Abstract
Incubation of human eosinophils in BSA-coated tissue culture plates resulted in time-dependent adhesion and attendant activation of the NADPH oxidase that were both inhibited (by >85%) by blocking antibodies raised against CD11b and CD18.
SB 203580, an inhibitor of p38 mitogen-activated protein (MAP) kinase, did not influence adhesion but inhibited superoxide anion generation (pIC50=−6.57).
PP1, an inhibitor of the src-family of protein tyrosine kinases, inhibited adhesion and CD11b/CD18-mediated superoxide anion generation with similar potencies (pEC50s=−5.53 and −5.99 respectively) suggesting that inhibition of the NADPH oxidase was a direct consequence of blocking adhesion.
The protein kinase C (PKC) inhibitors Ro-31 8220 (broad spectrum inhibitor), GF 109203X (inhibitor of conventional and novel isoforms) and Gö 6976 (inhibitor of conventional isoforms) suppressed adhesion-dependent NADPH oxidase activation (pIC50s=−6.61, −6.05 and −4.89 respectively) without affecting adhesion. Based upon the selectivity of these drugs PKCδ and PKCε are implicated in the suppression of oxidant production.
Wortmannin, an inhibitor of phosphatidylinositol 3-kinase (PtdIns 3-kinase), abolished superoxide anion production in adherent eosinophils (pEC50=−9.06). Similarly, CD11b/CD18-dependent adhesion was suppressed with the same potency (pEC50=−9.29) although the maximum effect did not exceed 50% implying that wortmannin also had an affect on those processes that govern adhesion-driven oxidase activation.
PD 098059 and piceatannol, inhibitors of MAP kinase kinase-1 and the syk tyrosine kinase respectively, had no effect on CD11b/CD18-mediated adhesion or NADPH oxidase activation.
The results of this study demonstrate that human eosinophils adhere to BSA-coated plastic by a CD11b/CD18-dependent mechanism, which is responsible for activation of the NADPH oxidase. Although the signalling pathway(s) utilized by CD11b/CD18 is still to be elucidated, the data presented herein implicate p38 MAP kinase, novel PKCs and PtdIns 3-kinase.
Keywords: Human adherent eosinophils, ‘outside-in' signalling, protein kinase C, p38 MAP kinase, adhesion (CD11b/CD18)-dependent NADPH oxidase activation
Introduction
Eosinophils are actively motile, terminally differentiated leukocytes derived from the bone marrow and have been identified in many mammalian and non-mammalian species (Giembycz & Lindsay, 1999). In humans, eosinophils were originally thought to be primarily involved in immune defence against parasitic infection. However, it is now recognized that they are implicated in a number of allergic and non-allergic diseases such as hypereosinophilic syndromes, eosinophilic pneumonia, atopic dermatitis, asthma and rhinitis. In inflammatory disorders, such as asthma, eosinophils migrate into the airways where they become activated and secrete a host of cytotoxic species including granule proteins and lipid mediators together with the generation of highly toxic free radicals that are formed enzymatically from molecular oxygen by the NADPH oxidase complex (Giembycz & Lindsay, 1999). Activation of eosinophils in the airways is thought to cause localized tissue injury, contraction of airways smooth muscle and increased bronchial responsiveness to a diverse range of spasmogens (Barnes, 1996; Seminario & Gleich, 1994; Desreumaux & Capron, 1996).
The release of leukotriene C4 (Anwar et al., 1994; Yoshida et al., 1995; Munoz et al., 1996), eosinophil peroxidase (Neeley et al., 1994), eosinophil-derived neurotoxin (Horie & Kita, 1994), interleukin-3, granulocyte/macropahge colony-stimulating factor (GM-CSF) (Anwar et al., 1993) and superoxide anions (Dri et al., 1991) is regulated by integrins upon their engagement by counter ligands expressed by extracellular matrix or plasma proteins. For example, platelet-activating factor and GM-CSF do not promote superoxide anion generation from eosinophils in suspension. However, a large and sustained activation of the NADPH oxidase occurs in the same eosinophils when they are adherent to albumin-coated polystyrene plates by a mechanism that is dependent upon the β2-integrin, CD18 (Horie & Kita, 1994). In another study, Laudanna et al. (1993) reported that coating plates with activating antibodies to β1- and β2-integrins triggers eosinophil spreading and oxidant generation. Similarly, an examination of the binding of eosinophils through the β1-integrin, very late antigen-4 (VLA4; α4β1; CD49d/CD29), revealed that adherence to vascular cell adhesion molecule-coated plates correlated with spontaneous production of superoxide and that this effect was increased by fMLP and inhibited by an antibody against CD18 (Nagata et al., 1995).
Collectively, the aforementioned reports suggest a central role for β1- and β2-integrins in the mechanism of eosinophil adhesion and NADPH oxidase activation. However, little is known of the biochemical pathways underlying these two responses in eosinophils. Furthermore, these studies are complicated by the need to differentiate between the pathways that increase receptor-ligand binding (‘inside-out' signalling) and those that mediate receptor-ligand-induced responses (‘outside-inK signalling) such as cell spreading, and subsequent degranulation and activation of the NADPH oxidase (Nathan, 1987).
In human neutrophils, physiological stimuli including cytokines, complement fragments, immune complexes, bacterial products and chemotaxins are required to stimulate adhesion. The intracellular pathways implicated in this ‘outside-in' signalling are under investigation and recent studies of β2 integrin-mediated adhesion have implicated phosphatidylinositol 3-kinase (PI 3-kinase) (Jones et al., 1998; Metzner et al., 1997), p38 mitogen-activated protein (MAP) kinase (Detmers et al., 1998), Rho GTPase and protein kinase C (PKC) ζ (Laudanna et al., 1998). Following adhesion, signals generated from integrin-ligand interactions ('outside-in') are propagated via the short cytoplasmic tails of integrin molecules. These tails have no intrinsic enzymatic activity but appear to function by coupling to cytoplasmic proteins that nucleate the formation of large multicomponent complexes containing both cytoskeletal elements and signalling enzymes (Aplin et al., 1998). Thus, neutrophil spreading and subsequent oxidant production is associated with the phosphorylation and/or activation of paxillin (Fuortes et al., 1994), MAP kinases (Rafiee et al., 1995), the src-related protein tyrosine kinases lyn, fgr and hck (Yan & Berton, 1996; Berton et al., 1994; Lowell et al., 1996), syk and focal adhesion kinases (Fernandez & Suchard, 1998; Yan et al., 1997).
In this study, we describe the development of an in vitro model of integrin-mediated NADPH oxidase activation in human peripheral blood eosinophils and the role of MAP kinases, the src and syk family of cytosolic protein tyrosine kinases, the PKC family and PtdIns 3-kinase in this response.
Methods
Isolation of human eosinophils
Human eosinophils were purified according to the method of Hansel et al. (1989). Briefly, venous blood (50–100 ml) was collected into 10–20 ml of acid citrate dextrose anticoagulant. White blood cells were obtained by sedimentation with 3% hydroxyethyl starch, layered onto Ficoll-Paque and centrifuged at 1300×g for 30 min at 18°C. The mononuclear cell layer was discarded and the pellet, containing granulocyte and red blood cells, was washed in HBSS. Contaminating red blood cells were lysed by hypotonic lysis. The remaining granulocytes were washed, counted and resuspended in 300 μl RPMI 1640 containing 2% foetal calf serum and 5 mM EDTA (RPMI/FCS/EDTA). Eosinophils were purified from neutrophils using immunomagnetic anti-CD16 antibody-conjugated microbeads (1 μl of beads per 2×106 neutrophils). Following the addition of beads, cells were incubated at 4°C for 40 min before resuspension in 6 ml RPMI/FCS/EDTA. The mixture was loaded onto a separation column positioned within a magnetic field and eluted with 40 ml RPMI/FCS/EDTA. The CD16 negative cells (>99% eosinophils), which are not retained by the column, were collected, washed in buffer A (HBSS, 10 mM HEPES, pH 7.4, 0.1% (w v-1) BSA), counted and resuspended at 107 cells ml−1.
