, along with their activity time courses (ATCs). (a) Glass-brain views (sagittal and transverse maximum intensity projections, no threshold applied) of occipital ICs isolated from one subject. All regions were stimulus-driven, were also isolated in the remaining subjects and had area-specific and significant inter subject correlations of their ATCs (Bartels & Zeki 2004a). Labels indicate the presumptive identity of the regions based on their anatomical locations. The colouring of the ICs shows the relative voxel contribution, using the colour scale shown on the right, with red indicating positive contribution, green neutral/no contribution, blue negative contribution. (b) The first 3 min of the ATCs associated with each of the ICs shown in (a). Note the temporally distinct involvement of each visual region. The scale of ICA–ATCs is arbitrary and was normalized to one for each. LOp, posterior part of the lateral occipital complex; LOl, lateral part of the lateral occipital complex, V5+: V5/MT together with parts of ventral occipital cortex and presumptive V3A (modified from Bartels & Zeki 2005).
Figure 9.
Anatomically corresponding areas in different brains had specific and significantly correlated ATCs during free viewing of the film. Inter subject correlations of anatomically corresponding areas were in fact higher than correlations of different areas within single brains (Bartels & Zeki 2004a). Here, this is illustrated on four cortical regions (auditory cortex, V1, V5 and V4). For the first three participants in our study, the ICs as well as the ATCs of these regions are shown. Maps and traces of each individual are colour-coded. The grey bars at the top and bottom indicate blank periods (black screen, no sound), which were not considered in further analyses (from Bartels & Zeki 2004a).
Figure 7.
Correlogram visualizing the BOLD signal correlations between the most active voxels of the ICs shown in figure 6 during free viewing of a film. Line thickness and colour code indicate the correlation strength. Same abbreviations as in figure 6. l, left; r, right (modified from Bartels & Zeki 2005).
Figure 8.

The same regions as shown in figure 6, superimposed onto a structural rendering of the subject's brain. Each region was identified by ICA in a separate IC, which was then colour-coded, intensity-thresholded (greater then 70% positive activation) and superimposed onto the subject's brain. Each area (IC) had an area-specific ATC that correlated significantly and selectively with anatomically corresponding areas (ICs) in the other eight subjects (see following figures). aCS, ventral lip of the anterior calcarine sulcus; Aud, auditory cortex (BA41 and 42); pc+rs, network containing precuneus (BA7) and retrosplenium (BA23 or 30); Wern, Wernicke's area (BA22) (from Bartels & Zeki 2004a).
