Abstract
This study investigated the effects of BIIE0246, a novel neuropeptide Y (NPY) Y2 receptor antagonist, on the inhibition of cholinergic neuroeffector transmission in rat heart and guinea-pig trachea and purinergic neuroeffector transmission in guinea-pig vas deferens produced by the NPY Y2 receptor agonist, N-acetyl [Leu28,31] NPY 24-36.
In pentobarbitone anaesthetized rats, supramaximal stimulation every 30 s, of the vagus nerve innervating the heart, increased pulse interval by approximately 100 ms. This response was attenuated by intravenous administration of N-acetyl [Leu28,31] NPY 24-36 (10 nmol kg−1).
Transmural stimulation of segments of guinea-pig trachea at 1 min intervals with 5 s trains of stimuli at 0.5, 5, 10, 20 and 40 Hz evoked contractions which were reduced in force by N-acetyl [Leu28,31] NPY 24-36 (2 μM).
In guinea-pig vasa deferentia, the amplitude of excitatory junction potentials evoked by trains of 20 stimuli at 1 Hz was reduced in the presence of N-acetyl [Leu28,31] NPY 24-36 (1 μM).
In all preparations BIIE0246 attenuated the inhibitory effect of N-acetyl [Leu28,31] NPY 24-36 but had no effect when applied alone.
The findings support the view that the nerve terminals of postganglionic parasympathetic and sympathetic neurones possess neuropeptide Y Y2 receptors which, when activated, reduce neurotransmitter release.
Keywords: BIIE0246, neuropeptide Y, Y2 receptor, neuroeffector junction, heart, trachea, vas deferens, guinea-pig, rat
Introduction
Neuropeptide Y (NPY) is a 36 residue polypeptide and a member of the pancreatic polypeptide family of peptides. It is ubiquitously distributed in the peripheral as well as the central nervous system and is involved in multiple physiological functions. Within the cardiovascular system, NPY is found co-localized with noradrenaline in most sympathetic nerve fibres (Lundberg et al., 1983). It is released together with noradrenaline during sympathetic nerve stimulation (Lundberg & Tatemoto, 1982), to act both postjunctionally and prejunctionally. At prejunctional receptors, NPY is proposed to inhibit neurotransmitter release. It does this at a number of nerve terminals, including those of peptidergic sensory neurones (Grundemar et al., 1990), and postganglionic sympathetic (Lundberg et al., 1985; Pernow et al., 1986; Lundberg & Stjarne, 1984) and parasympathetic (Stjernquist et al., 1983; Potter, 1985) neurones.
In these peripheral neurones, the inhibition of transmitter release has been attributed to the activation of a prejunctional NPY Y2 receptor (Wahlestedt & Hakanson, 1986; Westfall et al., 1990). Y2 receptor mRNA was identified by Northern hybridization in several human brain subregions but has been more difficult to detect in human peripheral tissues (Rose et al., 1995). Therefore, a peripheral neuropeptide Y Y2 receptor has been suggested with sequence identity divergent from the cloned Y2 receptor detected in the human brain (Rose et al., 1995).
In 1994, a shortened modified C-terminal peptide fragment of NPY, N-acetyl [Leu28,31] NPY 24-36, was developed as an agonist for the NPY Y2 receptor (Potter et al., 1994). N-acetyl [Leu28,31] NPY 24-36 competed for binding of 125I-PYY to Y2 receptors in SMS-KAN neuroblastoma cells with an IC50 of 3.9±0.4 nM, but was ineffective in displacing 125IPYY in SK-N-MC neuroblastoma cells expressing Y1 receptors (Potter et al., 1994). Additionally, N-acetyl [Leu28,31] NPY 24-36 has no affinity for the Y5 receptor at doses tested in the present study (unpublished). In vascular beds which receive both parasympathetic and sympathetic innervation, N-acetyl [Leu28,31] NPY 24-36 inhibited cholinergic mediated vasodilatation (Lacroix et al., 1994; (Mahns et al., 1998a). Similarly, in canine gracilis muscle, N-acetyl [Leu28,31] NPY 24-36 inhibited sympathetic (cholinergic) nerve evoked vasodilatation, but not sympathetic (adrenergic) vasoconstriction (Mahns et al., 1998b). However, N-acetyl [Leu28,31] NPY did inhibit sympathetic (adrenergic) nerve evoked vasoconstriction in the dog kidney (Mahns et al., 1999). At parasympathetic neuroeffector junctions in the heart, N-acetyl [Leu28,31] NPY 24-36 mimicked the activity of NPY by inhibiting cardiac vagal (cholinergic) activity (Potter et al., 1994). N-acetyl [Leu28,31] NPY 24-36 is therefore an effective pharmacological tool for characterizing NPY Y2 receptors, but the value of data could be further strengthened if this tool were to be combined with the use of a selective Y2 antagonist.