Measurement of superoxide anion generation and adhesion
Eosinophils (5×106 ml−1) were incubated with 10 μM Calcein-AM in buffer A for 30 min at 37°C, washed three times and resuspended in the same buffer at 5×106 ml−1. Aliquots (20 μl) of the cell suspension then were incubated in buffer A (+1 mM CaCl2/1 mM MgCl2), supplemented with 25 μM lucigenin and the relevant inhibitor/antibody in a total volume of 200 μl and seeded onto 96-well tisue culture plates coated with either BSA (0.1% w v−1) or FCS. Superoxide anion generation was monitored by lucigenin-enhanced chemiluminescence (Gyllenhammar, 1987) with a plate reading luminometer (Lucy II, Labtech Ltd., Uckfield, U.K.). At the appropriate time points, the number of adherent cells was determined by measuring the fluorescence of cellular Calcein-AM. Briefly, total fluorescence was measured at the outset of the experiment (Reading 1) and then at pre-determined time intervals (see text; Reading 2) using a Biolite F1 plate reader (λexcitation=485±20 nm; λemission=530±25 nm). Reading 2 was taken after non-adherent cells had been removed from the culture plates by gently washing in buffer A and the percentage of adherent eosinophils then was calculated by multiplying the ratio of fluorescence (Reading 2/Reading 1) by 100. Studies of the magnitude of the respiratory burst conducted in the presence and absence of eosinophils loaded with Calcein-AM showed that this fluorescent indicator had no adverse effects upon oxidant production or viability (data not shown).
Drugs and analytical reagents
PD 098059, SB 203580, piceatannol, wortmannin, Ro-31 8220, GF109203X and Gö 6976 were obtained from Calbiochem (Nottingham, U.K.). Flat clear-bottomed, white-walled 96-well tissue culture-treated plates, Ficoll-Paque and Calcein AM were purchased from Pharmacia (Uppsala, Sweden), Costar Ltd (Buckinghamshire, U.K.) and Molecular Probes (Eugene, Oregan, U.S.A.) respectively. Anti-CD16 microbeads and magnetic cell separation system were from Miltenyl Biotec (Surrey, U.K.). Blocking antibodies (6.5E, KIM225, MAX68P, MOPC21) to adhesion receptors and the inhibitor of the src-family of protein tyrosine kinases, PP1 (CP 118556), were kindly donated by Celltech Ltd (Slough, U.K.) and Pfizer (Groton, Connecticut, U.S.A.) respectively. All other reagents were purchased from Sigma (Poole, Dorset, U.K.).
Statistical analysis
Data points, bars and values in the text and Figure legends represent the mean±s.e.mean of ‘n' independent determinations taken from different cell preparations. Concentration-response curves were analysed by least-squares non-linear iterative regression with the ‘PRISM' curve fitting program (GraphPad software, CA, U.S.A.) and pEC50/pIC50 values were subsequently interpolated from curves of best-fit. Where appropriate, data were analysed non-parametrically using the Wilcoxon matched pairs test or Kruskal-Wallace one-way analysis of variance followed by Dunns multiple comparisons test. The null hypothesis was rejected when P<0.05.
Results
Characteristics of eosinophil adhesion and superoxide anion generation
The addition of human eosinophils to 96 well tissue-culture plates coated in BSA (0.1% w v−1) resulted in time-dependent adherence and the generation of superoxide anions as measured by lucigenin-enhanced chemiluminescence. A detectable and significant degree of adhesion was observed after a lag of approximately 20 min; thereafter, eosinophils adhered to the plastic rapidly over the duration of the experiment (Figure 1). Maximal adherence was achieved after approximately 100 min and the mean t1/2 of this response was 43 min. The generation of superoxide anions increased in parallel with adherence, although the response was transient. Thus, after a lag of 20 min the rate of oxidant production increased rapidly, peaked at 40–60 min and then declined towards baseline (Figure 1). In contrast, relatively few eosinophils adhered to plates coated with FCS (4.46±0.24% at 60 min, n=40) and only a modest respiratory burst was detected under those conditions (0.102±0.012 RLU at 60 min, n=40).
Figure 1.

Time-course of eosinophil adhesion and NADPH oxidase activation. Cells (105) were placed in 96-well tissue culture treated plates and adhesion (○) and NADPH oxidase activity (•) was monitored over a period of 100 min. Each data point represents mean±s.e.mean of five independent experiments using eosinophils purified from the blood of different donors. After 60 min, basal adhesion (per cent of total cells seeded) and respiratory burst, measured on FCS-coated plates, were 5.71±1.1% and 0.012±0.004 RLU respectively.
The nature of the adhesion of eosinophils to the tissue-culture plates and the requirement of that response for the activation of the NADPH oxidase was investigated using a panel of blocking antibodies raised against β1 (CD29) and β2 (CD18) integrins (Figure 2). Incubation of eosinophils with anti-CD18 (6.5E) and anti-CD11b (KIM225) antibodies suppressed adhesion by 63.2±13.6 and 53.9±16% respect-ively at 60 min. When both antibodies were used in combination a greater inhibitory effect (75.2±7.2%) was produced than when either of them was used alone (Figure 2). In contrast, neither a blocking antibody (MAX68P) to the α4 integrin, CD49d nor a non-specific, isotype-matched control antibody (MOPC21) affected eosinophil adhesion (Figure 2). Significantly, activation of the NADPH oxidase in eosinophils was dependent upon their prior adherence to the tissue-culture plates via a CD11b/CD18-dependent process. Thus, anti-CD18 and anti-CD11b antibodies inhibited superoxide anion generation by 82.8±3.6 and 75.6±4% respectively. When both antibodies were used in combination a greater inhibitory effect (86.9±2.3%) was elicited when compared to the effect of either antibody in isolation (Figure 2). The anti-CD49d and the isotype-matched, control antibodies did not affect the respiratory burst (Figure 2).
Figure 2.

Effect of integrin blocking antibodies on the adhesion and NADPH oxidase activity of human eosinophils. Eosinophils (105) were incubated for 5 min at 37°C with blocking antibodies (10 μg ml−1) to CD18 (6.5E), CD11b (KIM225), CD49d (MAX68P) or an isotype matched control (MOPC21) and then plated on to 96-well tissue culture plates and incubated for 60 min. Adherent cells (filled bars) and the maximal rate of NADPH oxidase activity (hatched bars) were determined and are expressed as a percentage of their respective responses obtained in untreated eosinophils. Each bar represents the mean±s.e.mean of five independent experiments using eosinophils purified from the blood of different donors (*P<0.05 and **P<0.001 compared to untreated eosinophils). Basal adhesion (per cent of total cells seeded) and respiratory burst, measured on FCS-coated plates, were 3.43±0.8% and 0.061±0.007 RLU respectively.
