Abstract
Endothelial dysfunction has been described with ageing but the mechanisms responsible have not been clearly elucidated and might be different from one vessel to the other. This study assesses the relative contribution of endothelial nitric oxide (NO) and cyclo-oxygenase (COX) metabolites in relaxation to acetylcholine with ageing in the aorta and the small mesenteric artery of the rat.
In the aorta and branch II or III of superior mesenteric artery (SMA), endothelium-dependent relaxation to acetylcholine was not different between 12–14 (adult) and 32-week-old rats whereas it was reduced at 70–100 (old) weeks of age.
Despite an increased endothelial NO-synthase protein expression, the NO-synthase inhibitor, NG-nitro-L-arginine-sensitive component of relaxation decreased with ageing.
In old rats, exposure to the COX inhibitor, indomethacin, but not the selective COX-2 inhibitor, NS-398, potentiated response to acetylcholine. The thromboxane A2/prostaglandin H2 receptor antagonist, GR 32191B enhanced relaxation to acetylcholine in aorta but it had no effect in SMA. Furthermore, acetylcholine increased thromboxane B2 production (enzymeimmunoassay) in aorta but not in SMA. Finally, Western blot analysis showed enhanced expression of COX-1 and 2 in the two arteries with ageing.
These results suggest that the decrease in acetylcholine-induced relaxation with ageing involves reduced NO-mediated dilatation and increased generation of vasoconstrictor prostanoids most likely from COX-1. They also point out vascular bed heterogeneity related to the nature of prostanoids involved between the aorta (i.e., thromboxane A2) and the SMA (unidentified) arteries even though increased expression of COX occurs in both vessels.
Keywords: Ageing, conductance and resistance arteries, endothelium, cyclo-oxygenase, nitric oxide
Introduction
Ageing is associated with marked changes in the cardiovascular system, especially at the level of the vascular wall. In addition to the possible role of intrinsic sympathetic nerves, the abnormalities of the vascular wall are accompanied with both structural and functional changes that can take place at the level of the endothelium, the vascular smooth muscle cells and the extracellular matrix of blood vessels.
Endothelial dysfunction has been described with ageing both in animals and human patients under basal conditions and in response to agonist or physical stimuli. With respect to endothelium-dependent relaxation to agonist such as acetylcholine (ACh), a reduced response has been found in different vascular beds including the human brachial artery (Taddei et al., 1995), the aorta (Küng & Lüscher, 1995), the carotid (Hongo et al., 1988) and the perfused mesenteric bed of the rat (Atkinson et al., 1994). The mechanisms responsible for this age-related endothelial dysfunction have not yet been clearly elucidated. Several hypothesis have been advanced in the aorta, including changes in activity or expression of endothelial NO-synthase (Cernadas et al., 1998; Barton et al., 1997) or increased participation of vasoconstrictor products from COX (Koga et al., 1989). Finally, alterations in lipid prooxidant-antioxidant equilibrium have also been reported to be associated with age-related endothelial dysfunction in the rat proximal tail artery (Rodriguez-Martinez et al., 1998). The present study was aimed to investigate the mechanisms involved in age-related endothelial dysfunction in the rat. The experiments were designed to study the relative contribution of NO-synthase and COX pathways using appropriate pharmacological inhibitors. Furthermore, expression of endothelial NO-synthase (eNOS) or different COX isoforms (i.e., the constitutive COX-1 and the inducible COX-2) were determined. Also, an anatomical heterogeneity of age-related endothelial dysfunction in the same animal has been reported in the rat (i.e., aorta and a muscular artery, the common femoral artery) (Barton et al., 1997). The existence of such anatomical heterogeneity was studied between the aorta and small mesenteric arteries which are known to play a role in the regulation of local blood flow.
Methods
Animals
Male Wistar rats from 12–14 (adult), 32 or 70–100 (old) week-old were bred in our institute from genitors provided by Iffa-Credo (Lyon, France). This investigation conforms to the authorization (number 01918) for the use of laboratory animals given by the French government (Department of Agriculture).
Mean arterial blood pressure was determined in pentobarbitone anaesthetized rats (60 mg kg−1) by direct measurement with a carotid catheter and a Statham 23 DB transducer.
Arterial preparation and mounting
The animals were killed by cervical dislocation and exsanguinated. At necropsy, no apparent pathology was noted in any animal. Thoracic aorta and branch II or III of superior mesenteric artery (SMA) (normalized internal diameter (μm): 172±8 and 198±12 for adult and old rats respectively) were carefully removed and segments of arteries (2–3 mm or 1.6–2.0 mm in length for aorta and for SMA, respectively) were mounted on myographs (for SMA: wire myographs as described by Mulvany & Halpern (1977) were used) filled with physiological salt solution (PSS) of the following composition (in mM): NaCl 119, KCl 4.7, MgSO4 1.17, KH2PO4 (1.18 and 0.4), NaHCO3 (25 and 14.9), CaCl2 (1.25 and 2.5) and glucose (11 and 5.5) for aorta and SMA respectively, under normalized tension as previously described (Andriambeloson et al., 1997; Martinez et al., 1996). Briefly, SMA were stretched to 200 and 400 mg for adult and old rats respectively, lengths that yielded circumferences equivalent to 90% of those which the vessels would have had with an intramural pressure of 100 Hg (i.e., the pressure corresponds to the physiological value). Aortic rings were stretched to 2 and 2.5 g respectively in adult and old rats, which yielded maximal contractile responses to high potassium chloride physiological salt solution. The PSS was continuously kept at 37°C and gassed with 95% O2 and 5% CO2 at pH 7.4. In some experiments, the endothelial layer was removed immediately after dissection either by gently rubbing the intimal surface with curved forceps for aorta or by intraluminal perfusion with 0.5% 3-[(3-cholamidopropyl) dimethylammonio]-1 propane sulphonate (CHAPS) in PSS for 25 s followed by repeated washing with PSS for SMA.
