Abstract
Lipoxin (LX) A4 and aspirin-triggered-LX (ATL) are endogenous lipid-derived mediators that regulate leukocyte trafficking via specific LXA4 receptors (ALX), and are involved in endogenous anti-inflammation and resolution. Both LXA4 and ATL are produced by rat tissues in vitro as well as in vivo. In rats, LXA4 and ATL exhibit potent physiological and pathophysiological roles. Thus, we set out to determine whether ALX is expressed in rat tissues and its potential role in modulating leukocyte trafficking with LXA4 and ATL.
In rats, a stable analog of ATL, when given intravenously with two consecutive doses at approximately 60 μg kg−1 each injection, significantly inhibited neutrophil infiltration (∼43%) and protein extravasation (∼42%) in a casein-induced peritonitis.
The rat orthologue of ALX was cloned from peripheral blood leukocytes encoding a putative G protein-coupled receptor (GPCR). It gave ∼74 and ∼84% homology, respectively to the deduced amino-acid sequences of the human and mouse ALX.
Tissue distribution analysis by RNase protection revealed that this rat receptor is expressed in tissues/cells, where LXA4 displays physiological and pathophysiological roles, namely, lung, kidney and leukocytes.
The rat orthologue of ALX gave specific radioligand binding with [3H]LXA4 and [125-Tyr]-annexin 1-derived peptide with apparent Kd values of 5 and 820 nM, respectively, that are at levels comparable to those of the human ALX.
Activation of rat ALX inhibited tumor necrosis factor alpha-mediated nuclear factor kappaB activity in a ligand-dependent manner utilizing a luciferase reporter gene system.
Together, these results are the first demonstration of a rat ALX that is conserved in both structure and function suggesting that ALX plays key roles in regulating effector immune responses from murine to human species.
Keywords: Lipoxin, aspirin-triggered lipoxin, ALX, GPCR, inflammation, annexin 1
Introduction
Lipoxin (LX) A4 and recently identified carbon-15 epimeric form, aspirin-triggered LXA4 (ATL or 15-epi-LXA4), exhibit potent ‘anti-PMN' actions and appear to serve as novel endogenous ‘stop signals' to regulate excessive leukocyte trafficking and promote resolution (reviewed recently in Serhan & Chiang, 2002). LXA4 and its carbon-15 epimeric counterpart ATL modulate leukocyte responses via interacting with a specific G-protein-coupled receptor (GPCR) denoted ALX. ALX was first identified in retinoic acid-differentiated HL-60 cells (Fiore et al., 1994) and also cloned in mouse (Takano et al., 1997) with high affinity to their endogenous lipid ligands (e.g. LXA4 and ATL) as well as their bioactive stable analogs. These compounds act in the subnanomolar-to-nanomolar range in both human cellular systems and murine models of acute inflammation and reperfusion injury. ALX also interacts with a wide panel of small peptides/proteins that give responses distinct from LXA4 in vitro and in vivo (Chiang et al., 2000; for a review, see Serhan & Chiang, 2002), suggesting that ALX may serve as a multirecognition receptor and can dictate different functional responses in vivo.
Lipoxins are evolutionarily conserved in species including rat and mouse as well as several species of fish and frogs (Serhan & Chiang, 2002). For the past decade, both LXA4 and ATL were shown to be generated by rat cell types and tissues as well as in vivo in rats during inflammation (Table 1). For example, in rats LXA4 is formed and elevated in viral-antibody-positive alveolar macrophage (Kim, 1990) as well as in basophilic leukemia (Ng et al., 1989) and kidney mesangial cells (Garrick et al., 1989). Also, LXA4 is produced in vivo during the course of inflammation such as in an experimental immune complex glomerulonephritis model (Papayianni et al., 1995; Munger et al., 1999) and in pleural exudate upon allergen challenge (Bandeira-Melo et al., 2000). In addition, LXA4 is formed in the rat brain and elevated in focal cerebral ischemia (Kim & Tominaga, 1989). Recently, aspirin-triggered 15-epi-LXA4 was shown to be produced in the rat kidney (Munger et al., 1999) and by the liver (Titos et al., 1999) in an aspirin-dependent fashion. Along these lines, it was reported that aspirin rapidly upregulates COX-2 expression in the stomach and causes a significant increase in gastric 15-epi-LXA4 production in rats (Fiorucci et al., 2002).