Effect of eosinophil number on superoxide anion generation and adherence
The magnitude of CD11b/CD18-induced superoxide anion generation was positively related to cell number with the maximum response achieved at approximately 0.8×105 to 105 eosinophils per well (Figure 3). Thus, at a seeding density of 0.2×105 eosinophils per well the respiratory burst amounted to 0.98 relative light units (RLU), which increased to 1.26, 2.65, 2.89 and 2.69 RLU at 0.4, 0.6, 0.8 and 1×105 cells per well respectively (Figure 3). In contrast, the percentage of eosinophils that adhered to the culture plate at each seeding density was invariant (range: 52.7–58.9%; Figure 3).
Figure 3.

Effect of eosinophil seeding density on eosinophil adhesion and NADPH oxidase activity. Cells (2×104–105) were placed in BSA-coated 96-well tissue culture treated plates and the maximum rate of NADPH oxidase activity (•) and the percentage of adherent eosinophils at 60 min (○) were measured. Each data point represents mean±s.e.mean of four independent experiment using eosinophils purified from the blood of different donors. Basal adhesion (per cent of total cells seeded) and respiratory burst, measured on FCS-coated plates, were 4.42±1.7% and 0.034±0.008 RLU respectively.
Comparison of CD11b/CD18-mediated superoxide anion generation with other stimuli
Using FCS-coated culture plates, which minimized the adhesion of eosinophil to less than 10%, the formylated tripeptide, fMLP (1 μM) failed to promote a respiratory burst (Figure 4a). However, in identical experiments using BSA-coated plates, to which approximately 40% of eosinophils adhered by a CD11b/CD18-dependent mechanism (Figure 4b), fMLP significantly enhanced (∼2 fold) NADPH oxidase activity over that produced by adhesion alone (Figure 4a). In contrast, the phorbol diester, PMA (100 nM) produced a robust (∼5 fold) stimulation of the NADPH oxidase over that mediated by CD11b/CD18 in culture plates that were coated with FCS and BSA (Figure 4a). This was associated with an equivalent degree (37–51%) of adhesion to both surfaces (Figure 4b).
Figure 4.

Comparative effects of CD11b/CD18, fMLP (1 μM) and PMA (100 nM) on eosinophil adhesion and NADPH oxidase activity. Cells (105) were placed in BSA- and FCS-coated 96-well tissue culture treated plates and the maximum rate of NADPH oxidase activity (a) and the percentage of adherent cells at 60 min (b) were measured. Each bar represents the mean±s.e.mean of seven independent experiments using eosinophils purified from the blood of different donors. *P<0.05, adherence and respiratory burst significantly greater than respective controls.
Role of MAP kinases in CD11b/CD18-mediated adhesion and superoxide anion generation
The MAP kinases are a group of proline-directed, serine/threonine-specific kinases that become activated following their dual phosphorylation at conserved threonine and tyrosine residues. Currently, three MAP kinase families have been biochemically classified: extracellular-regulated kinase-1 and 2 (ERK-1/2), c-jun N terminal kinase 46 and 54 and the p38 MAP kinase. To examine the role of ERK-1/2 and p38 MAP kinase in CD11b/CD18-dependent adhesion and superoxide generation of human eosinophils a pharmacological approach was adopted using selective inhibitors of these enzyme families.
PD 098059, which inhibits ERK-1 and ERK-2 indirectly by binding to the inactive form of MAP kinase kinase-1 thereby preventing its phosphorylation and activation by c-Raf (Alessi et al., 1995; Dudley et al., 1995), did not affect eosinophil adhesion or respiratory burst activity (Figure 5a) over a concentration range (300 pM–10 μM) that we have previously shown to inhibit ERK-1 and ERK-2 phosphorylation in eosinophils (Kankaanranta et al., 1999). In contrast, SB 203580 (100 pM–10 μM), an ATP binding site inhibitor of the α and β isoforms of p38 MAP kinase (Young et al., 1997; Tong et al., 1997) that has been shown to abolish MAPKAPK-2 phosphorylation in leukocytes (Dean et al., 1999; Lal et al., 1999), had no significant effect on CD11b/CD18-dependent eosinophil adhesion to the tissue-culture plates (Figure 3b) but inhibited the activation of the NADPH oxidase in a concentration-dependent manner (pIC50=−6.57±0.14). At the highest concentration (10 μM) of SB 203580 examined, the generation of superoxide anions was suppressed by 69±5% (Figure 5b).
Figure 5.

Effect of PD 098059 and SB 203580 on eosinophil adhesion and NADPH oxidase activity. Eosinophils (105) were seeded in BSA-coated 96-well tissue culture treated plates at 37°C containing PD 098059 (a), SB 203580 (b) or their respective vehicles. The number of adherent eosinophils after 60 min (○) and the maximal rate of NADPH oxidase activity (•) were measured and are expressed as a percentage of their respective responses obtained in untreated eosinophils. Each data point represents the mean±s.e.mean of five independent experiments using eosinophils purified from the blood of different donors. Basal adhesion (per cent of total cells seeded) and respiratory burst, measured on FCS-coated plates, were 3.56±0.66% and 0.12±0.024 RLU respectively.
Role of non-receptor-linked tyrosine kinases in CD11b/CD18-mediated adhesion and superoxide anion generation
The potential role of the src and syk families of cytosolic, or non-receptor-linked, protein tyrosine kinases in CD11b/CD18-mediated adhesion and superoxide generation was evaluated using the potent and selective membrane-permeable inhibitors, PP1 (Hanke et al., 1996) and piceatannol (Geahlen & McLaughlin, 1989), respectively.
PP1 (30 nM–100 μM) inhibited CD11b/CD18-mediated superoxide anion generation in a concentration-dependent manner with a pEC50 of −5.99±0.11; complete inhibition was achieved at 10 μM PP1 (Figure 6a). In contrast to the results obtained with SB 203580, PP1 also suppressed the adhesion of eosinophils to tissue-culture plates with a similar potency (pEC50=−5.53±0.11) suggesting that inhibition of the NADPH oxidase was a direct consequence of blocking adhesion (Figure 6a). However, only partial inhibition (∼70%) of adhesion was effected by PP1 implying that additional PP1-insensitive mechanisms are involved. In these experiments there was a tendency of PP1 to enhance adhesion at low concentrations although this failed to reach statistical significance (Figure 6a).
Figure 6.

Effect of PP1 and piceatannol on eosinophil adhesion and NADPH oxidase activity. Eosinophils (105) were seeded in BSA-coated 96-well tissue culture treated plates at 37°C containing PP1 (a), piceatannol (b) or their respective vehicles. The number of adherent eosinophils after 60 min (○) and the maximal rate of NADPH oxidase activity (•) were measured and are expressed as a percentage of their respective responses obtained in untreated eosinophils. Each data point represents the mean±s.e.mean of five independent experiments using eosinophils purified from the blood of different donors. Basal adhesion (per cent of total cells seeded) and respiratory burst, measured on FCS-coated plates, were and 4.09±0.37% and 0.103±0.022 RLU respectively.
Piceatannol (1 nM–10 μM) had no significant affect upon eosinophil adhesion or on the activity of the NADPH oxidase accept at the highest concentration (10 μM) studied, where it reduced superoxide anion generation by 44±16% (Figure 6b).