Challenges with either 1 μM (aorta) or 10 μM (SMA) noradrenaline (NA) were performed to test their maximal contractile capacity and to elicit reproducible contractile response. The presence of functional endothelium was assessed, in adult rats, by the ability of acetylcholine (ACh, 1 μM) to induce more than 50% relaxation of vessels pre-contracted with NA (1 μM (aorta) and 10 μM (SMA)). The absence of a relaxation response to ACh was taken as evidence that the vessel segments were functionally denuded of endothelium.
Relaxation experiments
Arteries with and without functional endothelium were pre-contracted at 80% of their maximal contraction with NA. The concentration of NA was adjusted for each preparation being 0.3 μM for the aorta and 3 μM for SMA in vessels with functional endothelium. In some experiments, the thromboxane agonist, (9,11-di-deoxy-11α, 9α-epoxymethano-prostaglandin F2α), U46619 (0.01 μM) was used to contract the aorta. When the contraction reached a plateau, cumulative addition of vasodilator agents was performed (i.e., ACh, the Ca2+ ionophore, calcimycin (A23187) and the NO donor, S-N-acetylpenicillamine (SNAP)). For SNAP, concentration-response curve was constructed only in vessels functionally denuded of their endothelium in order to test specifically the sensitivity of the smooth muscle to NO and to avoid the influence of the endothelium. Only one ring of aorta and SMA per rat was tested for a given protocol. Each artery was used with one of the above relaxing agents only. Concentration-response curves were constructed in the absence or in the presence of the indicated inhibitor(s). For the aorta, only one concentration-response curve was performed on each artery either in the presence or in the absence of inhibitor. For SMA, preliminary experiments showed that two consecutive concentration-response curves to ACh separated by a 45 min washout period were not significantly different in the absence of other treatment. The first curve was thus taken as control and the effect of the inhibitor was tested on the second curve. The following inhibitors were used: the NO synthase inhibitor, NG-nitro-L-arginine (L-NA, 30 μM), the superoxide anion and the hydrogen peroxide scavengers, superoxide dismutase (SOD, 100 u ml−1) and catalase (100 u ml−1), the non selective COX inhibitor, indomethacin (10 μM), the selective COX-2 inhibitor, N-(2-cyclohexyloxy-4-nitrophenyl) methanesulphonamide, (NS-398, 1 μM), the thromboxane A2/prostaglandin H2, Tp receptor antagonist, [1R-[1α (z),2β,3β m 5α]]-(+)-7-[5-[[(1,1′-biphenyl)-4-yl] methoxy]-3-hydroxy-2-(1-piperidinyl) cyclopentyl]-4-heptenoic acid, hydrochloride, (GR 32191B, 3 μM). When L-NA was used, the concentration of NA was adjusted in order to obtain the same level of pre-contraction. All the inhibitors were used at a maximally active concentration and were incubated with the tissue for 25 min before the pre-contraction with NA.
Thromboxane B2 (TXB2) production
To determine the production of TXB2, aortic rings or branches II or III of SMA with endothelium were placed in 1 ml PSS for 20 min at 37°C in a 95% O2–5% CO2 incubator. At the end of this period, NA (0.3 μM or 3 μM, aorta and SMA respectively) was added for 10 min followed by 1 μM ACh for 2 min. Then the medium was collected and TXB2 was measured by enzymeimmunoassay (EIA) system (R&D Systems, Abingdon, U.K.). TXB2 production was expressed as pg μg−1 DNA, DNA content being measured as described by Brunck et al. (1976).
Western-blotting with anti-eNOS, anti COX-1 and anti COX-2 antibodies
Aortic rings or branches II or III of SMA were homogenized and approximately 50 μg of total protein from supernatant fractions was loaded into 7% and 10% SDS-polyacrylamide gel to separate eNOS and COX-1 or COX-2, respectively. After electrophoresis, proteins were transferred into nitrocellulose membrane. Immunostaining of eNOS, COX-1 and COX-2 were achieved using specific monoclonal mouse anti-eNOS (Transduction Laboratories), anti-COX-1 and anti-COX-2 (Oxford Biochemical Research, Inc) antibodies and reacted with peroxidase-conjugated antimouse antibody. The blots were detected using an enhanced chemiluminescence assay (ECL, Amersham, U.K.) and evaluated by densitometry.
Drugs
Noradrenaline, acetylcholine, A23187, NG-nitro-L-arginine, superoxide dismutase, catalase and indomethacin were all obtained from Sigma-Aldrich (Saint-Quentin Fallavier, France). U46619 was obtained from Cayman Chemical Company (SPI Bio, Massy, France), S-N-acetylpenicillamine from Tocris (Bioblock Scientific, Illkirch, France). GR 32191B was a generous gift from Glaxo Research and Development, Hertfordshire, U.K.