Table 1.
Generation and actions of lipoxin A4 and ATL in rats
| System | LXA4 generation in | ATL generation in | Bioaction | Concentration/Dose |
|---|---|---|---|---|
| Kidney | Glomerulonephritis; mesangial cells (Garrick et al., 1989; Papayianni et al., 1995; Munger et al., 1999) | Glomerulonephritis (aspirin-dependent) (Munger et al., 1999) | Increase glomerular filtration rate and renal plasma flow rate in renal vasculature (Badr et al., 1987; Katoh et al., 1992) | |
| Lung | Pleuritis (Bandeira-Melo et al., 2000) | Shorten the duration of pleural exudation in pleuritis (Bandeira-Melo et al., 2000) | 0.5 μg mouse−1 by local injection | |
| Leukocytes | Basophilic leukemia cells; alveolar macrophage (Ng et al., 1989; Kim, 1990) | Stimulate nonphlogistic phagocytosis of apoptotic PMN bymacrophages (Mitchell et al., 2002) | 1 nM | |
| Inhibit PMN infiltration and proteinextravasation in peritonitis | 120 μg kg−1 intravenously | |||
| Gastrointestinal tract | Aspirin-induced gastric damage (Fiorucci et al., 2002) | Reduce the severity of gastric damage and suppress aspirin-induced leukocyte adherence (Fiorucci et al., 2002) | 2.5 μg kg−1 intraperitoneally | |
| Reduce L-NAME-induced leukocyte rolling and adherence in mesentery (Scalia et al., 1997) | ||||
| Liver | Hepatocytes; liver homogenates (ASA-treated) (Titos et al., 1999) | Reduce peroxisome proliferator-activated receptor α and cytokine-induced neutrophil chemoattractant-1 levels in hepatocytes (Planagumà et al., 2002) | 1 μM | |
| Brain | Focal cerebral ischemia (Kim & Tominaga, 1989) |
In rats, both LXA4 and ATL display potent actions in physiological and pathophysiological systems (Table 1), including the regulation of renal functions by increasing glomerular filtration rate and renal plasma flow rate (Badr et al., 1987; Katoh et al., 1992). Also, LXA4 mediates early resolution of allergic pleural edema observed during A. costaricensis infection (Bandeira-Melo et al., 2000). Furthermore, in gastrointestinal tract, LXA4 attenuates NG-nitro-L-arginine methyl ester (L-NAME)-induced leukocyte rolling and adherence in mesenteric microvasculature (Scalia et al., 1997) and recent evidence demonstrates that LXA4 reduces the severity of aspirin-induced gastric damage in rats by suppressing leukocyte adherence (Fiorucci et al., 2002). Taken together, LXA4 and ATL exhibit protective roles during inflammation and promote resolution in rats. Thus, it was of interest to determine whether ALX is functionally expressed in rats and its role in regulating PMN with LXA4 and ATL.