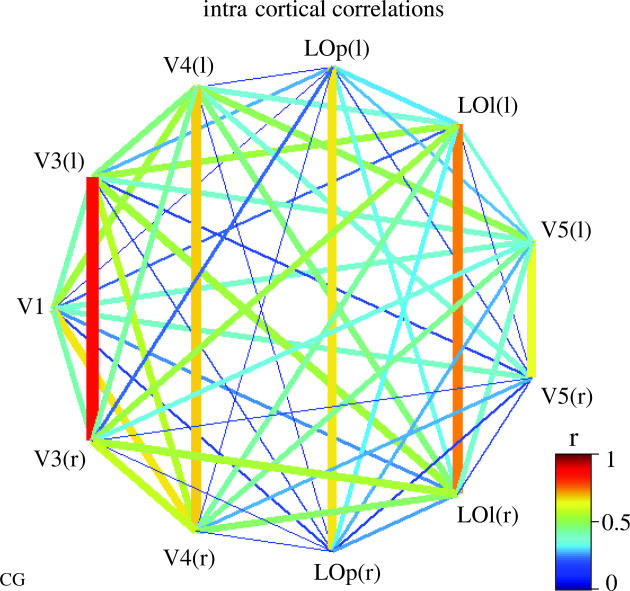
6. Intra cortical correlations during natural conditions: a guide for anatomical connectivity?
An additional property apparent in some of the ICs was that they contained not a single cluster of voxels, but several. Voxels that are present in a single IC are temporally dependent in relation to their relative activity within that IC. Note that not all voxels which have high temporal dependencies necessarily need to be coisolated in the same IC given that spatial ICA applies its independence criterion only to the spatial domain, not to the temporal one. Thus, multiple clusters in ICs reveal functional connectivity (i.e. temporal correlations between distinct regions; Friston et al. 1993), but do not necessarily reveal the full network involved (Bartels & Zeki 2005). In order to reveal the full extent of the functional connectivity of a given region isolated in an IC, we took the BOLD signal of its most active voxel in each of the subjects to create CMs. CMs show the temporal correlations of all voxels in the brain with the seed voxel. These CMs either contained the same set of clusters as was already apparent in the IC or, in some cases, additional clusters. CMs were generally nearly as specific as ICs, containing a few clearly isolated clusters of voxels. This indicated firstly that much of the dependence ICs were based on was due to second-order correlations. Secondly, it showed that dependencies across the brain were highly specific (see figure 12). Lastly, we note that the correlation of the BOLD time course of the hottest voxel of an IC with that of the IC-ATC was, on average, 0.9 or higher. Most frequently, the secondary clusters in CMs were simply the homologues of a single region, such that both left and right homologues of a given region appeared in a single IC (see figure 6), which indicated a high correlation between them. We believe it is no coincidence that these particularly strong correlations were observed between regions that are known to have strong direct anatomical connections through the corpus callosum (Zeki 1970; Pandya et al. 1971; Clarke & Miklossy 1990; Bartels & Zeki 2005). This is in accord with the finding that the significant correlation between homologue regions during rest is diminished in patients with agenesis of the corpus callosum (Quigley et al. 2003). In addition, several ICs contained tertiary clusters, indicating correlations between distinct regions within the same hemisphere. We hypothesize that these, too, may be related to anatomical connections. We illustrate this on the example of the regions shown in figure 12a. It shows ICs containing a region overlapping with Wernicke's area. These ICs were isolated in every subject, and their associated ATCs had specific inter subject correlations indicating a common functional involvement. In most subjects, this IC contained an additional cluster in a region close to Broca's area. CMs revealed that Wernicke's area correlated in every subject with the same set of regions, including putative Broca's area (BA 45), another prefrontal/premotor region in the vicinity of BA 6, and BA 39 in the parietal cortex, at a threshold of r > 0.4 (see figure 12b). Parallel to the bilateral visual areas, these specific correlations within language-related regions reflect the known direct anatomical connections between them (Kaas & Hackett 2000; Petrides & Pandya 2002; Scott & Johnsrude 2003). Furthermore, as described in detail in Bartels & Zeki (2005), we observed that correlations between regions that are known to be anatomically connected increased during natural viewing, while correlations between unconnected regions decreased during natural viewing in comparison to rest. We illustrate this using the example of correlations between visual regions with language-related regions that de-correlated during natural viewing, while correlations within the language network increased (figure 14). The correlations were based on the BOLD signal extracted from the hottest voxel of ICs containing visual or auditory regions. This double dissociation of correlations during natural viewing and rest between connected and unconnected regions led us to propose that intra cortical correlations during natural conditions may be indicative of anatomical connectivity (Bartels & Zeki 2005). Especially noteworthy is the consistent involvement of BA 39 in CMs using Wernicke's area as a seed. While BA 39 was not classically considered to have direct connections with Broca's area and Wernicke's area, a recent study using DTI