Expression of results and statistical analysis
Results were expressed as a relaxation per cent of the initial NA- or U46619-induced pre-contraction level. The concentration of NA (or U46619) was adjusted for each preparation to obtain 80% of the maximal response. Briefly, the 80% of the maximal response was estimated by performing a concentration-response curve to the agonist by cumulative addition of increasing concentrations on both types of vessels from adult and old rats. The values of contractions obtained with NA in vessels with endothelium (0.3 μM for aorta and 3 μM for SMA) were 2.4±0.26 (n=14), 2.0±0.16 (n=6) and 2.0±0.26 (n=13) g mg−1 dry tissue in aorta, and 3.2±0.35 (n=12), 2.7±0.25 (n=4) and 3.2±0.32 mN mm−1 (n=10) in SMA from 12–14, 32 and 70–100-week-old respectively (not significantly different with age). All results are expressed as mean±s.e.mean of n experiments, n represents the number of rats. Sensitivities to agonists are expressed as pD2 values, where pD2=−log EC50, EC50 being the molar concentration of the agonist that produces 50% of the maximal effect and were calculated by logit-log regression. Analysis of variance (MANOVA) and Student's t-test were used as appropriate for statistical analysis. Differences were considered significant when P<0.05.
Results
Body weight, and blood pressure
Body weight significantly increased with age (g) (405±17.1 (n=14), 518±16.0 (n=13) and 805±26.1 (n=15), P<0.001, 12–14, 32 and 70–100-week-old respectively). Mean blood pressure was not significantly different between 12–14, 32 and 70–100-week-old rats (114±9.2, 130±2.9 and 126±6.3 mmHg).
Endothelium-dependent relaxation
ACh produced relaxation in a concentration-dependent manner both in aortic rings and in the SMA with endothelium precontracted with NA (Figure 1A,B) but it failed to produce relaxation in endothelium-denuded arteries (not shown). The concentration-response curves to ACh were not significantly different in aortic rings and in the SMA from 12–14 and 32-week-old rats (Figure 1A,B) but were dramatically decreased in 70–100 compared to 12–14 and 32-week-old rats in both arteries (P<0.001) (Figure 1A,B). Therefore, the following experiments were performed in arteries taken from 12–14 (adult) and 70–100 (old) week-old rats. Also, the endothelium-dependent relaxation produced by A23187 was reduced in the aorta and the SMA precontracted with NA in old versus adult rats (Table 1).
Figure 1.
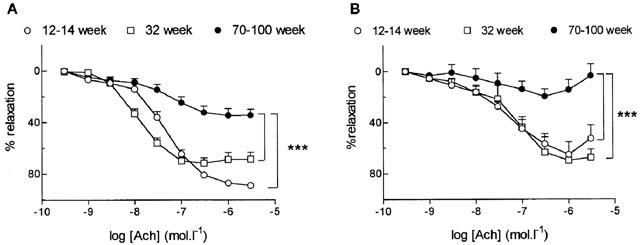
Influence of age on relaxation to acetylcholine (ACh) in the aorta (A) and the small mesenteric artery (B) from 12–14, 32 and 70–100-week-old rats. Results are expressed as a relaxation per cent of the initial noradrenaline-induced precontraction level. Data are mean±s.e.mean of 6–14 arterial segments. ***P<0.001, 12–14 and 32 week versus 70–100-week-old rats.
Table 1.
pD2 values and maximal effects of A23187, ACh and SNAP in aorta and small mesenteric (SMA) arteries from adult and old rats after pre-contraction to noradrenaline

The NO-synthase inhibitor, L-NA (30 μM), significantly inhibited ACh-induced relaxation in both type of arteries from adult rats (P<0.001, P<0.05, aorta and SMA respectively) (Figure 2A,B). In old rats, the same concentration of L-NA abolished the relaxation to ACh in aorta (P<0.01) but it had no effect in SMA (Figure 2C,D).
Figure 2.
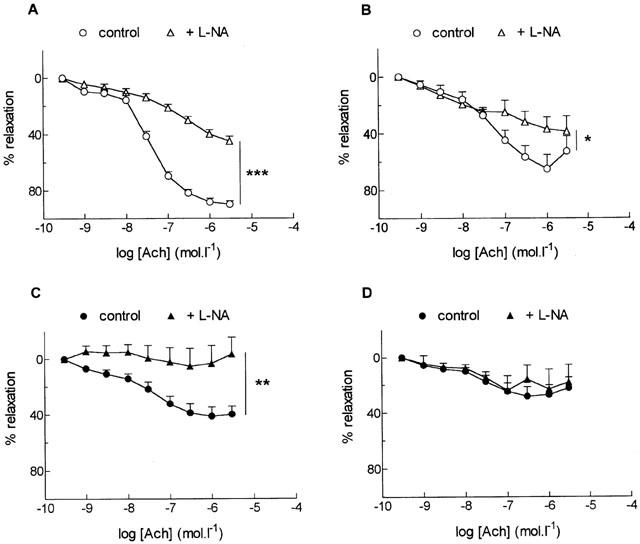
Effect of the nitric oxide synthase inhibitor, NG-nitro-L-arginine (L-NA, 30 μM), on relaxation to acetylcholine (ACh) in the aorta (A, C) and the small mesenteric artery (B, D) from adult (A, B) and old (C, D) rats. Results are expressed as a relaxation per cent of the initial noradrenaline-induced precontraction level. Data are mean±s.e.mean of 6–7 arterial segments. ***P<0.001, **P<0.01, *P<0.05, with versus without L-NA.