Role of protein kinase C isoenzymes in CD11b/CD18-mediated adhesion and superoxide anion generation
Three PKC inhibitors (Ro-31 8220, GF109203X and Gö 6976) were used in an attempt to dissect which isoenzymes might play a role in CD11b/CD18-mediated adhesion and superoxide anion generation. These drugs were selected on the basis of the PKC isoenzymes they selectively inhibit. Thus, Ro-31 8220 is a broad-spectrum PKC inhibitor (Davis et al., 1989) while GF109203X shows selectivity for the conventional and novel isoforms (cPKC and nPKC families respectively) and Gö 6976 is reported to selectively block the activity of cPKC family members (Martiny-Baron et al., 1993).
As shown in Figure 7, Ro-31 8220 (30 nM–10 μM) and GF 109203X (30 nM–100 μM) inhibited CD11b/CD18-mediated superoxide anion generation in a concentration-dependent manner (pIC50s=−6.61±0.11 and −6.05±0.2 respectively). However, concentrations of Ro-31 8220 (3 μM) and GF 109203X (10 μM) that abolished the respiratory burst, failed to significantly affect the adhesion of eosinophils to the tissue culture plates. In fact, there was a tendency of the former inhibitor to enhance this response (Figure 7a,b). Gö 6976 also suppressed superoxide anion generation in a concentration-dependent manner in the absence of a detectable affect on adhesion. However, the pIC50 of Gö 6976 (−4.89±0.56) was low relative to the potency of Ro-31 8220 and GF 109203X (Figure 7c).
Figure 7.
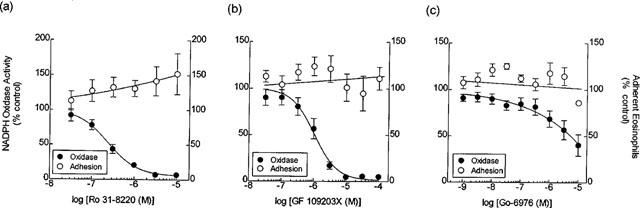
Effect of Ro-31 8220, GF 109203X and Gö 6976 on eosinophil adhesion and NADPH oxidase activity. Eosinophils (105) were seeded in BSA-coated 96-well tissue culture treated plates at 37°C containing Ro-31 8220 (a), GF 109203X (b), Gö 6976 (c) or their respective vehicles. The number of adherent eosinophils after 60 min (○) and the maximal rate of NADPH oxidase activity (•) were measured and are expressed as a percentage of their respective responses obtained in untreated eosinophils. Each data point represents the mean±s.e.mean of five independent experiments using eosinophils purified from the blood of different donors. Basal adhesion (per cent of total cells seeded) and respiratory burst, measured on FCS-coated plates, were 5.36±0.51% and 0.077±0.017 RLU respectively.
Role of PtdIns 3-kinase in CD11b/CD18-mediated adhesion and superoxide anion generation
Wortmannin (Arcaro & Wymann, 1993) was used to investigate the role of PtdIns 3-kinases in CD11b/CD18-mediated adhesion of, and superoxide generation by, human eosinophils. As illustrated in Figure 8, wortmannin (10 pM–1 μM) elicited a concentration-dependent inhibition both of NADPH oxidase activity and adhesion. For both responses the pEC50 values were not significantly different (−9.06±0.23 and −9.27±0.29 respectively) although the maximum inhibition of CD11b/CD18-mediated adhesion effected by wortmannin amounted to 50.4±7.7% under conditions where superoxide anion production was abolished.
Figure 8.

Effect of wortmannin on eosinophil adhesion and NADPH oxidase activity. Eosinophils (105) were seeded in BSA-coated 96-well tissue culture treated plates at 37°C containing wortmannin or its vehicle. The number of adherent eosinophils after 60 min (○) and the maximal rate of NADPH oxidase activity (•) were measured and are expressed as a percentage of their respective responses obtained in untreated eosinophils. Each data point represents the mean±s.e.mean of five independent experiments using eosinophils purified from the blood of different donors. Basal adhesion (per cent of total cells seeded) and respiratory burst, measured on FCS-coated plates, were 3.83±0.49% and 0.16±0.029 RLU respectively.
Discussion
A number of studies have demonstrated that the NADPH oxidase in human eosinophils is activated following ligation of the β2-integrin, CD11b/CD18. However, little is known of the intracellular mechanisms that mediate this physiological/pathophysiological response. In the experiments described herein, we report the development of an in vitro model to directly examine signalling by CD11b/CD18 and demonstrate, using a pharmacological approach, a role for nPKCs, p38 MAP kinase and PtdIns 3-kinase in CD11b/CD18-mediated superoxide anion generation.
Using a panel of blocking antibodies to β1- and β2-integrins, we initially set out to characterize the interaction of human eosinophils with tissue culture plates and how this effect relates to the production of a respiratory burst. The demonstration that 6.5E and KIM225 (anti-CD18 and anti-CD11b antibodies respectively) markedly suppressed eosinophil adhesion strongly implicates the β2 integrin CD11b/CD18 in this response. The observation that neither MAX68P, an antibody against CD49d, nor an isotype-matched control antibody (MOPC21), affected the process of eosinophil adhesion excludes a role for VLA-4 and a non-specific antibody effect. These data also support the idea that the binding of human eosinophils to tissue culture plates reflects a specific interaction involving the β2 integrin. Furthermore, the additional finding that superoxide anion generation followed a similar time-course to adhesion, together with the ability of 6.5E and KIM225 also to attenuate this response demonstrates that activation of the NADPH oxidase is dependent upon ligation of CD11b/CD18.
A consistent finding in these experiments was that the percentage of eosinophils that adhered to BSA-coated 96-well tissue culture plates varied significantly (30–60%) between donors and that on no occasion was 100% adherence observed. The inability of all eosinophils to stick to BSA-coated plastic is consistent with the results of similar experiments using other substrates (see Walsh et al., 1993) although why this occurs is unclear. One simple explanation is that too many eosinophils were seeded per well such that only a proportion of those gained access to the BSA. However, no evidence was found to support this idea. Indeed, the percentage of eosinophils that adhered to BSA remained invariant over a range of seeding densities in which the number of eosinophils was reduced from 105 to 0.2×105 cells per well. An alternative explanation is eosinophil heterogeneity. In this scenario only a certain proportion of eosinophils within each preparation express the necessary adhesion molecules to bind to BSA. It has been known for sometime that human blood eosinophils are physically, morphologically and functionally heterogeneous and that the expression of many cell surface epitopes varies between phenotypes (see Giembycz & Lindsay, 1999). In this respect, it is significant that cell surface CD18 is markedly (∼50%) lower in eosinophils of low buoyant density (Hartnell et al., 1990), which are considered to represent an activated phenotype associated with eosinophilia, when compared to their autologous normodense counterparts. Moreover, it has been proposed that low density eosinophils in the circulation have undergone down-regulation of their capacity to adhere to the vascular endothelium (Hartnell et al., 1990). Thus, given that the method of eosinophil purification used in the present study does not distinguish between eosinophil phenotypes, hypodense cells will be present in all preparations, and their number will vary significantly depending on whether or not donors have eosinophil-associated disease. Clearly, this could have an impact on the percentage of eosinophils able to adhere to BSA-coated plastic via a CD18-dependent mechanism.
fMLP failed to stimulate the NADPH oxidase in eosinophils seeded in FCS-coated plates but effectively primed CD11b/CD18-induced oxidative metabolism and adherence over the basal levels. These results are consistent with previous studies where fMLP is reported to increase the expression of CD11b/CD18 on human eosinophils and enhance adhesion to human umbilical vein endothelial cells (Neeley et al., 1993; Kimani et al., 1988). The phorbol ester, PMA, also activated the NADPH oxidase and effected adhesion of eosinophils but these effects differed from fMLP-induced responses. In particular, PMA evoked a respiratory burst in eosinophils regardless of whether FCS or BSA was used as substrate. One interpretation of this result is that PMA is active on non-adherent cells. However, PMA also augmented the adhesion of eosinophils to FCS-coated plates to a level that was not significantly different from that achieved with BSA. Thus, the role of adhesion molecules in PMA-induced oxidase activation in human eosinophils is equivocal and requires formal investigation.