and track tracing methods revealed it to be a directly connected part of the language network (Catani et al. 2005). Our CMs revealed the same result, highlighting the huge potential of natural viewing CMs as hypothesis-generating tools for the mapping of anatomical connectivity. Apart from our experimental evidence, the relation of natural viewing correlations to anatomical connectivity does also make sense at a conceptual level. The brain evolved and develops during exposure to natural conditions. Its anatomical connectivity is therefore likely to reflect the degree of communication between distinct regions during these conditions. Thus, it seems plausible that intra cortical correlations reflect anatomical connectivity most accurately when they are measured during more natural conditions. This is not to say that correlations during these conditions invariably reveal anatomical ones. Firstly, one has to be aware of potential artefactual correlations (e.g. induced by head movements, breathing, changes in heart rate, etc.) combined with common vascularization. These artefacts affect all imaging studies and are particularly dangerous to those relying on temporal dependencies of any kind. Secondly, some anatomical connections may not be apparent at all through correlations, and some correlations may arise for other reasons. Methods relying on temporal correlations, of course, can only reveal anatomical connections that are active. Each area of the cerebral cortex has multiple outputs to different destinations, both cortical and sub cortical. It is not clear whether all these outputs are engaged when a given area is active. Indeed, previous studies have shown that distinct tasks or stimuli may lead to distinct degrees of functional connectivity (i.e. correlation) between regions (Buchel & Friston 1997; Friston & Buchel 2000; Friston et al. 2003). In view of this, we hypothesize that natural conditions are most suitable for eliciting correlations that reflect the underlying anatomical connectivity. Alternatively, one could say that the conditions should be as varied as possible in order to learn about all possible interactions of an area. Our results show that intra cortical correlations during natural conditions are more specific with respect to the known anatomical connectivity than those observed during the resting state, which have been implied to be indicative of anatomical connectivity previously (e.g. Lowe et al. 1998; Cordes et al. 2000; Hampson et al. 2002; Koch et al. 2002; Quigley et al. 2003; Young et al. 2003). As we are only beginning to understand the relation between the BOLD signal measured using fMRI and the underlying neural activity (Logothetis 2003), any inference on connectivity can only provide hypotheses rather than conclusive evidence. Despite this, such hypotheses are valuable, especially in the human brain, as we have only limited knowledge about its anatomical connectivity. DTI is a non invasive technique that is more directly based on anatomical connectivity by exploiting the diffusion preference of water molecules along myelinated fibre bundles (Parker et al. 2002). Unfortunately, while this method delivers impressive results, it is mostly limited to connections that originate in sub cortical nuclei or white matter. The numerous fibre crossings near the cortex are difficult to resolve within the dimensions of DTI-weighted image voxels, and thus make cortico-cortical tracing difficult, even though better solutions are in sight (Tuch et al. 2003) and first impressive results have been published as described above (Catani et al. 2005). We therefore envisage that DTI and connectivity patterns inferred on the basis of temporal correlations during natural conditions may be suited to complement each other for the charting of cortico-cortical anatomical connectivity in vivo. Our conceptual understanding and our experimental evidence suggest that correlations during natural conditions may be better suited as indicators of anatomical connections than other, more artificial, experimental settings (Bartels & Zeki 2005).
Figure 14.
Effects of film viewing and rest periods on BOLD correlations between anatomically connected (within the language network) and non connected regions (between visual regions and language network). All correlations were calculated for within-hemispheric pairs of areas, error bars indicate ±s.e.m. (n=13 hemispheres; from Bartels & Zeki 2005). Asterisks indicate that the correlations differed with a significance of p < 0.005 for area-weighted statistics (t-test).