The combination of the superoxide anions and the hydrogen peroxide scavengers, SOD (100 u ml−1) and catalase (100 u ml−1), did not affect ACh responses in both type of arteries (Table 1). The efficacy of SOD was demonstrated by its ability to inhibit the contractile response to pyrogallol, a generator of superoxide anions in aortic rings from adult rats.
Both in aorta and in SMA, the concentration-response curves to the NO donor, SNAP, were not significantly different in vessels without functional endothelium from adult versus old rats (Table 1).
In adult rats, the non selective COX inhibitor, indomethacin (10 μM), did not affect significantly relaxation to ACh in both types of arteries (Figure 3A,B) but, it significantly potentiated the response to ACh both in aorta and in SMA from old rats (P<0.05, P<0.01) (Figure 3C,D).
Figure 3.
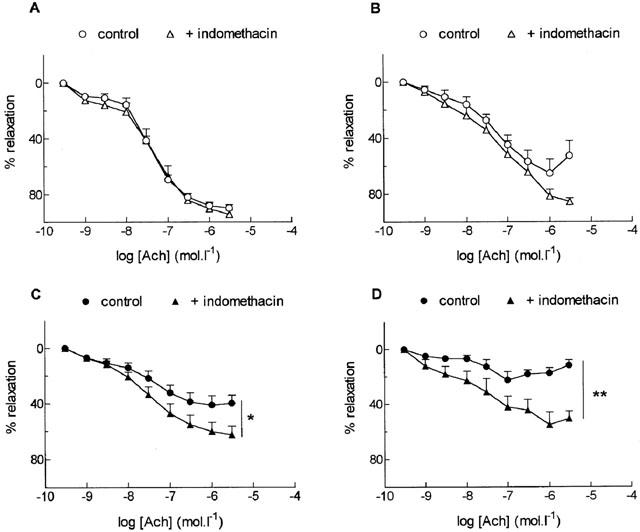
Effect of the cyclo-oxygenase inhibitor, indomethacin (10 μM), on relaxation to acetylcholine (ACh) in the aorta (A, C) and the small mesenteric artery (B, D) from adult (A, B) and old (C, D) rats. Results are expressed as a relaxation per cent of the initial noradrenaline-induced precontraction level. Data are mean±s.e.mean of 6–7 arterial segments. **P<0.01, *P<0.05, with versus without indomethacin.
Exposure to both L-NA (30 μM) and indomethacin (10 μM) did not result in further alteration of relaxation compared to L-NA alone (in the aorta from both adult and old rats and in SMA from adult rats) or to indomethacin alone (in old rats) (not shown).
As illustrated in Figure 4, NS-398 (1 μM), a selective COX-2 inhibitor, did not affect significantly relaxation to ACh in both types of arteries from adult (Figure 4A,B) and in SMA from old rats (Figure 4D). In the aorta from old rats, NS-398 slightly but significantly inhibited relaxation to ACh (P<0.05) (Figure 4C).
Figure 4.

Effect of the selective cyclo-oxygenase-2 inhibitor, NS-398 (1 μM), on relaxation to acetylcholine (ACh) in the aorta (A, C) and the small mesenteric artery (B, D) from adult (A, B) and old (C, D) rats. Results are expressed as a relaxation per cent of the initial noradrenaline (for SMA) or U46619 (for aorta)-induced precontraction level. Data are mean±s.e.mean of 5–6 arterial segments. *P<0.05, with versus without NS-398.
As illustrated in Figure 5, GR 32191 B (3 μM), a thromboxane A2/prostaglandin H2 receptor antagonist, significantly potentiated the response to ACh in aorta (P<0.05, Figure 5A) but it did not modify ACh-induced relaxation in SMA (Figure 5B) from old rats. In aorta and SMA from adult rats, the responses to ACh were not significantly modified in the presence of GR 32191 B (3 μM) (data not shown).
Figure 5.
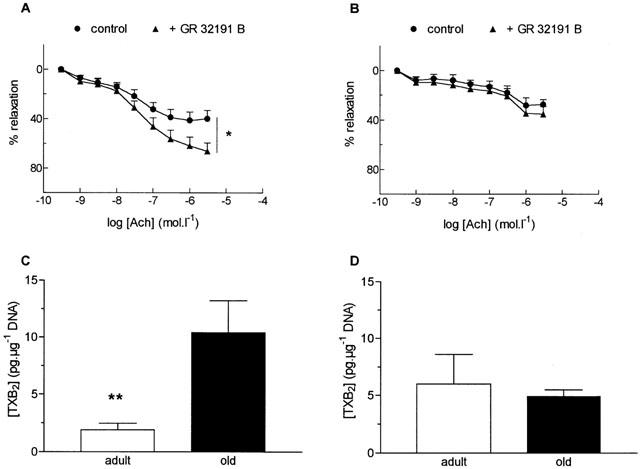
Effect of the thromboxane A2/prostaglandin H2 receptor antagonist, GR 32191 B (3 μM), on relaxation responses to acetylcholine (ACh) in the aorta (A) and the small mesenteric artery (B) from old rats and thromboxane B2 (TXB2) production of aortic (C) and SMA (D) rings from old rats exposed to noradrenaline (0.3 μM) and ACh (1 μM). Results are expressed as a relaxation per cent of the initial noradrenaline-induced precontraction level. Data are mean±s.e.mean of 8–9 arterial segments for relaxation experiments and 10 aortic and five SMA rings for TXB2 determination. *P<0.05, with versus without GR 32191 B, **P<0.01, adult versus old rats.