It is clear from the above discussion that eosinophil adhesion and subsequent oxidant generation did not require the addition of an exogenous stimulus (so-called ‘inside-out' signalling) and so permitted a pharmacological examination of the mechanism of CD11b/CD18-mediated ‘outside-in' signalling. The identification of putative MAP kinase phosphorylation sites on p47phox, a cytosolic component of the NADPH oxidase (el Benna et al., 1994; 1996), prompted an examination of the role of these kinases in adhesion and/or adhesion-dependent superoxide generation. SB 203580, an inhibitor of the α- and β-isoforms of the p38 MAP kinase family, inhibited superoxide anion generation in a concentration-dependent manner but had no significant effect on CD11b/CD18-dependent adhesion. These results clearly demonstrate that SB 203580 suppresses activation of the NADPH oxidase directly and implicates p38 MAP kinase in CD11b/CD18-mediated ‘outside-in' signalling. Indeed, the ability of SB 203580 to suppress superoxide anion generation in human eosinophils occurred over a concentration range similar to a variety of p38 MAP kinase-driven responses including CD11b/CD18-dependent adhesion and adhesion-dependent oxidative burst of human neutrophils in response to lipopolysacchide and tumour necrosis factor-α (Detmers et al., 1998), fMLP-induced NADPH oxidase activation in non-adherent human neutrophils (Nick et al., 1997), interleukin-6 generation from interleukin-1β-stimulated human fibroblast-like synoviocytes (Miyazawa et al., 1998), the phosphorylation, in intact cells, of mitogen-activated protein kinase-activated protein kinase-2 (MAPKAPK-2; Miyazawa et al., 1998), a down-stream substrate of p38 MAP kinase, and the phosphorylation of heat-shock protein-27, a down-stream substrate of MAPKAPK-2 (Ridley et al., 1997). However, it is noteworthy that in the study by Detmers et al. (1998), the ability of SB 203580 to prevent the activation of the NADPH oxidase was secondary to the inhibition of adhesion suggesting that different mechanisms regulate superoxide anion generation between adherent granulocytes. As illustrated in Figure 5b, SB 203580 only partially suppressed CD11b/CD18-mediated superoxide anion generation even at high concentrations (10 μM) where selectivity for p38 MAP kinase is presumably lost, implying that other mechanisms also contribute to this response. The possible role of MEK-1 was excluded based on the consistent finding that PD 098059 had no affect on oxidant production even at concentrations (10 μM) that we have shown previously to inhibit the dual phosphorylation of ERK-1 and ERK-2 in eosinophils (Lindsay et al., 1998; Kankaanranta et al., 1999). This conclusion also is consistent with results obtained with fMLP-induced NADPH oxidase activation in non-adherent human neutrophils where PD 098059 similarly is inactive (Kuroki & O'Flaherty, 1997).
Previous studies have found that the binding of eosinophils through VLA4 (Nagata et al., 1995) and CD11b/CD18 (Kato et al., 1998) results in protein tyrosine phosphorylation. A number of candidate kinases could account for this effect including members of the syk and/or src-families of cytosolic protein tyrosine kinases. Indeed, on the basis of studies with selective inhibitors, evidence has been published implicating the syk and src families in ‘outside-in' signalling in a host of cells (Aplin et al., 1998) including neutrophils (Yan & Berton, 1996; Berton et al., 1994; Lowell et al., 1996; Fernandez & Suchard, 1998). To assess if tyrosine phosphorylation could be involved in CD11b/CD18-mediated superoxide anion generation in human adherent eosinophils, a similar pharmacological approach was adopted. However, no evidence was found to implicate either enzyme family. Thus, although PP1, a selective inhibitor of the src-related family of protein tyrosine kinases (Hanke et al., 1996), inhibited NADPH oxidase activation, it also prevented adhesion with equal potency suggesting a causal relationship between these responses. This conclusion is consistent with the results obtained with neutrophils isolated from hck−/− fgr−/− double-knockout mice that were unable to generate oxidants when plated on to surfaces coated with extracellular matrix proteins or murine ICAM-1 in the presence of TNF and fMLP (Lowell et al., 1996). Similarly, superoxide anion generation was not observed in neutrophils from the same double mutant animals following cross-linking of β2-subunits with surface-bound monoclonal antibodies, when compared to the wide-type mice (Lowell et al., 1996). In both cases, photomicroscopy revealed that impaired NADPH oxidase activity was due to defective spreading and tight adhesion (Lowell et al., 1996). A similar conclusion was reached with a naturally occurring inhibitor of syk, piceatannol (Geahlen & McLaughlin, 1989), which had no effect upon eosinophil adhesion or superoxide anion generation (accept at a high concentration). This was somewhat unexpected given that piceatannol is reported to attenuate syk activity, cell spreading and H2O2 production in fMLP-stimulated human neutrophils with an IC50 of approximately 1 μM (Fernandez & Suchard, 1998). However, this apparent discrepancy might relate simply to differences in activating stimulus, given that fMLP acts through a G-protein-coupled receptor (unlike CD11b/CD18). Alternatively, these data might be explained by a more fundamental difference between eosinophils and neutrophils in the mechanism of NADPH oxidase activation.
Protein kinase C is a generic term that represents an increasingly diverse superfamily of cell signalling enzymes (Nishizuka, 1992; 1995) that are implicated in eosinophil adhesion (Dobrina et al., 1991), degranulation (Kroegel et al., 1989) and NADPH oxidase activation (Sedgwick et al., 1990). Currently, at least eleven isoforms of PKC have been identified that, on the basis of molecular structure and biochemical properties, have been categorized into three groups: cPKCs (α, βI, βII and γ), which are Ca2+- and phospholipid-dependent; nPKCs (δ, ε, ζ and θ), which lack the Ca2+-binding region and are, therefore, Ca2+-independent; and atypical PKCs (aPKCs; ι, μ and ζ), which lack both the Ca2+- and diacylglycerol/phorbol ester binding-site (Nishizuka, 1992; 1995). We have reported previously that human eosinophils express representatives of the conventional (α, β1, βI1), novel (δ, ε) and atypical (ι, μ, ζ) PKC subtypes (Evans et al., 1999), and other studies have established that these three broad classes of enzymes can be differentiated by the use of the family-selective inhibitors, Ro-31 8220 (broad spectrum), GF109203X (cPKC and nPKC-selective) and Gö 6976 (cPKC-selective) (Davis et al., 1989; Martiny-Baron et al., 1993).