7. Conclusion
In this paper, we have reviewed a new approach to mapping functional subdivisions of the brain. It does not rely on anatomical markers nor on hypothesis-driven experiments. Rather, it relies on a marker that is present and unique to every functional subdivision of the brain; namely, its ACT during natural conditions (Bartels & Zeki 2004a,b, 2005). Chronoarchitectonic maps, therefore, mirror traditional ones. However, because they collapse all the known and unknown functional and structural properties of cerebral architecture onto a single and measurable factor, they may be easier to derive, even for uncharted regions. In addition, because the temporal fingerprint is a direct consequence of function, it also reflects functional similarity (or functional connectivity) and during natural conditions, this may be indicative of anatomical connections between areas (Bartels & Zeki 2005). The chronoarchitectonic approach therefore constitutes the logical development of one of the principal themes of cortical studies; namely, the attempt to identify distinct parts of the cerebral cortex and assign specific functions to each. It distinguishes and defines areas based on the timing of activity in them in relation to the timing of activity in other areas, without a priori knowledge about anatomical location or function. Its weakness, which it shares with other anatomical mapping techniques (e.g. the cyto- or myeloarchitectonic techniques), is that it does not allow one to infer specific functions for the areas that it maps. Paradoxically, this may also be its main strength since it does not depend upon the simplistic and sometimes erroneous attribution of a single function. Nevertheless, a reverse correlation of a region's ATC with the stimulus may generate a hypothesis with regard to its preferred feature (Hasson et al. 2004), which should then be validated statistically using a formal hypothesis-led regression against the intensity of the feature under concern (Bartels & Zeki 2003). We have demonstrated that a multitude of distinct regions can be identified across the whole brain, even within the visual cortex, based entirely on their differential ATCs during natural viewing. Functionally corresponding regions did not only correspond anatomically across subjects, but also had highly area-specific temporal inter subject correlations. In contrast, distinct functional subdivisions, even within visual cortex, had surprisingly low temporal correlations, even within a single brain. In fact, our results show that natural conditions lead to a decorrelation of activity between distinct regions compared with rest (Bartels & Zeki 2004a, 2005). This makes it easier for the ICA to identify voxels belonging to a given subdivision and to segregate them into separate components. At the same time, it also makes functionally connected (i.e. temporally correlated) regions stand out. In fact, our results also showed that regions with known substantial anatomical connections increased the correlations of their ATCs during natural viewing compared with the rest, revealing a double dissociation as a function of anatomical connectivity and stimulation condition. Temporal correlations during natural conditions may thus be indicative of anatomical connectivity, which we demonstrate for the case of regions involved in the processing of language. Most importantly, our results reveal a high degree of temporal independence of distinct cortical regions during natural processing, which not only confirms the view of a high degree of functional specialization even within the visual cortex, but also indicates that natural conditions may enhance regional specificity (Zeki 1978; Zeki & Bartels 1998b). Other types of natural stimuli may be more suitable to obtain differential activity in other parts of the brain, and future algorithms may deliver better results than the ICA used here. However, we hope that the general principle of chronoarchitectonic mapping may serve to identify novel cortical subdivisions and their connectivity. The results obtained may then inspire future, hypothesis-led experiments to illuminate the precise functional involvement of these regions.
Acknowledgments
This work was supported by the Wellcome Trust, London. A.B. is supported by the Swiss National Science Foundation.
Glossary
- ATC
activity time course
- BOLD
blood oxygenation dependent
- CM
correlation map
- DTI
diffusion tensor imaging
- EEG
electroencephalogram
- fMRI
functional magnetic resonance imaging
- ICA
independent component analysis
- PCA
principal component analysis
- SPM
statistical parametric mapping
Footnotes
One contribution of 12 to a Theme Issue ‘Cerebral cartography 1905–2005’.