TXB2 production (Figure 5C)
Assay of TXB2, the stable product of TXA2, showed increased production in aorta from old rats exposed to NA and ACh (P<0.01). In contrast, no change in TXB2 production was observed after stimulation with NA and ACh in SMA from old rats.
Western-blot analysis of eNOS, COX-1 and COX-2
The 140 kDa eNOS isoform was expressed in aorta and SMA from adult rats (Figure 6A). This expression was increased in both type of arteries from old rats. The increase of eNOS expression was 3.3±0.66 (n=3, P<0.05) and 3.7±0.72 (n=3, P<0.05) fold higher in aorta and in SMA respectively.
Figure 6.

Representative Western-blot of e-NOS (A), COX-1 (B) and COX-2 (C) protein expression in aortic and small mesenteric artery (SMA) segments from adult and old rats. Similar results were obtained in three separate experiments.
The data in Figure 6B,C show increased labelling of a 70 kDa protein by both anti COX-1 (6B) and anti COX-2 (6C) antibodies with ageing. With COX-1 antibody, the increase was 2.6±0.23 (n=3, P<0.05) and 2.1±0.42 (n=3, P<0.05) fold higher in aorta and in SMA respectively. Finally, COX-2 (70 kDa) was weakly expressed in aorta and SMA from adult rats (Figure 6C). However in vessels from old rats, COX-2 expression was observed markedly in aorta (fold increase: 9.3±0.32, n=4, P<0.001) and at a lower level in SMA (fold increase: 4.2±0.46, n=4, P<0.001).
Discussion
The present study suggests that the reduced endothelium-dependent relaxation to ACh with ageing results from an alteration in the balance between endothelial NO and vasoconstrictor products from COX, both in the conductance and the small arteries. In addition they show that the relative importance of these two mechanisms is different in aorta and in SMA, and that the COX vasoconstrictor products are different in the two arteries.
The concept of generalized endothelial dysfunction with ageing is extensively documented in vessels from different species including humans (Taddei et al., 1995; Küng & Lüscher, 1995; Haidet et al., 1995). However, most of the results come from conductance arteries and to the best of our knowledge only one report studied the possible anatomical heterogeneity of age-related endothelial dysfunction in the same animal (Barton et al., 1997). In the latter report the vascular effects of ageing with regard to the NO, SOD and endothelin-1 pathways were investigated in two different arteries, the aorta and the common femoral artery, a muscular artery. The data showed that relaxation to acetylcholine was impaired in the aorta but not in the femoral artery of the rat and this difference has been related to lower NO-synthase mRNA expression and superoxide dismutase activity in the aorta. In the present work, comparison was performed between the aorta and a small artery, the SMA. Generalization about the respective roles of small arteries is not possible but there is evidence showing that the SMA participate actively in the physiological regulation of mesenteric bed resistance (Mulvany & Halpern, 1977; Mulvany & Aalkjaer, 1990; Warshaw et al., 1979).
Endothelium-dependent relaxation to ACh was impaired both in the aorta and the SMA with ageing in accordance with previous observations reported in aorta (Barton et al., 1997; Shirasaki et al., 1986; Tschudi et al., 1996) and perfused mesenteric bed (Atkinson et al., 1994) of the rat. It should be noted that the endothelium-dependent relaxations to ACh were not significantly different in aortic rings and SMA from 12–14 week and 32-week-old rats whereas they were dramatically decreased at 70–100 compared to 12–14 and 32-week-old rats. Thus, the reduced endothelial response to ACh is most likely related to senescence as it is not present in both young adult (12–14 week) and more mature (32 week) rats. Therefore, this study focused on the young adult (adult, 12–14 week) and the senescent (old, 70–100 week) rats.
An increase in contractility of the vessels to NA cannot account for the reduced endothelial relaxation because the level of pre-contractions were matched between arteries from adult and old rats. In addition, age-related decreased endothelial relaxation was also observed in vessels pre-contracted with another vasoconstrictor agonist, U46619, in the aorta. A decrease in the number of muscarinic receptors activated by ACh, agonist affinity to its receptor, or a default in the muscarinic receptor signal transduction can be ruled out. Indeed, the endothelium-dependent relaxation to the calcium ionophore, A23187, which did not involve receptor stimulation, was also impaired in both types of arteries with ageing.
The most relevant hypothesis to explain the reduced relaxation to both ACh and A23187 might be an impairment of either the generation (i.e. synthesis or release) of, or response to endothelial relaxant factors with ageing. Also, an alteration of the balance between endothelial relaxant and constricting factors can be advanced.
One of the major endothelial relaxant factors is NO formed by eNOS by conversion of L-arginine, the participation of which has been reported to be altered with ageing in different blood vessels (Barton et al., 1997; Tschudi et al., 1996). In the present study a decreased sensitivity of vascular smooth muscle to NO appears not to be involved as relaxations of endothelium denuded preparations to the NO donor, SNAP, were not affected by ageing both in aorta and SMA. In accordance with these results, no evidence for an impaired responsiveness of vascular smooth muscle to NO donors has been found in rat aorta with age (Barton et al., 1997).