Recently, Doods et al. (1999) reported a non-peptide molecule, BIIE0246 ((S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6h) -oxodibenz [b,e] azepin-11-yl]-1-piperazinyl]-2-oxo-ethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3-H-1,2,4-triazol-4-y]ethyl]-argininamid), as a potent NPY Y2 receptor antagonist. The structure of BIIE0246 was designed based on knowledge obtained from the known interactions of NPY at the Y2 receptor. In rat vas deferens, BIIE0246, shifted the inhibitory effect of exogenously applied NPY on neurally evoked contraction to the right in a concentration dependent manner. Additionally, BIIE0246 completely displaced the specific binding of [125I]·-NPY at human Y2 receptors expressed in SMS-KAN cell lines, with an IC50 of 3.3 nM. No displacement was observed at human and rat Y1, Y4 or Y5 receptors (Doods et al., 1999). A binding affinity study confirmed BIIE0246 as selective for the NPY Y2 receptor (Dumont et al., 2000). In a recent study in rat hippocampal slices, BIIE0246 at a concentration of 1 μM completely antagonized the inhibitory effects of 0.3 μM neuropeptide Y on synaptic transmission (Weiser et al., 2000). A subsequent study in rat hypothalamic slices showed BIIE0246 at 1 – 10 μM, prevented 0.1 μM NPY (13-36) induced reduction in K+-stimulated neuropeptide release (King et al., 2000).
The present study was undertaken to determine if BIIE0246 could antagonize functional responses attributed to activation of Y2 receptors, at cholinergic and purinergic peripheral autonomic neuroeffector junctions, by the agonist N-acetyl [Leu28,31] NPY 24-36. The bioassays used are the N-acetyl [Leu28,31] NPY 24-36 induced inhibition of: (1) vagally-mediated bradycardia in the heart of anaesthetized rats; (2) neurally-evoked smooth muscle contraction in guinea-pig trachea; (3) excitatory junction potential (e.j.p) amplitude in guinea-pig vas deferens. In all these preparations activation of prejunctional NPY Y2 receptors is believed to reduce neurotransmitter release.
Methods
In-vivo anaesthetised rat-cardiac vagal stimulation
Adult female inbred Wistar rats (200 – 250 g) were anaesthetized with sodium pentobarbitone (Nembutal, Boehringer-Ingelhiem; 60 mg kg−1, i.p.). The trachea was cannulated and attached to a positive pressure rodent ventilator (Ugo Basile 6025). The left femoral artery was cannulated for continuous recording of arterial blood pressure via a Statham physiological pressure transducer (P23XL) which was connected to one channel of a pen recorder (Graphtec 7400). Temperature was kept at 35±1°C and blood gases were monitored using a Corning 278 blood gas analyser. Subcutaneous needle electrodes were used to record the electrocardiogram (ECG) which was displayed on a storage oscilloscope (Gould 1401). The ECG was used to obtain beat-by-beat pulse interval (PI) following processing with Neurolog modules (Digitimer, England NL 200, 303, 601) and was recorded on the pen recorder. Both vagus nerves were cut to eliminate vagally mediated reflex effects on the heart. The cardiac end of the right vagus was stimulated every 30 s at a supramaximal voltage of 7.5 V, at a frequency of 2 – 2.5 Hz for 5 s using a square wave stimulator (Grass SD9). The frequency of the stimulation that increased pulse interval by approximately 100 ms was chosen. The right femoral vein was cannulated for administration of drugs. As a measure of drug activity at prejunctional Y2 receptors we measured maximal inhibition of the increase in pulse interval (ΔPI) evoked by stimulation of the vagus nerve. For pressor action we monitored change in blood pressure. Previous studies have shown that these parameters give reliable measures of the actions of NPY at pre- and postjunctional receptors (Potter et al., 1989).
To compare the activity of N-acetyl [Leu28,31] NPY24-36 before and after administration of BIIE0246, a dose of N-acetyl [Leu28,31] NPY24-36 (10 nmol kg−1) was chosen that was previously shown to give a sub-maximal inhibitory effect (i.e. ∼50% of control ΔPI). Following a bolus dose of BIIE0246 (0.1 μmol kg−1 n=3, and 1 μmol kg−1 n=4), N-acetyl [Leu28,31] NPY24-36 was given at specified time intervals (see Figure 2).
Figure 2.
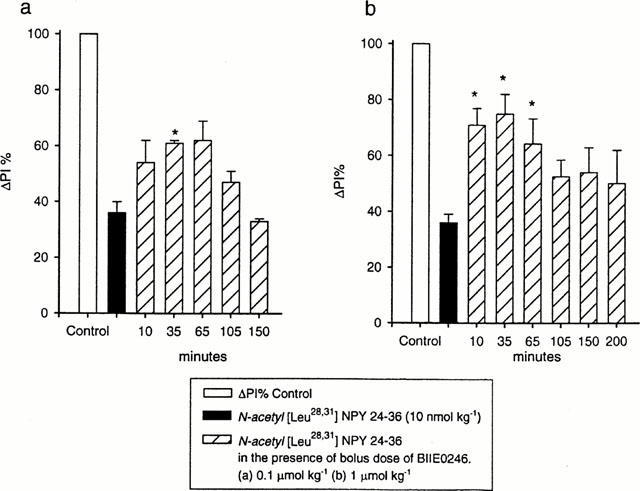
Results from two groups of rats investigating the effects of 0.1 μmol kg−1 (a) and 1 μmol kg−1 (b) BIIE0246. In each histogram ΔPI is attenuated following intravenous injection of N-acetyl [Leu28,31] NPY 24-36 (10 nmol kg−1). Each time interval represents an injection of N-acetyl [Leu28,31] NPY 24-36. The inhibitory effect of N-acetyl [Leu28,31] NPY 24-36 on ΔPI was reduced in the presence of a bolus dose of the antagonist, BIIE0246 (0.1 μmol kg−1, n=3) (a). The antagonism was greater following BIIE0246 1 μmol kg−1 (n=4) (b). Statistical comparisons were made with paired Student t-tests. *P<0.05.