References
- Bartels A, Zeki S. The architecture of the colour centre in the human visual brain: new results and a review. Eur. J. Neurosci. 2000a;12:172–193. doi: 10.1046/j.1460-9568.2000.00905.x. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000b;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Functional brain mapping during free viewing of natural scenes. Hum. Brain Mapp. 2003;21:75–83. doi: 10.1002/hbm.10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The chronoarchitecture of the human brain—natural viewing conditions reveal a time-based anatomy of the brain. NeuroImage. 2004a;22:419–433. doi: 10.1016/j.neuroimage.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The chronoarchitecture of the human brain: functional anatomy based on natural brain dynamics and the principle of functional independence. In: Frackowiak R, Friston K, Frith C, Dolan R, Zeki S, editors. Human brain function. 2nd edn. Academic Press; London: 2004b. pp. 201–229. [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004c;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Brain dynamics during natural viewing conditions—a new guide for mapping connectivity in vivo. NeuroImage. 2005;24:339–349. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F, Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bell A.J, Sejnowski T.J. An information maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. Dritte Mitteilung: Die Rindenfelder der niederen Affen. J. Psychol. Neurol. 1905;4:177–226. [Google Scholar]
- Brodmann K. J. A. Barth; Leipzig: 1909. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. [Google Scholar]
- Buchel C, Friston K.J. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cereb. Cortex. 1997;7:768–778. doi: 10.1093/cercor/7.8.768. [DOI] [PubMed] [Google Scholar]
- Buchel C, Holmes A.P, Rees G, Friston K.J. Characterizing stimulus–response functions using nonlinear regressors in parametric fMRI experiments. NeuroImage. 1998;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D, Adali T, McGinty V.B, Pekar J.J, Watson T.D, Pearlson G.D. fMRI activation in a visual-perception task: network of areas detected using the general linear model and independent components analysis. NeuroImage. 2001;14:1080–1088. doi: 10.1006/nimg.2001.0921. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D, Adali T, Pearlson G.D, Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001a;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D, Adali T, Pearlson G.D, Pekar J.J. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum. Brain Mapp. 2001b;13:43–53. doi: 10.1002/hbm.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D, Pekar J.J, McGinty V.B, Adali T, Watson T.D, Pearlson G.D. Different activation dynamics in multiple neural systems during simulated driving. Hum. Brain Mapp. 2002;16:158–167. doi: 10.1002/hbm.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A.W. Cambridge University Press; Cambridge, UK: 1905. Histological studies on the localisation of cerebral function. [Google Scholar]
- Castelo-Branco M, Formisano E, Backes W, Zanella F, Neuenschwander S, Singer W, Goebel R. Activity patterns in human motion-sensitive areas depend on the interpretation of global motion. Proc. Natl Acad. Sci. USA. 2002;99:13 914–13 919. doi: 10.1073/pnas.202049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones D.K, Ffytche D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Clarke S, Miklossy J. Occipital cortex in man: organization of callosal connections, related myelo- and cytoarchitecture, and putative boundaries of functional visual areas. J. Comp. Neurol. 1990;298:188–214. doi: 10.1002/cne.902980205. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton V.M, Arfanakis K, Wendt G.J, Turski P.A, Moritz C.H, Quigley M.A, Meyerand M.E. Mapping functionally related regions of brain with functional connectivity MR imaging. Am. J. Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- DeYoe E.A, Van Essen D.C. Segregation of efferent connections and receptive field properties in visual area 2 of the macaque. Nature. 1985;317:58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- Esposito F, Formisano E, Seifritz E, Goebel R, Morrone R, Tedeschi G, Di Salle F. Spatial independent component analysis of functional MRI time-series: to what extent do results depend on the algorithm used? Hum. Brain Mapp. 2002;16:146–157. doi: 10.1002/hbm.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffytche D.H, Howard R.J, Brammer M.J, David A, Woodruff P, Williams S. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat. Neurosci. 1998;1:738–742. doi: 10.1038/3738. [DOI] [PubMed] [Google Scholar]