Reduced endothelium-dependent relaxation in aged rats may be due to a decreased participation of endothelium-derived NO. In vessels from adult rats, the NO-synthase inhibitor, L-NA, used at a concentration of 30 μmol l−1 in order to avoid unspecific effect of this compound on smooth muscle cells (Wang & Pang, 1994), reduced ACh-induced vasodilatation in both type of arteries confirming that endothelial NO was involved. In vessels from old rats, L-NA abolished the response to ACh in aorta but it had no effect in SMA suggesting a reduced endothelial NO-mediated vasodilation with ageing. These results are in accordance with observations reported by Dohi et al. (1990) in perfused mesenteric artery of the rat.
Decreased participation of endothelial NO may result from an increased NO breakdown due to an augmented production of O2− (Gryglewski et al., 1986; Rubanyi & Vanhoutte, 1986), an alteration of antioxidant defence systems or an increased production of reactive oxygen species different from O2− (Beckman & Ames, 1998). In the present study, the use of O2− and H2O2 scavengers, SOD and catalase, did not improve ACh-induced endothelial vasodilatation in both type of arteries. These results ruled out an increased NO breakdown by extracellular O2− even though a participation of intracellular reactive oxygen species insensitive to exogenous SOD cannot be excluded. On the other hand, a decrease in the release of endothelial NO might occur with ageing. Indeed, direct measurement using porphyrinic NO microsensor showed a decreased endothelial NO release in aorta and pulmonary artery of the rat with ageing (Tschudi et al., 1996). Reduced endothelial NO release with ageing might result from reduced expression of eNOS. Such observations have been reported at the level of eNOS mRNA expression in rat aorta (Barton et al., 1997). On the contrary, in the present study, Western blot analysis showed that eNOS protein expression was increased rather than reduced both in aorta and SMA from old rats. A possible explanation for the different results could be that Barton et al. (1997) quantified eNOS mRNA whereas eNOS proteins were studied here. Nevertheless, the age-related reduced participation of endothelial NO cannot be attributed to a decreased expression of eNOS protein both in aorta and SMA. Thus, the simplest and most parsimonious conclusion is that the origin of reduced participation of endothelial NO vasodilatation takes place at the level of the activity of the enzyme. Indeed, a decrease of eNOS activity has been reported in aorta of old rats (Cernadas et al., 1998; Chou et al., 1998). The present study extends the finding to small arteries such as the SMA.
Another possible explanation for the reduced endothelium-dependent relaxation with ageing, independently from or in addition to a decreased contribution of NO, might be an increased production of endothelium-derived constricting factors. The most likely candidates are vasoconstrictor products from endothelial COX such as TXA2 and PGH2 (Maclouf et al., 1998; Vane et al., 1998). Indirect evidence for the involvement of TXA2 as the endothelial vasoconstrictor metabolite from COX has been reported in aorta (Koga et al., 1989) but not in cerebral arteries (Mayhan et al., 1990) from old rats. In the present study, endothelium-dependent relaxation to ACh was potentiated after blockade of COX by indomethacin both in aorta and SMA from old but not adult rats. Thus, the reduced endothelium-dependent relaxation with ageing is associated with the participation of vasoconstrictor products from COX both in the aorta and the SMA.
We cannot exclude that vasoconstrictor prostanoids might originate from an extra-endothelial source like the medial or the adventitial layer. However, contractile response to NA in endothelium-denuded aorta and SMA were not different between adult and old rats. In addition, indomethacin did not modify NA response both in aorta and SMA without functional endothelium from old rats. Thus, the balance between vasoconstrictor and vasodilator protanoids released and its functional consequences are not relevant in altering responses in the used vessels. Furthermore, ACh-induced relaxation was completely dependent on the presence of the endothelium in vessels pre-contracted with NA and it was not able to produce contraction in endothelium denuded arteries (not shown). Thus, it is most likely that ageing is associated with the participation of endothelial vasoconstrictor products from COX activated by ACh both in the aorta and the small mesenteric artery.
The release of endothelial vasoconstrictor products from COX was associated with an increased expression of COX-1 and COX-2 proteins both in the aorta and in the SMA. Such an increase in COX-1 and COX-2 expression in the vascular wall of old rats has never been reported yet. Caution should be made for a direct correlation between functional data showing an increased generation of vasoconstrictor products from COX and enhanced COX expression, as COX-1 and COX-2 are able to release both vasodilatory and vasoconstrictor products. To assess the nature of the COX isoform involved in the release of vasoconstrictor prostanoids, the effect of the selective COX-2 inhibitor, NS-398, on endothelium-dependent relaxation to ACh was studied. Unlike indomethacin, a non-selective COX-1 and COX-2 inhibitor, NS-398 was unable to potentiate ACh relaxation in SMA from old rats. Moreover, NS-398 slightly but significantly inhibited rather than potentiated relaxation to ACh in aorta from old rats. Thus, it is likely that COX-2 generates predominantly vasodilatory products, at least in the aorta from old rats and that the balance of vasoactive products released through COX-1 is probably shifted towards vasoconstrictor prostanoids. The increased expression of COX-2 and subsequent release of vasodilatory products in vessels from old rats (at least in the aorta) may play a compensatory role for the reduced endothelial NO-mediated vasodilation and increased generation of vasoconstrictor prostanoids from COX-1. Indeed, upregulation of COX-2 leading to an overproduction of vasodilatory prostanoids (i.e. prostacyclin) after chronic inhibition of NO-synthase has been reported in mesenteric artery of the rat (Henrion et al, 1997).