Guinea-pig trachea
Guinea-pigs (400 – 500 g) were killed by an overdose of pentobarbitone sodium (100 mg kg−1, i.p.) and a 5 mm section of trachea was dissected and cut into zig-zag strips (Emmerson & Mackay, 1979). The strips were mounted in a 15 ml organ bath and attached at one end to a force-displacement transducer (Grass FT03). The organ bath was kept at 37°C and contained physiological saline (mM): NaCl 119; KCl 4.6; CaCl2 1.5; MgCl2 1.2; NaHCO3 15; Na2HPO4 1.2; D-glucose 6. The solution was bubbled continuously with 95% O2 and 5% CO2 to give a pH of 7.4. The physiological saline solution contained 1 μM indomethacin to improve reproducibility of the contractile response (Fernandes et al., 1994). Initial tension was adjusted to 5 mN, followed by an equilibration period of 45 – 60 min. Platinum electrodes surrounded the tracheal segment in a ring formation for transmural stimulation. The electrodes were attached to a Grass S88 stimulator and 5 s trains of biphasic, square wave stimuli of ∼60 V, 1 ms pulse were given every min at increasing frequencies of 0.5, 5, 10, 20 and 40 Hz, to obtain a stimulus frequency response curve. Contractions were recorded on a Grass polygraph (series 7). The NPY receptor agonists were added to the bath and left in contact with the tissue until maximal inhibition was obtained. In experiments with BIIE0246, this agent was applied for 20 min prior to the addition of N-acetyl [Leu28,31] NPY24-36.
Guinea-pig vas deferens
Male guinea-pigs (180 – 300 g) were killed by an overdose of pentobarbitone sodium (100 mg kg−1, i.p.) and the vasa deferentia were removed. Individual tissues were pinned to the Sylgard (Dow Corning Corporation, Midland, MI, U.S.A.) coated base of a 1 ml recording chamber. The tissue was superfused continuously at 3 – 5 ml min−1 with physiological saline solution (mM): NaCl 133.4; KCl 4.7; CaCl2 2; MgCl2 1.2; NaH2P04 1.3, NaHCO3 16.3 and D-glucose 7.8. The solution was bubbled continuously with a mixture of 95% O2 and 5% CO2 (to pH 7.4) and maintained at 35 – 36°C. In all experiments, phentolamine (1 μM) was added to the physiological saline to block the effects of α-adrenoceptor-mediated autoinhibition of neurotransmitter release. Postganglionic sympathetic nerve fibres were excited by electrical stimulation through a pair of ring electrodes positioned around the prostatic end of the vas deferens. For each impalement, the resting membrane potential was determined upon withdrawal of the microelectrode.
Conventional glass micro-electrodes filled with 0.5 M KCl (resistances 120 – 180 MΩ) and connected to an Axoclamp bridge amplifier (Axon Instruments, Inc., Foster City, U.S.A.) were used for intracellular recording of excitatory junction potentials (e.j.ps). The stimulation parameters (pulse width 0.5 – 1 ms, voltage 5 – 12 V) were adjusted to evoke e.j.ps of approximately 10 mV amplitude during continuous stimulation at 1 Hz in order to reduce the effects of non-linear summation on e.j.p amplitude. Once set, the stimulus parameters were not changed for the duration of the experiment. All experiments were carried out in single impalements during which the tissues were stimulated at 2 min intervals with trains of 20 pulses at 1 Hz. Drugs were added to the superfusing solution at the required concentration and were left in contact with the tissue for a period of 20 min. The electrophysiological signals were digitized (sampling frequencies of 0.2 kHz) and stored using a Powerlab recording system (ADInstruments, Castle Hill, NSW 2154, Australia). The amplitude and time constant of decay of e.j.ps were determined using the program Igor Pro (Wavemetrics, Lake Oswego, OR, U.S.A.). To assess the effects of the drugs, the relative changes in the mean amplitude of the last 5 e.j.ps in the train were compared.
The limited amount of BIIE0246 available restricted both the numbers of animal experiments conducted in each preparation and number of concentrations tested.
Data analysis
All results are presented as the mean±s.e.mean. In each of the tissues the effects of N-acetyl [Leu28,31] NPY 24-36 in the presence and absence of BIIE0246 were compared using repeated measures ANOVA and Student's paired t-tests. Where appropriate the P values were corrected using the Dunn-Sidak method. P values <0.05 were considered significant.