- Flechsig P. Developmental (myelogenetic) localisation of the cerebral cortex in the human subject. Lancet. 1901;2:1027–1029. [Google Scholar]
- Frackowiak R, Friston K, Frith C, Dolan R, Mazziotta J.C, editors. Human brain function. Academic Press; London: 1997. [Google Scholar]
- Friston K.J. Modes or models: a critique on independent component analysis for fMRI. Trends Cogn. Sci. 1998;2:373–374. doi: 10.1016/s1364-6613(98)01227-3. [DOI] [PubMed] [Google Scholar]
- Friston K.J, Buchel C. Attentional modulation of effective connectivity from V2 to V5/MT in humans. Proc. Natl Acad. Sci. USA. 2000;97:7591–7596. doi: 10.1073/pnas.97.13.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J, Frith C.D, Liddle P.F, Frackowiak R.S.J. Functional connectivity—the principal-component analysis of large (Pet) data sets. J. Cereb. Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Friston K.J, Frith C.D, Frackowiak R.S.J, Turner R. Characterizing dynamic brain responses with fMRI—a multivariate approach. NeuroImage. 1995;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Friston K.J, Holmes A.P, Poline J.B, Grasby P.J, Williams S.C.R, Frackowiak R.S.J, Turner R. Analysis of fMRI time-series revisited. NeuroImage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Friston K.J, Williams S, Howard R, Frackowiak R.S.J, Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Friston K.J, Harrison L, Penny W. Dynamic causal modeling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Gu H, Engelien W, Feng H, Silbersweig D.A, Stern E, Yang Y. Mapping transient, randomly occurring neuropsychological events using independent component analysis. NeuroImage. 2001;14:1432–1443. doi: 10.1006/nimg.2001.0914. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson B.S, Skudlarski P, Gatenby J.C, Gore J.C. Detection of functional connectivity using temporal correlations in MR images. Hum. Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Henson R.N, Price C.J, Rugg M.D, Turner R, Friston K.J. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. NeuroImage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Jen L.S, Zeki S. High cytochrome-oxidase content of the V5 complex of macaque monkey visual-cortex. J. Physiol. Lond. 1984;348:23. [Google Scholar]
- Kaas J.H, Hackett T.A. Subdivisions of auditory cortex and processing streams in primates. Proc. Natl Acad. Sci. USA. 2000;97:11 793–11 799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Kantola J.H, Jauhiainen J, Hyvarinen A, Tervonen O. Independent component analysis of nondeterministic fMRI signal sources. NeuroImage. 2003;19:253–260. doi: 10.1016/s1053-8119(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Büchel C, Zeki S, Frackowiak R.S.J. Human brain activity during spontaneously reversing perception of ambiguous figures. Proc. R. Soc. B Biol. Sci. 1998;265:2427–2433. doi: 10.1098/rspb.1998.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M.A, Norris D.G, Hund-Georgiadis M. An investigation of functional and anatomical connectivity using magnetic resonance imaging. NeuroImage. 2002;16:241–250. doi: 10.1006/nimg.2001.1052. [DOI] [PubMed] [Google Scholar]
- Livingstone M.S, Hubel D.H. Anatomy and physiology of a color system in the primate visual cortex. J. Neurosci. 1984;4:309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N.K. The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M.J, Mock B.J, Sorenson J.A. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lumer E.D, Friston K.J, Rees G. Neural correlates of perceptual rivalry in the human brain. Science. 1998;280:1930–1934. doi: 10.1126/science.280.5371.1930. [DOI] [PubMed] [Google Scholar]
- Lungwitz W. Zur myeloarchitektonischen Untergliederung der menschlichen Area praeoccipitalis (Area 19 Brodmann) J. Psychol. Neurol. 1937;47:607–639. [Google Scholar]