The results obtained with the aforementioned PKC inhibitors provide persuasive evidence that specific PKC isoforms are involved in the mechanism of CD11b/CD18-mediated NADPH oxidase activation in human eosinophils. Thus, while all three inhibitors blocked superoxide anion generation, none of them suppressed adhesion. The isoenzyme family responsible for this effect was inferred from the IC50 values obtained for each inhibitor. Thus, Ro-31 8220 (IC50∼0.25 μM) and GF 109203X (IC50∼1 μM) effectively suppressed oxidant production with potencies similar to those found for inhibition of fMLP-induced activation of the NADPH oxidase in human neutrophils (respective IC50's=0.2 and 0.85 nM; Lindsay et al., 1996; Wenzel-Seifert et al., 1994); in contrast, Gö 6976 was a relatively weak inhibitor (IC50∼13 μM). Since Ro 31-8220, GF 109203X and Gö 6976 all are bisindolylmaleimide derivatives with similar physical characteristics, these data suggest that the α, β1 and βI1 isoforms (cPKCs) in the eosinophils are unlikely to be involved in the adhesion-dependent activation of the NADPH oxidase. Additional support for this proposal comes from studies with macrophages where Gö 6976 abolished PMA-induced arachidonic acid release at a concentration (1 μM) that had only a modest inhibitory effect (∼30%) on CD11b/CD18-dependent superoxide anion production reported herein (Lin & Chen, 1998). Thus, it is suggested that CD11b/CD18-mediated NADPH oxidase activation requires the participation of nPKC family members of which the δ- and ε-subtypes have been identified in human eosinophils (Evans et al., 1999). In this respect, Kato et al. (1998) have reported that the adherence of IL-5-treated human eosinophils to protein-coated tissue culture plates via β2-integrins is associated with inositol phosphate accumulation. Clearly, this effect would provide the necessary diglyceride for the activation of nPKCs although the extent to which this accounts for CD11b/CD18-dependent signalling in the present study requires formal investigation. Again, differences in cell type and/or stimulus apparently dictate the subtype or complement of PKCs involved in a specific response given that fMLP, immune complex and PMA activate the NADPH oxidase, but not adhesion, in HL60 cells, by a PKCβ-dependent mechanism (Korchak et al., 1998).
Previous studies, using wortmannin, have implicated PtdIns 3-kinase in CD11b expression (Powell et al., 1999), chemotaxis (Palframan et al., 1998) and NADPH oxidase activation in human non-adherent eosinophils in response to agonists that act through G-protein-coupled receptors (Elsner et al., 1996). The experiments described in the present report extend those studies by demonstrating that wortmannin abolished superoxide anion production in adherent eosinophils with a potency (IC50∼0.9 nM) comparable to its ability to inhibit purified PtdIns 3-kinase (IC50's=2–5 nM) (Powis et al., 1994). This is an important observation since it reduces the possibility that wortmannin is working non-specifically on other enzymes such as myosin light chain kinase (Nakanishi et al., 1992) and PtdIns 4-kinase (Nakanishi et al., 1995) that are sensitive only to high nanomolar concentrations of the drug. It is possible that part of the inhibitory effect of wortmannin on superoxide anion generation is due to the inhibition of eosinophil adhesion since the EC50 values for both responses were almost identical (0.9 and 0.5 nM respectively). However, the finding that CD11b/CD18-dependent adhesion was maximally suppressed by only 50% implies that either a threshold level of adhesion is required to mount an oxidase response or that wortmannin also has an affect on those processes that govern ‘outside-in' (CD11b/CD18-driven) oxidase activation.
In conclusion, the experiments described herein demonstrate that human eosinophils adhere to BSA-coated tissue culture plastic by a CD11b/CD18-dependent mechanism that is responsible for the subsequent activation of the NADPH oxidase. Furthermore, although the precise ‘outside-in' signalling pathway(s) responsible for this effect still is to be elucidated, the results obtained with the selective inhibitors used in this study implicate p38 MAP kinase, nPKCs and PtdIns 3-kinase.
Acknowledgments
The authors gratefully acknowledge the British Lung Foundation [BLF], the Wellcome Trust (Grant #056814) and GlaxoWellcome for financial support, and Dr Martyn Robinson (Celltech Therapeutics Ltd, Slough) for the generous gift of the antibodies used in these studies.
Abbreviations
- ERK
extracellular-regulated kinase
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- H2O2
hydrogen peroxide
- MAPKAPK-2
mitogen-activated protein kinase-activated protein kinase-2
- MAP kinase
mitogen-activated protein kinase
- PKC
protein kinase C
- PtdIns 3-kinase
phosphatidylinositol 3-kinase
- VLA4
very late antigen-4
References
- ALESSI D.R., CUENDA A., COHEN P., DUDLEY D.T., SALTIEL A.R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- ANWAR A.R., MOQBEL R., WALSH G.M., KAY A.B., WARDLAW A.J. Adhesion to fibronectin prolongs eosinophil survival. J. Exp. Med. 1993;177:839–843. doi: 10.1084/jem.177.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANWAR A.R., WALSH G.M., CROMWELL O., KAY A.B., WARDLAW A.J. Adhesion to fibronectin primes eosinophils via α4β1 (VLA-4) Immunology. 1994;82:222–228. [PMC free article] [PubMed] [Google Scholar]
- APLIN A.E., HOWE A., ALAHARI S.K., JULIANO R.L. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol. Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- ARCARO A., WYMANN M.P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem. J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES P.J. Pathophysiology of asthma. Br. J. Clin. Pharmacol. 1996;42:3–10. doi: 10.1046/j.1365-2125.1996.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTON G., FUMAGALLI L., LAUDANNA C., SORIO C. β2-integrin-dependent protein tyrosine phosphorylation and activation of the fgr protein tyrosine kinase in human neutrophils. J. Cell Biol. 1994;126:1111–1121. doi: 10.1083/jcb.126.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS P.D., HILL C.H., KEECH E., LAWTON G., NIXON J.S., SEDGWICK A.D., WADSWORTH J., WESTMACOTT D., WILKINSON S.E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989;259:61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- DEAN J.L., BROOK M., CLARK A.R., SAKLATVALA J. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J. Biol. Chem. 1999;274:264–269. doi: 10.1074/jbc.274.1.264. [DOI] [PubMed] [Google Scholar]
- DESREUMAUX P., CAPRON M. Eosinophils in allergic reactions. Curr. Opin. Immunol. 1996;8:790–795. doi: 10.1016/s0952-7915(96)80006-9. [DOI] [PubMed] [Google Scholar]
- DETMERS P.A., ZHOU D., POLIZZI E., THIERINGER R., HANLON W.A., VAIDYA S., BANSAL V. Role of stress-activated mitogen-activated protein kinase (p38) in β2-integrin-dependent neutrophil adhesion and the adhesion-dependent oxidative burst. J. Immunol. 1998;161:1921–1929. [PubMed] [Google Scholar]