Drugs
BIIE0246 was a gift from Dr H. Doods (Boehringer Ingelheim Pharma, Biberach, Germany). N-acetyl [Leu28,31] NPY 24-36 was custom synthesized by Chiron Technologies Pty. Ltd. (Clayton, Victoria, Australia). Other drugs commercially available were: human pancreatic polypeptide (Peninsula Laboratories, Inc., Belmont, CA, U.S.A.); human [Leu31, Pro34] NPY (Auspep, Parkville, Victoria, Australia); phentolamine mesylate (Ciba, Pendle Hill, NSW, Australia) and indomethacin (Sigma-Aldrich Pty. Ltd., Castle Hill, NSW, Australia).
Results
Effects of BIIE0246 on inhibition of vagally induced bradycardia
In the anaesthetized rat, stimulation of the cardiac vagus nerve every 30 s increased PI. The response was attenuated following a 10 nmol kg−1 dose of the NPY Y2 receptor agonist N-acetyl [Leu28,31] NPY24-36 (Figure 1a). N-acetyl [Leu28,31] NPY24-36 had no affect on resting PI (Figure 1a) or blood pressure (not shown). In three animals, following a bolus dose of BIIE0246 (0.1 μmol kg−1), the inhibitory effect of N-acetyl [Leu28,31] NPY24-36 on vagal action was significantly attenuated (Figures 1b and 2a). The effect of the antagonist was maximal 35 min post-injection (Figure 1b). Approximately 2 h after injection of the antagonist the full inhibitory effect of N-acetyl [Leu28,31] NPY24-36 on ΔPI returned to pretreatment values (Figure 1c). Figure 2a shows the time-related data for the group of animals treated with 0.1 μmol kg−1 BIIE0246. In four animals a higher dose of BIIE0246 (1 μmol kg−1) had maximal effect 10 min after injection. The reduction in the inhibitory activity of N-acetyl [Leu28,31] NPY24-36 was greater in magnitude at the higher dose of antagonist (Figure 2b). Significant changes in the duration of the antagonistic action of BIIE0246 lasted ∼30 min at the lower concentration and ∼65 min for the higher concentration. BIIE0246 alone had no effect on ΔPI, resting pulse interval or blood pressure (not shown).
Figure 1.

Effects of an intravenous, bolus injection of N-acetyl [Leu28,31] NPY24-36 (10 nmol kg−1) on increase in pulse interval evoked by vagal stimulation in an anaesthetized rat. N-acetyl [Leu28,31] NPY24-36 attenuated the increase in pulse interval (a). Following addition of a bolus injection of BIIE0246 (0.1 μmol kg−1) the inhibitory effect was attenuated. Maximal effect was 35 min after injection of BIIE0246 (b). 105 min following injection of BIIE0246, the full inhibitory effect of the agonist was restored (c). Lines drawn above traces show control level of increase in pulse interval evoked by vagal stimulation.
Effect of BIIE0246 on inhibition of cholinergic mediated contraction of smooth muscle in guinea-pig trachea
Transmural nerve stimulation (0.5 – 40 Hz) of the tracheal strip evoked a contraction that increased in force as the frequency of stimulation was raised. These contractions were completely abolished by 10 μM atropine except at the highest frequency (40 Hz) where approximately 10% of the contraction remained. There was no relaxation of the tissue. These findings indicate that the contractions are due to activation of the parasympathetic nerves.
Figure 3 shows polygraph traces from one experiment recorded under control conditions (Figure 3a), 5 min following application of N-acetyl [Leu28,31] NPY (2 μM, Figure 3b) and 45 min after wash out of the peptide from the bath (Figure 3c).
Figure 3.
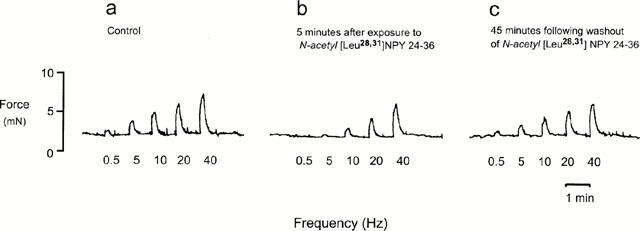
Polygraph trace from one animal showing contractions of trachea strip evoked by transmural nerve stimulation at increasing frequencies (a) were attenuated in the presence of N-acetyl [Leu28,31] NPY 24-36 (2 μM) (b). The inhibitory response was maximal 5 – 10 min after addition of peptide and returns to control levels 45 min after peptide is washed from the bath (c).
Pancreatic polypeptide (PP, human) and [Leu31Pro34] NPY were used to investigate the presence of other neuropeptide Y receptors in the guinea-pig tracheal preparation. Following application of PP (2 μM), contractions were attenuated. The small but significant (P<0.05) inhibitory effect was present for all but the highest stimulus frequency (40 Hz). [Leu31Pro34] NPY (2 μM) produced a similar inhibitory response to that of PP. These effects were compared to those of N-acetyl [Leu28,31] NPY 24-36 in the same tissue. Figure 4 shows summary data for the group (n=8 – 12).