- Makeig S, Jung T.P, Bell A.J, Ghahremani D, Sejnowski T.J. Blind separation of auditory event-related brain responses into independent components. Proc. Natl Acad. Sci. USA. 1997;94:10 979–10 984. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung T.P, Enghoff S, Townsend J, Courchesne E, Sejnowski T.J. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Malach R, Levy I, Hasson U. The topography of high-order human object areas. Trends Cogn. Sci. 2002;6:176–184. doi: 10.1016/s1364-6613(02)01870-3. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, et al. A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM) Phil. Trans. R. Soc. B. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M.J. Detection of consistently task-related activations in fMRI data with hybrid independent component analysis. NeuroImage. 2000;11:24–35. doi: 10.1006/nimg.1999.0518. [DOI] [PubMed] [Google Scholar]
- McKeown M.J, Jung T.P, Makeig S, Brown G, Kindermann S.S, Lee T.W, Sejnowski T.J. Spatially independent activity patterns in functional MRI data during the Stroop color naming task. Proc. Natl Acad. Sci. USA. 1998;95:803–810. doi: 10.1073/pnas.95.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M.J, Makeig S, Brown G.G, Jung T.P, Kindermann S.S, Bell A.J, Sejnowski T.S. Analysis of fMRI data by blind separation into independent spatial components. Hum. Brain Mapp. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M.J, Hansen L.K, Sejnowski T.J. Independent component analysis of functional MRI: what is signal and what is noise? Curr. Opin. Neurobiol. 2003;13:620–629. doi: 10.1016/j.conb.2003.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Baese A, Wismueller A, Lange O. Comparison of two exploratory data analysis methods for fMRI: unsupervised clustering versus independent component analysis. IEEE Trans. Inf. Technol. Biomed. 2004;8:387–398. doi: 10.1109/titb.2004.834406. [DOI] [PubMed] [Google Scholar]
- Meyer-Baese A, Lange O, Wismuller A, Ritter H. Model-free functional MRI analysis using topographic independent component analysis. Int. J. Neural Syst. 2004;14:217–228. doi: 10.1142/S0129065704002017. [DOI] [PubMed] [Google Scholar]
- Moutoussis K, Zeki S. A direct demonstration of perceptual asynchrony in vision. Proc. R. Soc. B. 1997;264:393–399. doi: 10.1098/rspb.1997.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada T, Suzuki K, Fujii Y, Matsuzawa H, Kwee I.L. Independent component-cross correlation-sequential epoch (ICS) analysis of high field fMRI time series: direct visualization of dual representation of the primary motor cortex in human. Neurosci. Res. 2000;37:237–244. doi: 10.1016/s0168-0102(00)00122-x. [DOI] [PubMed] [Google Scholar]
- Nybakken G.E, Quigley M.A, Moritz C.H, Cordes D, Haughton V.M, Meyerand M.E. Test-retest precision of functional magnetic resonance imaging processed with independent component analysis. Neuroradiology. 2002;44:403–406. doi: 10.1007/s00234-001-0722-6. [DOI] [PubMed] [Google Scholar]
- Pandya D.N, Karol E.A, Heilbronn D. The topographical distribution of interhemispheric projections in the corpus callosum of the rhesus monkey. Brain Res. 1971;32:31–43. doi: 10.1016/0006-8993(71)90153-3. [DOI] [PubMed] [Google Scholar]
- Parker G.J, et al. Initial demonstration of in vivo tracing of axonal projections in the macaque brain and comparison with the human brain using diffusion tensor imaging and fast marching tractography. NeuroImage. 2002;15:797–809. doi: 10.1006/nimg.2001.0994. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D.N. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Quigley M.A, Haughton V.M, Carew J, Cordes D, Moritz C.H, Meyerand M.E. Comparison of independent component analysis and conventional hypothesis-driven analysis for clinical functional MR image processing. Am. J. Neuroradiol. 2002;23:49–58. [PMC free article] [PubMed] [Google Scholar]
- Quigley M, Cordes D, Turski P, Moritz C, Haughton V, Seth R, Meyerand M.E. Role of the corpus callosum in functional connectivity. Am. J. Neuroradiol. 2003;24:208–212. [PMC free article] [PubMed] [Google Scholar]
- Roland P, et al. A database generator for human brain imaging. Trends Neurosci. 2001;24:562–564. doi: 10.1016/s0166-2236(00)01924-x. [DOI] [PubMed] [Google Scholar]