- DOBRINA A., MENEGAZZI R., CARLOS T.M., NARDON E., CRAMER R., ZACCHI T., HARLAN J.M., PATRIARCA P. Mechanisms of eosinophil adherence to cultured vascular endothelial cells. Eosinophils bind to the cytokine-induced ligand vascular cell adhesion molecule-1 via the very late activation antigen-4 integrin receptor. J. Clin. Invest. 1991;88:20–26. doi: 10.1172/JCI115278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRI P., CRAMER R., SPESSOTTO P., ROMANO M., PATRIARCA P. Eosinophil activation on biologic surfaces. Production of O2− in response to physiologic soluble stimuli is differentially modulated by extracellular matrix components and endothelial cells. J. Immunol. 1991;147:613–620. [PubMed] [Google Scholar]
- DUDLEY D.T., PANG L., DECKER S.J., BRIDGES A.J., SALTIEL A.R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL BENNA J., FAUST L.P., BABIOR B.M. The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation. Phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. J. Biol. Chem. 1994;269:23431–23436. [PubMed] [Google Scholar]
- EL BENNA J., FAUST R.P., JOHNSON J.L., BABIOR B.M. Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. Phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase. J. Biol. Chem. 1996;271:6374–6378. doi: 10.1074/jbc.271.11.6374. [DOI] [PubMed] [Google Scholar]
- ELSNER J., HOCHSTETTER R., KIMMIG D., KAPP A. Human eotaxin represents a potent activator of the respiratory burst of human eosinophils. Eur. J. Immunol. 1996;26:1919–1925. doi: 10.1002/eji.1830260837. [DOI] [PubMed] [Google Scholar]
- EVANS D.J., LINDSAY M.A., WEBB B.L.J., KANKAANRANTA H., GIEMBYCZ M.A., O'CONNOR B.J., BARNES P.J. Expression and activation of protein kinase C-ζ in eosinophils after allergen challenge. Am. J. Physiol. 1999;277:L233–L239. doi: 10.1152/ajplung.1999.277.2.L233. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ R., SUCHARD S.J. Syk activation is required for spreading and H2O2 release in adherent human neutrophils. J. Immunol. 1998;160:5154–5162. [PubMed] [Google Scholar]
- FUORTES M., JIN W.W., NATHAN C. β2 integrin-dependent tyrosine phosphorylation of paxillin in human neutrophils treated with tumor necrosis factor. J. Cell Biol. 1994;127:1477–1483. doi: 10.1083/jcb.127.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEAHLEN R.L., MCLAUGHLIN J.L. Piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene) is a naturally occurring protein-tyrosine kinase inhibitor. Biochem. Biophys. Res. Commun. 1989;165:241–245. doi: 10.1016/0006-291x(89)91060-7. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., LINDSAY M.A. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51:213–339. [PubMed] [Google Scholar]
- GYLLENHAMMAR H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J. Immunol. Methods. 1987;97:209–213. doi: 10.1016/0022-1759(87)90461-3. [DOI] [PubMed] [Google Scholar]
- HANKE J.H., GARDNER J.P., DOW R.L., CHANGELIAN P.S., BRISSETTE W.H., WERINGER E.J., POLLOK B.A., CONNELLY P.A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of lck- and fyn-dependent T cell activation. J. Biol. Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- HANSEL T.T., POUND J.D., PILLING D., KITAS G.D., SALMON M., GENTLE T.A., LEE S.S., THOMPSON R.A. Purification of human blood eosinophils by negative selection using immunomagnetic beads. J. Immunol. Methods. 1989;122:97–103. doi: 10.1016/0022-1759(89)90339-6. [DOI] [PubMed] [Google Scholar]
- HARTNELL A., MOQBEL R., WALSH G.M., BRADLEY B., KAY A.B. Fcγ and CD11/CD18 receptor expression on normal density and low density human eosinophils. Immunology. 1990;69:264–270. [PMC free article] [PubMed] [Google Scholar]
- HORIE S., KITA H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte/macrophage colony-stimulating factor and platelet-activating factor. J. Immunol. 1994;152:5457–5467. [PubMed] [Google Scholar]
- JONES S.L., KNAUS U.G., BOKOCH G.M., BROWN E.J. Two signaling mechanisms for activation of αMβ2 avidity in polymorphonuclear neutrophils. J. Biol. Chem. 1998;273:10556–10566. doi: 10.1074/jbc.273.17.10556. [DOI] [PubMed] [Google Scholar]
- KANKAANRANTA H., DE SOUZA P.M., SALMON M., BARNES P.J. , GIEMBYCZ M.A., LINDSAY M.A. SB 203580, an inhibitor of p38 mitogen-activated protein kinase, augments constitutive apoptosis of human eosinophils. J. Pharmacol. Exp. Ther. 1999;290:621–628. [PubMed] [Google Scholar]
- KATO M., ABRAHAM R.T., OKADA S., KITA H. Ligation of the β2 integrin triggers activation and degranulation of human eosinophils. Am. J. Respir. Cell Mol. Biol. 1998;18:675–686. doi: 10.1165/ajrcmb.18.5.2885. [DOI] [PubMed] [Google Scholar]
- KIMANI G., TONNESEN M.G., HENSON P.M. Stimulation of eosinophil adherence to human vascular endothelial cells in vitro by platelet-activating factor. J. Immunol. 1988;140:3161–3166. [PubMed] [Google Scholar]
- KORCHAK H.M., ROSSI M.W., KILPATRICK L.E. Selective role for β-protein kinase C in signaling for O2− generation but not degranulation or adherence in differentiated HL60 cells. J. Biol. Chem. 1998;273:27292–27299. doi: 10.1074/jbc.273.42.27292. [DOI] [PubMed] [Google Scholar]
- KROEGEL C., PLEASS R., YUKAWA T., CHUNG K.F., WESTWICK J., BARNES P.J. Characterization of platelet-activating factor-induced elevation of cytosolic free calcium concentration in eosinophils. FEBS Lett. 1989;243:41–46. doi: 10.1016/0014-5793(89)81214-1. [DOI] [PubMed] [Google Scholar]
- KUROKI M., O'FLAHERTY J.T. Differential effects of a mitogen-activated protein kinase kinase inhibitor on human neutrophil responses to chemotactic factors. Biochem. Biophys. Res. Commun. 1997;232:474–477. doi: 10.1006/bbrc.1997.6296. [DOI] [PubMed] [Google Scholar]
- LAL A.S., CLIFTON A.D., ROUSE J., SEGAL A.W., COHEN P. Activation of the neutrophil NADPH oxidase is inhibited by SB 203580, a specific inhibitor of SAPK2/p38. Biochem. Biophys. Res. Commun. 1999;259:465–470. doi: 10.1006/bbrc.1999.0759. [DOI] [PubMed] [Google Scholar]
- LAUDANNA C., MELOTTI P., BONIZZATO C., PIACENTINI G., BONER A., SERRA M.C., BERTON G. Ligation of members of the β1 or the β2 subfamilies of integrins by antibodies triggers eosinophil respiratory burst and spreading. Immunology. 1993;80:273–280. [PMC free article] [PubMed] [Google Scholar]
- LAUDANNA C., MOCHLY-ROSEN D., LIRON T., CONSTANTIN G., BUTCHER E.C. Evidence of ζ protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J. Biol. Chem. 1998;273:30306–30315. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- LIN W.W., CHEN B.C. Distinct PKC isoforms mediate the activation of cPLA2 and adenylyl cyclase by phorbol ester in RAW 264.7 macrophages. Br. J. Pharmacol. 1998;125:1601–1609. doi: 10.1038/sj.bjp.0702219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDSAY M.A., DANIELS I., FLETCHER J. Investigation of the role of protein kinase C and tyrosine kinases during the rapid and sustained release of superoxide from adherent human neutrophils. Biochem. Soc. Trans. 1996;24:67S. doi: 10.1042/bst024067s. [DOI] [PubMed] [Google Scholar]