Figure 4.

Effects of transmural parasympathetic nerve stimulation at increasing frequencies, shown as a percentage of the control response (obtained in the absence of peptide). The values shown were measured 10 min after the application of the peptides. At this time the effects of PP (2 μM) and the [Leu31, Pro34] NPY (2 μM) on neurally evoked contractions are maximal. The NPY Y2 receptor agonist, N-acetyl [Leu28,31] NPY 24-36 (2 μM) had a maximal effect 5 – 10 min after introduction of peptide to the bath. Peptides are given in equimolar concentration. There is a significant difference (P<0.05, n=8 – 12) in the inhibitory response of the NPY Y2 receptor agonist and both PP and the NPY Y1 receptor agonist at all frequencies except 40 Hz. Statistical comparisons were made with paired Student's t-test.
At 5 and 10 min following addition of N-acetyl [Leu28,31] NPY24-36, the force of the contractions evoked by each frequency of stimulation, except 40 Hz, was reduced (Figure 5). Following wash out of the peptide, the force of the contractions gradually returned to control levels over an approximate 40 min period. In the presence of BIIE0246 (0.1 μM), the inhibitory effect of N-acetyl [Leu28,31] NPY 24-36 was significantly reduced (Figure 5). No direct effects by BIIE0246 on the contractile response were observed.
Figure 5.

Effects on transmural parasympathetic nerve stimulation at increasing frequencies after application N-acetyl [Leu28,31] NPY 24-36 (2 μM) alone, and in the presence of BIIE0246 (0.1 μM). Results are shown as a percentage of the control response (obtained in the absence of peptide). N-acetyl [Leu28,31] NPY 24-36 significantly attenuated contractions evoked at all frequencies except 40 Hz (P<0.05, n=9). The effect was attenuated in the presence of BIIE0246 (P<0.05, n=9). Statistical comparisons were made with paired Student's t-test.
Effects of N-acetyl [Leu28,31] NPY 24-36 and BIIE0246 on e.j.p amplitude
In guinea-pig vas deferens treated with phentolamine (1 μM), trains of 20 stimuli at 1 Hz evoked e.j.ps which facilitated in amplitude to reach a plateau level after about the fifteenth stimulus (Figure 6a). Application of N-acetyl [Leu28,31] NPY 24-36 (1 μM) reduced the amplitude of e.j.ps evoked during the trains of stimuli (Figure 6a,b) and this effect of the NPY Y2 receptor agonist was reduced by the subsequent addition of 0.1 or 0.5 μM BIIE0246 (Figure 6a,b). Application of BIIE0246 (0.1 and 0.5 μM) alone had no effect on e.j.p amplitude (Figure 6b). Both N-acetyl [Leu28,31] NPY 24-36 and BIIE0246 applied alone or together had no effect on resting membrane potential or the e.j.p time constant of decay (Table 1), indicating that the effects of these agents are not due to changes in the electrical properties of the smooth muscle.
Figure 6.

The effects of N-acetyl [Leu28,31] NPY 24-36 and BIIE0246 on e.j.ps evoked by trains of 20 stimuli at 1 Hz in the guinea-pig vas deferens. (a) Traces recorded before and during the sequential application of N-acetyl [Leu28,31] NPY 24-36 and BIIE0246 (0.5 μM) in a single impalement. (b) Histogram showing the mean per cent change in the amplitude of the last 5 e.j.ps in the train produced by the sequential addition of N-acetyl [Leu28,31] NPY 24-36 (1 μM) followed by BIIE0246 (0.1 μM, n=6; 0.5 μM, n=5) and BIIE0246 alone (0.1 μM, n=6; 0.5 μM, n=5). Statistical comparisons were made with one-sided paired t-tests. *P<0.05, **P<0.01.
Table 1.
Effects of N-acetyl [Leu28,31] NPY24-36 and BIIE0246 on resting membrane potential (r.m.p) and e.j.p time constant of decay (τe.j.p) in the guinea-pig vas deferens

Discussion
The primary objective of this study was to determine if BIIE0246 could effectively antagonize functional responses attributed to activation of peripheral Y2 receptors by N-acetyl [Leu28,31] NPY 24-36. In all three preparations the attenuating effect of the neuropeptide Y Y2 receptor agonist N-acetyl [Leu28,31] NPY24-36, on responses to neurotransmitter release, was reduced in the presence of a novel Y2 receptor antagonist, BIIE0246. The magnitude and duration of responses were dependent on dose or concentration of antagonist applied, and in none of the assays did the antagonist alone have an effect.
In the heart and trachea, NPY Y2 receptors are most likely located presynaptically on the parasympathetic nerve terminal where, when activated, they inhibit the release of acetylcholine. In the heart, NPY was proposed as the mediator of sympathetic nerve mediated inhibition of vagal action because the inhibitory effect of sympathetic stimulation could be mimicked by exogenous NPY (Potter, 1985). N-acetyl [Leu28,31] NPY24-36 in turn, mimics the inhibitory effect of NPY on cardiac vagal activity (Potter et al., 1994). In this study, BIIE0246 attenuated the inhibitory effect of N-acetyl [Leu28,31] NPY24-36 on stimulus-evoked vagal bradycardia. These results confirm the presence of functional Y2 receptors at parasympathetic neuroeffector junctions in the rat heart.