The nature of COX vasoconstrictor metabolites involved was further investigated using the Tp receptor antagonist, GR32191 B. Interestingly, GR32191 B significantly enhanced ACh-induced relaxation in aorta but not in SMA from old rats. Moreover, assay of TXB2, the stable product of TXA2, showed that ACh was able to increase its production in aorta but not in SMA from old rats. Taken together, the above results provide direct evidence for the nature of endothelial vasoconstrictor prostanoids activated by ACh, most likely from COX-1, being TXA2 acting on Tp receptors in aorta from old rats. Also, the data show for the first time a differential involvement of endothelial vasoconstrictor products from COX-1 in small mesenteric arteries from old rats. The COX-1 metabolites did not belong to TXA2 or other prostanoids acting on Tp receptors in the SMA. Thus, the present study highlights a vascular bed heterogeneity in the mechanism of age-related endothelial dysfunction between the aorta and the SMA, the respective role of which is very different in the regulation of blood flow.
One of the main vasoconstrictor prostanoids produced includes TXA2 as shown in the aorta from old rats. However, under certain circumstances with increasing lipid accumulation, the pattern of COX products may change due to the different lipid substrates, and much more hydroxyoctadecadienoic (HODE), hydroxyeicosatetraenoic (HETE) acids and isoprostane vasoconstrictor metabolites may be produced (Bishop-Baley et al., 1999). This might be the case for the endothelial COX metabolites released by ACh in the SMA from old rats.
In conclusion, the present results shed light on the alteration in the equilibrium between endothelial derived NO and vasoconstrictor products from COX in the age-related endothelial dysfunction in the aorta and the small mesenteric artery. They also point out vascular bed heterogeneity related to the nature of COX metabolites involved despite the fact that both the aorta and the small mesenteric artery exhibit increased expression of COX with ageing.
Acknowledgments
The authors express gratitude to Dr M.C. Martínez for carefully reading the manuscript. Also, we thank D. Wagner and C. Untereiner for technical assistance. R.L. Matz was supported by a doctoral grant from Ministère de L'Education Nationale et de la Recherche (France). Parts of this study have been presented in abstract form at the British Pharmacological Society, Edinburgh, UK, September 2–4, 1997 (Alvarez de Sotomayor M. et al., 1997) and in invited oral communication at the French Pharmacological Society, Nancy, France, March 23–25, 1998.
Abbreviations
- ACh
acetylcholine
- A23187; calcimycin
calcium ionophore
- COX
cyclo-oxygenase
- EC50
molar concentration of the agonist that produces 50% of the maximal effect
- ECL
enhanced chemiluminescence assay
- EIA
enzyme immuno assay
- eNOS
endothelial nitric oxide synthase
- GR 32191 B
[1R-[1α(z),2β,3βm 5α]]-(+)-7-[5-[[(1,1′-biphenyl)-4-yl]methoxy]-3-hydroxy-2-(1-piperidinyl) cyclopentyl]-4-heptenoic acid hydrochloride, TP receptor antagonist
- H2O2
hydrogen peroxide
- HETE
hydroxyoctadecadienoic acid
- HODE
hydroxyeicosatetraenoic acid
- L-NA
NG-nitro-L-arginine, nitric oxide synthase inhibitor
- NA
noradrenaline
- NO
nitric oxide
- NS-398: N-(2-cyclohexyloxy-4-nitrophenyl) methanesulphonamide
selective cyclo-oxygenase-2 inhibitor
- O2−
superoxide anion
- pD2
−log EC50
- PGH2
prostaglandin H2
- PSS
physiological salt solution
- SDS
sodium dodecyl sulphate
- s.e.m.
standard error of the mean
- SMA
small mesenteric artery, branch II or III of superior mesenteric artery
- SNAP
S-N-acetylpenicillamine, nitric oxide donor
- SOD
superoxide dismutase
- TXA2: thromboxane A2; TXB2
thromboxane B2
- U46619
9,11-di-deoxy-11α,9α-epoxymethano-prostaglandin F2α.