Here, we report that ATL modulated PMN infiltration in acute inflammation in rats and cloning of a functional rat ALX from leukocytes. The rat receptor shares high homology with both human and mouse ALX (74 and 84%, respectively) in deduced amino-acid sequences and is expressed in rat tissues/cells, where LXA4 displays potent bioactions. In addition, we recently found that ALX directly interacts with both LXA4 as well as the annexin 1-derived peptide (Perretti et al., 2002). Annexin 1 (ANXA1) is a highly abundant PMN-derived protein that is induced in vivo by glucocorticoids (Flower & Rothwell, 1994). Both ANXA1 as well as its enzymatically generated ANXA1-derived peptide (e.g. peptide Ac2-26, Ac-AMVSEFLKQAWFIENEEQEYVQTVK) are potent inhibitors of PMN transmigration and phagocytosis in vitro and in vivo contributing to the in vivo actions of glucocorticoid (Lim et al., 1998; Maridonneau-Parini et al., 1989). Results of the present report demonstrate that the rat ALX gives ligand-dependent inhibition of tumor necrosis factor alpha- (TNF-α) mediated nuclear factor-kappaB (NF-κB) activation. Hence, the identified rat receptor is a functional ALX that is conserved in both primary structure as well as function in vivo in inflammation.
Methods
Materials
Ac2-26 peptide and [125I-Tyr]Ac2-26 were prepared by custom synthesis with Phoenix Pharmaceuticals, Inc. and purified by HPLC (Belmont, CA, U.S.A.). [11,12-3H]LXA4-methyl ester was prepared with Schering AG (Berlin, Germany) essentially as in Chiang et al. (2000) and was a gift of Dr. H.D. Perez at Berlex Biosciences (Richmond, CA, U.S.A.). The oligonucleotides were synthesized by GIBCO-BRL Life Technologies. Human embryonic kidney (HEK) 293 cells were from American Type Culture Collection (Rockville, MD, U.S.A.). DMEM medium and fetal bovine serum (FBS) were from BioWhittaker.
Casein-induced peritonitis
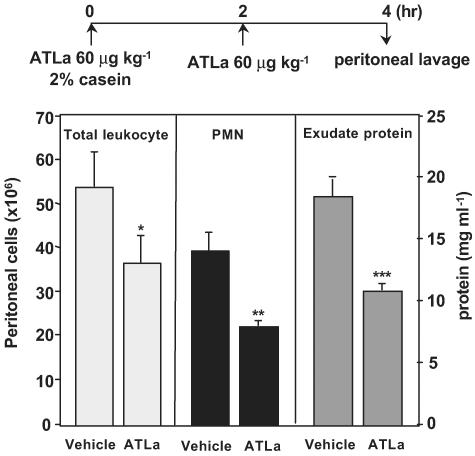
Male Sprague–Dawley rats with an average weight of ∼320 g were anesthetized by isoflurane and injected with ATLa (60 μg kg−1) intravenously immediately before induction of peritonitis by an intraperitoneal injection of 10 ml of 2% casein (Sigma, St. Louis). The second dose of ATLa (60 μg kg−1) was given intravenously after 2 h. The rats were euthanized 2 h later with an overdose of isoflurane in accordance with Harvard Medical Area Standing Committee on Animals (protocol nos. 1610 and 2570) and peritoneal lavages were obtained (see experimental timeline in Figure 1). Polymorphonuclear cells were stained with Türk solution and numerated by light microscopy. Exudate protein was determined by BioRad DC (Detergent Compatible) protein assay reagent (BioRad, Hercules, CA, U.S.A.).
Figure 1.
ATL analog inhibits rat PMN infiltration in casein-induced peritonitis. Rats were injected with ATLa (∼60 μg kg−1) intravenously immediately before induction of peritonitis by intraperitoneal injection of casein. The second injection (∼60 μg kg−1) was given 2 h later (see experimental timeline). Peritoneal lavages were collected and cells numerated. Results represent the mean±s.e.m. from n=3 (*P=0.04, **P=0.01, ***P<0.01).
Cloning and plasmid construction
Blood from male Sprague–Dawley rats were collected into heparin. Red blood cells were lysed with NH4Cl and the remaining cells (leukocytes) were used to isolate total RNA using TriZol Reagent (Life Technology, Burlington, ON, Canada). cDNA was transcribed from rat leukocyte RNA with SuperScript II (Life Technology) and used as template for subsequent polymerase chain reactions (PCR) using Taq and Pwo DNA polymerase (8 : 1, both from Roche Diagnostics, Laval, QC, Canada). DNA sequencing was carried out from three independent amplifications. The following oligonucleotides were synthesized and PCR product was first cloned into pCR2.1 vector (Invitrogen, Carlsbad, CA, U.S.A.). After the sequence was confirmed, the insert was released by HindIII and EcoRV, blunted and subcloned into the EcoRV site of pcDNA3 vector (Invitrogen).