- LINDSAY M.A., HADDAD E.B., ROUSELL J., TEIXEIRA M.M., HELLEWELL P.G., BARNES P.J., GIEMBYCZ M.A. Role of the mitogen-activated protein kinases and tyrosine kinases during leukotriene B4-induced eosinophil activation. J. Leukocyte Biol. 1998;64:555–562. doi: 10.1002/jlb.64.4.555. [DOI] [PubMed] [Google Scholar]
- LOWELL C.A., FUMAGALLI L., BERTON G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J. Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINY-BARON G., KAZANIETZ M.G., MISCHAK H., BLUMBERG P.M., KOCHS G., HUG H., MARME D., SCHACHTELE C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- METZNER B., HEGER M., HOFMANN C., CZECH W., NORGAUER J. Evidence for the involvement of phosphatidylinositol 4,5-bisphosphate 3-kinase in CD18-mediated adhesion of human neutrophils to fibrinogen. Biochem. Biophys. Res. Commun. 1997;232:719–723. doi: 10.1006/bbrc.1997.6350. [DOI] [PubMed] [Google Scholar]
- MIYAZAWA K., MORI A., MIYATA H., AKAHANE M., AJISAWA Y., OKUDAIRA H. Regulation of interleukin-1β-induced interleukin-6 gene expression in human fibroblast-like synoviocytes by p38 mitogen-activated protein kinase. J. Biol. Chem. 1998;273:24832–24838. doi: 10.1074/jbc.273.38.24832. [DOI] [PubMed] [Google Scholar]
- MUNOZ N.M., RABE K.F., NEELEY S.P., HERRNREITER A., ZHU X.D., MCALLISTER K., MAYER D., MAGNUSSEN H., GALENS S., LEFF A.R. Eosinophil VLA-4 binding to fibronectin augments bronchial narrowing through 5-lipoxygenase activation. Am. J. Physiol. 1996;14:L587–L594. doi: 10.1152/ajplung.1996.270.4.L587. [DOI] [PubMed] [Google Scholar]
- NAGATA M., SEDGWICK J.B., BATES M.E., KITA H., BUSSE W.W. Eosinophil adhesion to vascular cell adhesion molecule-1 activates superoxide anion generation. J. Immunol. 1995;155:2194–2202. [PubMed] [Google Scholar]
- NAKANISHI S., CATT K.J., BALLA T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositol phospholipids. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKANISHI S., KAKITA S., TAKAHASHI I., KAWAHARA K., TSUKUDA E., SANO T., YAMADA K., YOSHIDA M., KASE H., MATSUDA Y. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J. Biol. Chem. 1992;267:2157–2163. [PubMed] [Google Scholar]
- NATHAN C.F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J. Clin. Invest. 1987;80:1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEELEY S.P., HAMANN K.J., DOWLING T.L., MCALLISTER K.T., WHITE S.R., LEFF A.R. Augmentation of stimulated eosinophil degranulation by VLA-4 (CD49d)-mediated adhesion to fibronectin. Am. J. Respir. Cell Mol. Biol. 1994;11:206–213. doi: 10.1165/ajrcmb.11.2.8049081. [DOI] [PubMed] [Google Scholar]
- NEELEY S.P., HAMANN K.J., WHITE S.R., BARANOWSKI S.L., BURCH R.A., LEFF A.R. Selective regulation of expression of surface adhesion molecules Mac-1, L-selectin, and VLA-4 on human eosinophils and neutrophils. Am. J. Respir. Cell Mol. Biol. 1993;8:633–639. doi: 10.1165/ajrcmb/8.6.633. [DOI] [PubMed] [Google Scholar]
- NICK J.A., AVDI N.J., YOUNG S.K., KNALL C., GERWINS P., JOHNSON G.L., WORTHEN G.S. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J. Clin. Invest. 1997;99:975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIZUKA Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- NISHIZUKA Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- PALFRAMAN R.T., COLLINS P.D., WILLIAMS T.J., RANKIN S.M. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood. 1998;91:2240–2248. [PubMed] [Google Scholar]
- POWELL W.S., GRAVEL S., HALWANI F. 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of L-selectin shedding, surface expression of CD11b, actin polymerization, and calcium mobilization in human eosinophils. Am. J. Respir. Cell Mol. Biol. 1999;20:163–170. doi: 10.1165/ajrcmb.20.1.3141. [DOI] [PubMed] [Google Scholar]
- POWIS G., BONJOUKLIAN R., BERGGREN M.M., GALLEGOS A., ABRAHAM R., ASHENDEL C., ZALKOW L., MATTER W.F., DODGE J., GRINDEY G. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3- kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- RAFIEE P., LEE J.K., LEUNG C.C., RAFFIN T.A. TNFα induces tyrosine phosphorylation of mitogen-activated protein kinase in adherent human neutrophils. J. Immunol. 1995;154:4785–4792. [PubMed] [Google Scholar]
- RIDLEY S.H., SARSFIELD S.J., LEE J.C., BIGG H.F., CAWSTON T.E., TAYLOR D.J., DEWITT DL., SAKLATVALA J. Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase: regulation of prostaglandin H synthase-2, metalloproteinases, and IL-6 at different levels. J. Immunol. 1997;158:3165–3173. [PubMed] [Google Scholar]
- SEDGWICK J.B., GEIGER K.M., BUSSE W.W. Superoxide generation by hypodense eosinophils from patients with asthma. Am. Rev. Respir. Dis. 1990;142:120–125. doi: 10.1164/ajrccm/142.1.120. [DOI] [PubMed] [Google Scholar]
- SEMINARIO M.C., GLEICH G.J. The role of eosinophils in the pathogenesis of asthma. Curr. Opin. Immunol. 1994;6:860–864. doi: 10.1016/0952-7915(94)90005-1. [DOI] [PubMed] [Google Scholar]
- TONG L., PAV S., WHITE D.M., ROGERS S., CRANE K.M., CYWIN C.L., BROWN M.L., PARGELLIS C.A. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nature (Struct. Biol). 1997;4:311–316. doi: 10.1038/nsb0497-311. [DOI] [PubMed] [Google Scholar]
- WALSH G.M., WARDLAW A.J., KAY A.B.Eosinophil accumulation, secretion and activation Immunopharmacology of Eosinophils 1993Academic Press, London; 73–90.In: Smith, H. & Cook, R.M. (eds) [Google Scholar]
- WENZEL-SEIFERT K., SCHACHTELE C., SEIFERT R. n-Protein kinase C isoenzymes may be involved in the regulation of various neutrophil functions. Biochem. Biophys. Res. Commun. 1994;200:1536–1543. doi: 10.1006/bbrc.1994.1625. [DOI] [PubMed] [Google Scholar]
- YAN S.R., BERTON G. Regulation of Src family tyrosine kinase activities in adherent human neutrophils. Evidence that reactive oxygen intermediates produced by adherent neutrophils increase the activity of the p58c-fgr and p53/56lyn tyrosine kinases. J. Biol. Chem. 1996;271:23464–23471. doi: 10.1074/jbc.271.38.23464. [DOI] [PubMed] [Google Scholar]
- YAN S.R., HUANG M., BERTON G. Signaling by adhesion in human neutrophils: activation of the p72syk tyrosine kinase and formation of protein complexes containing p72syk and Src family kinases in neutrophils spreading over fibrinogen. J. Immunol. 1997;158:1902–1910. [PubMed] [Google Scholar]
- YOSHIDA K., SUKO M., MATSUZAKI G., SUGIYAMA H., OKUDAIRA H., ITO K. Effect of fibronectin on the production of leukotriene C4 by eosinophils. Int. Arch. Allergy Immunol. 1995;108 Suppl 1:50–51. doi: 10.1159/000237203. [DOI] [PubMed] [Google Scholar]
- YOUNG P.R., MCLAUGHLIN M.M., KUMAR S., KASSIS S., DOYLE M.L., MCNULTY D., GALLAGHER T.F., FISHER S., MCDONNELL P.C., CARR S.A., HUDDLESTON M.J., SEIBEL G., PORTER T.G., LIVI G.P., ADAMS J.L., LEE J.C. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J. Biol. Chem. 1997;272:12116–12121. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]