References
- ALVAREZ DE SOTOMAYOR M., SCHOTT C., STOCLET J.C., ANDRIANTSITOHAINA R.Effect of ageing on endothelial function and vascular reactivity in rat conductance and resistance arteries Br. J. Pharmacol. 1997122238P(abstract) [Google Scholar]
- ANDRIAMBELOSON E., KLESCHYOV A.L., MULLER B., BERETZ A., STOCLET J.C., ANDRIANTSITOHAINA R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br. J. Pharmacol. 1997;120:1053–1058. doi: 10.1038/sj.bjp.0701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATKINSON J., TATCHUM-TALOM R., CAPDEVILLE-ATKINSON C. Reduction of endothelial function with age in the mesenteric arterial bed of the normotensive rat. Br. J. Pharmacol. 1994;111:1184–1188. doi: 10.1111/j.1476-5381.1994.tb14870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTON M., COSENTINO F., BRANDES R.P., MOREAU P., SHAW S., LUSCHER T.F. Anatomic heterogeneity of vascular aging. Role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- BECKMAN K.B., AMES B.N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- BISHOP-BAILEY D., HLA T., MITCHELL J.A. Cyclo-oxygenase-2 in vascular smooth muscle (Review) Int. J. Mol. Med. 1999;3:41–48. doi: 10.3892/ijmm.3.1.41. [DOI] [PubMed] [Google Scholar]
- BRUNCK C.F., JONES K.C., JONES T.W. Assay of nanogram quantities of DNA in cellular homogenates. Anal. Biochem. 1976;72:497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- CERNADAS M.R., SANCHEZ DE MIGUEL L., GARCIA-DURAN M., GONZALEZ-FERNANDEZ F., MILLAS I., MONTON M., RODRIGO J., RICO L., FERNANDEZ P., DE FRUTOS T., RODRIGUEZ-FEO J.A., GUERRA J., CARAMELO C., CASADO S., LOPEZ-FARRE A. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ. Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- CHOU T.C., YEN M.H., LI C.Y., DING Y.A. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–648. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- DOHI Y., LUSCHER T.F. Aging differentially affects direct and indirect actions of endothelin-1 in perfused mesenteric arteries of the rat. Br. J. Pharmacol. 1990;100:889–893. doi: 10.1111/j.1476-5381.1990.tb14110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRYGLEWSKI R.J., PALMER R.M., MONCADA S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- HAIDET G., WENNBERG P.W., RECTOR T.S. Aging and vasoreactivity: in vivo responses in beagle hindlimb. Am. J. Physiol. 1995;268:H92–H99. doi: 10.1152/ajpheart.1995.268.1.H92. [DOI] [PubMed] [Google Scholar]
- HENRION D., DECHAUX E., DOWELL F.J., MACLOUF J., SAMUEL J.L., LEVY B.I., MICHEL J.B. Alteration of flow-induced dilatation in mesenteric resistance arteries of L-NAME treated rats and its partial association with induction of cyclo-oxygenase-2. Br. J. Pharmacol. 1997;121:83–90. doi: 10.1038/sj.bjp.0701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONGO K., NAKAGOMI T., KASSEL N.F., SASAKI T., LEHMAN M., VOLLMER D.G., TSUKAHARA T., OGAWA H., TORNER J. Effects of aging and hypertension on endothelium-dependent vascular relaxation in rat carotid artery. Stroke. 1998;19:892–897. doi: 10.1161/01.str.19.7.892. [DOI] [PubMed] [Google Scholar]
- KOGA T., TAKATA Y., KOBAYASHI K., TAKISHITA S., YAMASHITA Y., FUJISHIMA M. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the aorta of the rat. Hypertension. 1989;14:542–548. doi: 10.1161/01.hyp.14.5.542. [DOI] [PubMed] [Google Scholar]
- KUNG C.H.F., LUSCHER T.F. Different mechanisms of endothelial dysfunction with aging and hypertension in rat aorta. Hypertension. 1995;25:194–200. doi: 10.1161/01.hyp.25.2.194. [DOI] [PubMed] [Google Scholar]
- MACLOUF J., FOLCO G., PATRONO C. Eicosanoids and iso-eicosanoids: constitutive, inducible and transcellular biosynthesis in vascular disease. Thromb. Haemost. 1998;79:691–705. [PubMed] [Google Scholar]
- MARTINEZ M.C., MULLER B., STOCLET J.C., ANDRIANTSITOHAINA R. Alteration by lipopolysaccharide of the relationship between intracellular calcium levels and contraction in rat mesenteric artery. Br. J. Pharmacol. 1996;118:1218–1222. doi: 10.1111/j.1476-5381.1996.tb15526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYHAN W.G., FARACI F.M., BAUMBACH G.L., HEISTAD D.D. Effects of aging on responses of cerebral arterioles. Am. J. Physiol. 1990;258:H1138–H1143. doi: 10.1152/ajpheart.1990.258.4.H1138. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., AALKJAER C. Structure and function of small arteries. Physiol. Rev. 1990;70:921–961. doi: 10.1152/physrev.1990.70.4.921. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-MARTINEZ M.A., ALONSO M.J., REDONDO J., SALAICES M., MARIN J. Role of lipid peroxidation and the glutathione-dependent antioxidant system in the impairment of endothelium-dependent relaxations with age. Br. J. Pharmacol. 1998;123:113–121. doi: 10.1038/sj.bjp.0701595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBANYI G.M., VANHOUTTE P.M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am. J. Physiol. 1986;250:H822–H1143. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- SHIRASAKI Y., SU C., LEE J.F., KOLM P., CLINE W.H., ALLEN-NICKOLS G., JR Endothelial modulation of vascular relaxation to nitrovasodilators in aging and hypertension. J. Pharmacol. Exp. Ther. 1986;239:861–866. [PubMed] [Google Scholar]
- TADDEI S., VIRDIS A., MATTEI P., GHIADONI L., GENNARI A., FASOLO C.B., SUDANO C.B., SALVETTI A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- TSCHUDI M.R., BARTON M., BERSINGER N.A., MOREAU P., COSENTINO F., NOLL G., MALINSKI T., LUSCHER T.F. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J. Clin. Invest. 1996;98:899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANE J.R., BAKHLE Y.S., BOTTING R.M. Cyclooxygenases 1 and 2. Ann. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- WANG Y.X., PANG C.C.Y. NG-nitro-L-arginine contracts vascular smooth muscle by an endothelium-independent mechanism. J. Cardiovasc. Pharmacol. 1994;24:59–63. doi: 10.1097/00005344-199407000-00011. [DOI] [PubMed] [Google Scholar]
- WARSHAW D.M., MULVANY M.J., HALPERN W. Mechanical and morphological properties of arterial resistance vessels in young and old spontaneously hypertensive rats. Circ. Res. 1979;45:250–259. doi: 10.1161/01.res.45.2.250. [DOI] [PubMed] [Google Scholar]