- Salami M, Itami C, Tsumoto T, Kimura F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc. Natl Acad. Sci. USA. 2003;100:6174–6179. doi: 10.1073/pnas.0937380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky M.T, Wang Y, Hanes D.P, Thompson K.G, Leutgeb S, Schall J.D, Leventhal A.G. Signal timing across the macaque visual system. J. Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Scott S.K, Johnsrude I.S. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 2003;26:100–107. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki S. Segregation of pathways leading from area V2 to areas V4 and V5 of macaque monkey visual cortex. Nature. 1985;315:322–325. doi: 10.1038/315322a0. [DOI] [PubMed] [Google Scholar]
- Spiridon M, Kanwisher N. How distributed is visual category information in human occipito-temporal cortex? An fMRI study. Neuron. 2002;35:1157–1165. doi: 10.1016/s0896-6273(02)00877-2. [DOI] [PubMed] [Google Scholar]
- Stone J.V, Porrill J, Porter N.R, Wilkinson I.D. Spatiotemporal independent component analysis of event-related fMRI data using skewed probability density functions. NeuroImage. 2002;15:407–421. doi: 10.1006/nimg.2001.0986. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kiryu T, Nakada T. Fast and precise independent component analysis for high field fMRI time series tailored using prior information on spatiotemporal structure. Hum. Brain Mapp. 2002;15:54–66. doi: 10.1002/hbm.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch D.S, Reese T.G, Wiegell M.R, Wedeen V.J. Diffusion MRI of complex neural architecture. Neuron. 2003;40:885–895. doi: 10.1016/s0896-6273(03)00758-x. [DOI] [PubMed] [Google Scholar]
- Van De Ven V.G, Formisano E, Prvulovic D, Roeder C.H, Linden D.E. Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum. Brain Mapp. 2004;22:165–178. doi: 10.1002/hbm.20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C. Windows on the brain: the emerging role of atlases and databases in neuroscience. Curr. Opin. Neurobiol. 2002;12:574–579. doi: 10.1016/s0959-4388(02)00361-6. [DOI] [PubMed] [Google Scholar]
- Vogt C, Vogt O. Allgemeinere Ergebnisse unserer Hirnforschung. Vierte Mitteilung. Die physiologische Bedeutung der architektonischen Rindenfelderung auf Grund neuer Rindenreizungen. J. Psychol. Neurol. 1919;25:399–462. [Google Scholar]
- von Economo C, Koskinas G. Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen. Springer; Berlin: 1925. Individuelle und physiologische Bedeutung der Areale. [Google Scholar]
- Watson J.D.G, Myers R, Frackowiak R.S.J, Hajnal J.V, Woods R.P, Mazziotta J.C, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb. Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Wang Y, Felleman D.J. A spatially organized representation of colour in macaque cortical area V2. Nature. 2003;421:535–539. doi: 10.1038/nature01372. [DOI] [PubMed] [Google Scholar]
- Young J.P, Geyer S, Grefkes C, Amunts K, Morosan P, Zilles K, Roland P.E. Regional cerebral blood flow correlations of somatosensory areas 3a, 3b, 1, and 2 in humans during rest: a PET and cytoarchitectural study. Hum. Brain Mapp. 2003;19:183–196. doi: 10.1002/hbm.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S.M. Interhemispheric connections of prestriate cortex in the monkey. Brain Res. 1970;19:63–75. doi: 10.1016/0006-8993(70)90237-4. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Functional specialization in the visual cortex of the monkey. Nature. 1978;274:423–428. doi: 10.1038/274423a0. [DOI] [PubMed] [Google Scholar]
- Zeki S, Bartels A. The asynchrony of consciousness. Proc. R. Soc. B. 1998a;265:1583–1585. doi: 10.1098/rspb.1998.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Bartels A. The autonomy of the visual systems and the modularity of conscious vision. Phil. Trans. R. Soc. B. 1998b;353:1911–1914. doi: 10.1098/rstb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Bartels A. The clinical and functional measurement of cortical (in-) activity in the visual brain, with special reference to the two subdivisions (V4 and V4α) of the human colour centre. Phil. Trans. R. Soc. B. 1999;354:1371–1382. doi: 10.1098/rstb.1999.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Clarke S. Architecture, connectivity, and transmitter receptors of human extrastriate visual cortex: comparison with nonhuman primates. In: Rockland K.S, Kaas J.H, Peters A, editors. Extrastriate cortex in primates, vol. 12, 2nd edn. Plenum Press; New York: 1997. pp. 673–742. [Google Scholar]