In guinea-pig trachea, N-acetyl [Leu28,31] NPY 24-36 reduced the size of contractions evoked by stimulation of the parasympathetic nerves. The contractions were cholinergic in nature and were abolished by the application of atropine. The attenuating effect on contractile force produced by the agonist was reduced in the presence of BIIE0246. A previous study (Grundemar, 1997) using a similar preparation suggested that NPY inhibited stimulus evoked tracheal contractions primarily by acting at NPY Y2 receptors, but also by acting on NPY Y1 receptors. In that study [Leu31, Pro34] NPY and the Y4 receptor agonist PP were used. As [Leu31, Pro34] NPY inhibited contractions to a small extent and PP had no effect, Grundemar suggested that Y1 receptors contributed to the inhibitory effect evoked by exogenous application of NPY in the trachea. [Leu31, Pro34] NPY has previously been reported as being selective for the NPY Y1 receptor (Fuhlendorff et al., 1990), but subsequent studies report [Leu31, Pro34] NPY also binding to Y4 receptors (Bard et al., 1995). In the present study, both PP and [Leu31, Pro34] NPY produced a small, but significant, inhibitory effect on contractions. While [Leu31, Pro34] NPY can bind with relatively high affinity to 125I-PYY labelled Y4 receptors, it is not as potent an agonist as PP in functional assays (Pheng et al., 1999). The data is consistent with the possibility that both Y4 and Y1 receptors are present in this preparation. The present study and the work by Grundemar do however indicate the inhibitory effect of NPY on contractions are being mediated primarily by NPY Y2 receptors.
At sympathetic neuroeffector junctions in guinea-pig vas deferens, application of N-acetyl [Leu28,31] NPY 24-36 reduced e.j.p amplitude, an effect antagonized by BIIE0246. As e.j.ps are mediated by ATP (Sneddon, 1992), these findings are consistent with previous reports that activation of prejunctional NPY receptors by exogenously applied NPY inhibits ATP release in the guinea-pig vas deferens (Ellis & Burnstock 1990; Cheung & Dukkipati 1991). The present study indicates that the NPY receptors present on the sympathetic nerve terminals in the guinea-pig vas deferens are Y2 receptors. Importantly, BIIE0246 applied alone had no effect on e.j.p amplitude indicating that, under the conditions of the experiment, endogenous NPY does not play a role in modulating neurotransmitter release in the guinea-pig vas deferens.
Relatively high amounts of the antagonist were used in this study to block the effects of the agonist compared to antagonist affinity in Y2 cell lines (Doods et al., 1999). However, the concentrations of the antagonist used here did fall within the range used in previously reported functional assays (Weiser et al., 2000; King et al., 2000). Consideration should be given to the choice of agonist used to evoke a functional response. Many of the NPY fragments used currently in assays are not full agonists at the Y2 receptor (Smith-White et al., 1998). It is possible the higher concentrations of BIIE0246 are needed to block effects of the specific NPY Y2 agonist N-acetyl [Leu28,31] NPY 24-36 than are needed to block effects of the partial agonist (13-36) NPY as used in in vitro studies.
The findings of this study confirm that the NPY Y2 receptor agonist N-acetyl [Leu28,31] NPY 24-36 activates Y2 receptors to inhibit cholinergic neuroeffector transmission in rat heart and guinea-pig trachea, and purinergic neuroeffector transmission in guinea-pig vas deferens. The selective NPY Y2 receptor antagonist, BIIE0246, will now make it possible to examine the participation of Y2 receptors in functional responses, in other assays and species, evoked by exogenous and nerve released neuropeptide Y.
Acknowledgments
We thank Ms Tamara Powell for expert technical assistance and Dr Henri Doods for the gift of BIIE0246. The National Health and Medical Research Council support this work.
Abbreviations
- BIIE0246
(S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6h)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxo-ethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3-H-1,2,4-triazol-4-y]ethyl]-argininamid
- N-acetyl
[Leu28,31] NPY 24-36, N-terminal acetylated leucine24,31, neuropeptide Y 24-36
- NPY
neuropeptide Y
- PP
pancreatic polypeptide
- PYY
peptide YY
- SK-N-MC
human neuroblastoma cell line
- SMS-KAN
human neuroblastoma cell line
References
- BARD J.A., WALKER M.W., BRANCHEK T.A., WEINSHANK R.L. Cloning and functional expression of a human Y4 subtype receptor for pancreatic polypeptide, neuropeptide Y, and peptide YY. J. Biol. Chem. 1995;270:26762–26765. doi: 10.1074/jbc.270.45.26762. [DOI] [PubMed] [Google Scholar]
- CHEUNG D.W., DUKKIPATI R. An electrophysiological study of neuropeptide Y on sympathetic neurotransmission in guinea-pig vas deferens. J. Pharmacol. Exp. Ther. 1991;257:979–983. [PubMed] [Google Scholar]
- DOODS H., GAIDA W., WIELAND H.A., DOLINGER H., SCHNORRENBERG G., ESSER F., ENGEL W., WOLFGANG E., RUDOLF K. BIIE0246: A selective and high affinity neuropeptide Y Y2 receptor antagonist. Eur. J. Pharmacol. 1999;384:R3–R5. doi: 10.1016/s0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., CADIEUX A., DOODS H., PHENG L.H., ABOUNDER R., HAMEL E., JACQUES D., REGOLI D., QUIRION R. BIIE0246, a potent and highly selective non-peptide neuropeptide Y Y2 receptor antagonist. Br. J. Pharmacol. 2000;129:1075–1088. doi: 10.1038/sj.bjp.0703162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS J.L., BURNSTOCK G. Neuropeptide Y modulation of sympathetic co-transmission in the guinea-pig vas deferens. Br. J. Pharmacol. 1990;100:457–462. doi: 10.1111/j.1476-5381.1990.tb15828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMERSON, MACKAY The zig-zag tracheal tracheal strip. J. Pharm. Pharmacol. 1979;252:358–364. doi: 10.1111/j.2042-7158.1979.tb13666.x. [DOI] [PubMed] [Google Scholar]
- FERNANDES L.B., HUBBARD W.C., UNDEM B.J. Release of inflammatory mediators from guinea pig trachea by electrical field stimulation lack of neuronal involvement. J. Pharmacol. Exp. Ther. 1994;270:1116–1170. [PubMed] [Google Scholar]
- FUHLENDORFF J., GETHER U., AAKERLUND L., LANGELAND-JOHANSEN N., THOGERSEN H., MELBERG S.G., OLSEN U.B., THASTRUP O., SCHWARTZ T.W. [Leu31, Pro34]neuropeptide Y: a specific Y1 receptor agonist. P.N.A.S (U.S.A.) 1990;87:182–186. doi: 10.1073/pnas.87.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNDEMAR L. Characterization of the receptor response for the neuropeptide Y-evoked suppression of parasympathetically-mediated contractions in the guimea pig trachea. Reg. Pept. 1997;71:97–101. doi: 10.1016/s0167-0115(97)01023-9. [DOI] [PubMed] [Google Scholar]
- GRUNDEMAR L., GRUNDSTROM N., JOHANSSON I.G., ANDERSSON R.G., HAKANSON R. Suppression by neuropeptide Y of capsaicin-sensitive sensory nerve-mediated contraction in guinea-pig airways. Brit. J. Pharmacol. 1990;99:473–476. doi: 10.1111/j.1476-5381.1990.tb12952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING P.J., WILLIAMS G., DOODS H., WIDDOWSON P.S. Effect of a selective neuropeptide Y Y2 receptor antagonist, BIIE0246 on neuropeptide Y release. Eur. J. Pharmacol. 2000;396:R1–R3. doi: 10.1016/s0014-2999(00)00230-2. [DOI] [PubMed] [Google Scholar]
- LACROIX J.S., ULMAN L.G., POTTER E.K. Modulation by neuropeptide Y of parasympathetic nerve-evoked nasal vasodilatation via Y2 prejunctional receptor. Br. J. Pharmacol. 1994;113:479–484. doi: 10.1111/j.1476-5381.1994.tb17014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG J.M., STJARNE L. Neuropeptide Y (NPY) depresses the secretion of 3H-noradrenaline and the contractile response evoked by field stimulation, in rat vas deferens. Acta Physiol. Scand. 1984;120:477–479. doi: 10.1111/j.1748-1716.1984.tb07410.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M., TATEMOTO K. Pancreatic polypeptide family (APP, BPP, NPY and PYY) in relation to sympathetic vasoconstriction resistant to alpha-adrenoceptor blockade. Acta Physiol. Scand. 1982;116:393–402. doi: 10.1111/j.1748-1716.1982.tb07157.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M., TERENIUS L., HOKFELT T., GOLDSTEIN M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci. Lett. 1983;42:167–172. doi: 10.1016/0304-3940(83)90401-9. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M., TORSSELL L., SOLLEVI A., PERNOW J., THEODORSSON NORHEIM E., ANGGARD A., HAMBERGER B. Neuropeptide Y and sympathetic vascular control in man. Reg. Pept. 1985;13:41–52. doi: 10.1016/0167-0115(85)90085-0. [DOI] [PubMed] [Google Scholar]
- MAHNS D.A., KELLY C., MCCLOSKEY D.I., POTTER E.K. NPY Y2 receptor agonist, N-acetyl [Leu28,Leu31]NPY24-36, reduces renal vasoconstrictor activity in anaesthetised dogs. J. Auton. Nerv. Syst. 1999;78:10–17. doi: 10.1016/s0165-1838(99)00056-9. [DOI] [PubMed] [Google Scholar]
- MAHNS D.A., LACROIX J.S., POTTER E.K. Inhibition of vagal vasodilatation by a selective neuropeptide Y Y2 receptor agonist in the bronchial circulation of anaesthetised dogs. J. Auton. Nerv. Syst. 1998a;73:80–85. doi: 10.1016/s0165-1838(98)00086-1. [DOI] [PubMed] [Google Scholar]
- MAHNS D.A., REVINGTON M.L., RUNCIE M.J., MCCLOSKEY D.I., POTTERE K. Inhibition of sympathetic cholinergic vasodilatation by a selective NPY Y2 receptor agonist in the gracilis muscle of anaesthetised dogs. J. Auton. Nerv. Syst. 1998b;68:14–20. doi: 10.1016/s0165-1838(97)00111-2. [DOI] [PubMed] [Google Scholar]
- PERNOW J., SARIA A., LUNDBERG J.M. Mechanisms underlying pre- and postjunctional effects of neuropeptide Y in sympathetic vascular control. Acta Physiol. Scand. 1986;126:239–249. doi: 10.1111/j.1748-1716.1986.tb07811.x. [DOI] [PubMed] [Google Scholar]
- PHENG L.H., PERRON A., QUIRION R., CADIEUX A., FAUCHERE J.L., DUMONT Y., REGOLI D. Neuropeptide Y-induced contraction is mediated by neuropeptide Y Y2 and Y4 receptors in the rat colon. Eur. J. Pharmacol. 1999;374:85–91. doi: 10.1016/s0014-2999(99)00296-4. [DOI] [PubMed] [Google Scholar]
- POTTER E.K. Prolonged non-adrenergic inhibition of cardiac vagal action following sympathetic stimulation: neuromodulation by neuropeptide Y. Neurosci. Lett. 1985;54:117–121. doi: 10.1016/s0304-3940(85)80065-3. [DOI] [PubMed] [Google Scholar]
- POTTER E.K., BARDEN J.A., MCCLOSKEY M.J., SELBIE L.A., TSENG A., HERZOG H., SHINE J. A novel neuropeptide Y analog, N-acetyl [Leu28, Leu31]neuropeptide Y-(24-36), with functional specificity for the presynaptic (Y2) receptor. Eur. J. Pharmacol. 1994;267:253–262. doi: 10.1016/0922-4106(94)90148-1. [DOI] [PubMed] [Google Scholar]
- POTTER E.K., MITCHELL L., MCCLOSKEY M.J., TSENG A., GOODMAN A.E., SHINE J., MCCLOSKEY D.I. Pre- and postjunctional actions of neuropeptide Y and related peptides. Reg. Pept. 1989;25:167–177. doi: 10.1016/0167-0115(89)90258-9. [DOI] [PubMed] [Google Scholar]
- ROSE P.M., FERNANDES P., LYNCH J.S., FRAZIER S.T., FISHER S.M., KODUKULA K., KIENZLE B., SEETHALA R. Cloning and functional expression of a cDNA encoding a human type 2 neuropeptide Y receptor. J. Biol. Chem. 1995;270:22661–22664. doi: 10.1074/jbc.270.39.22661. [DOI] [PubMed] [Google Scholar]
- SMITH-WHITE M., MORIARTY M.J., POTTER E.K. A comparison of actions of neuropeptide Y (NPY) agonists and antagonists at NPY Y1 and Y2 receptors in anaesthetized rats. Neuropeptide. 1998;32:109–118. doi: 10.1016/s0143-4179(98)90025-7. [DOI] [PubMed] [Google Scholar]
- SNEDDON P. Suramin inhibits excitory junction potentials in guinea pig vas deferens. Br. J. Pharmacol. 1992;222:171–174. doi: 10.1111/j.1476-5381.1992.tb14469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STJERNQUIST M., EMSON P., OWMAN C., SJOBERG N.O., SUNDLER F., TATEMOTO K. Neuropeptide Y in the female reproductive tract of the rat. Distribution of nerves and motor effects. Neurosci. Lett. 1983;39:279–284. doi: 10.1016/0304-3940(83)90313-0. [DOI] [PubMed] [Google Scholar]
- WAHLESTEDT C., HAKANSON R. Effects of neuropeptide Y (NPY) at the sympathetic neuroeffector junction. Can pre- and postjunctional receptors be distinguished. Med. Biol. 1986;64:85–88. [PubMed] [Google Scholar]
- WEISER T., WIELAND H.A., DOODS H. Effects of the neuropeptide Y Y2 receptor antagonist BIIE0246 on presynaptic inhibition by neuropeptide Y in rat hippocampal slices. Eur. J. Pharmacol. 2000;404:133–136. doi: 10.1016/s0014-2999(00)00478-7. [DOI] [PubMed] [Google Scholar]
- WESTFALL T.C., HAN S.P., CHEN X.L., DEL VALLE K., CURFMAN M., CIARLEGLIO A., NAES L. Presynaptic peptide receptors and hypertension. Ann. N.Y. Acad. Sci. 1990;604:372–388. doi: 10.1111/j.1749-6632.1990.tb32006.x. [DOI] [PubMed] [Google Scholar]