- Primer 1:
5′-caatggtggttgtctccatc-3′ (mouse sequence 86–105).
- Primer 2:
5′-aagaaggaagccacaactcc-3′ (mouse sequence 733–752).
- Primer 3:
5′-cagctggttgtgcagacaaaatg-3′ (mouse sequence –20 to +3).
- Primer 4:
5′-ctgtgaaagagaagtcagccaaagcta-3′ (rat sequence).
- Primer 5:
5′-ccgtccattaagagtccttac-3′ (rat sequence).
- Primer 6:
5′-catcccacagccccctcctcctca-3′ (mouse sequence 1054–1077).
- Primer 7:
5′-atggaagccaactattccatc-3′ (rat sequence).
- Primer 8:
5′-tcatattgcttttatatcaatgtt-3′ (rat sequence).
Ribonuclease (RNase) protection Assay
The PCR product of primers 3 and 4 was cloned into pCR2.1. and used as template for the probe. RNase protection assay was performed as described previously (Takano & Cybulsky, 2000). In brief, total RNA (5–20 μg) was hybridized with 32P-labeled antisense cRNA probes for 16 h at 55°C. Unhybridized probes were digested with RNaseA and RNaseT1, and the RNases were then digested with proteinase K. After phenol/chloroform extraction and ethanol precipitation, the hybrids were denatured at 85°C for 3 min and electrophoresed on 6% polyacrylamide gels. After drying, gels were exposed to X-ray film at −85°C for 48 h.
Cell culture and transfection
Human embryonic kidney (HEK) 293 cells were cultured in DMEM media supplemented with 10% FBS. For stable transfection, cells were transfected with plasmid DNA (1 μg) using SuperFect transfection reagent (Qiagen, CA, U.S.A.). After 48 h, cells were placed in DMEM with 0.5 mg ml−1 Geneticin (GIBCO/BRL, Grand Island, NY, U.S.A.) to select cells stably expressing transfected receptors.
Specific radioligand binding
[3H]LXA4 and [125I-Tyr]Ac2-26 binding was performed with HEK293 cells transfected with rat recombinant ALX. Cells were suspended in Dulbecco's phosphate buffered saline with CaCl2 and MgCl2 (DPBS††). Aliquots (0.5 × 106 cells ml−1) were incubated with 1 nM of [11,12-3H] LXA4 (∼60,000 c.p.m., specific activity ∼10 Ci mmol−1) or 30 nM of [125I-Tyr]Ac2-26 (∼80,000 c.p.m., specific activity ∼1171.5 Ci mmol−1) in the absence or presence of increasing concentrations of unlabeled compounds for 40 min at 4°C. The bound and unbound radioligands were separated by filtration through Whatman GF/C glass microfiber filters (Fisher, Pittsburgh, PA, U.S.A.). Nonspecific binding was determined in the presence of 1 μM of unlabeled LXA4 for [3H]LXA4 binding and 100 μM unlabeled Ac2-26 for [125I-Tyr]Ac2-26 binding.
NF-κB-dependent luciferase reporter gene
HEK293 cells were seeded into 24-well plates at a density of 1 × 105 cells per well and cultured overnight in DMEM with 10% fetal bovine serum. For transient transfection, 0.1 μg of NF-κB luciferase reporter plasmid (Stratagene, La Jolla, CA, U.S.A.) was cotransfected with 0.8 μg of expression plasmid pcDNA3 with rat ALX or without (mock) using SuperFect transfection reagent. At 24 hours after transfection, cells were treated with 15-epi-LXA4 or vehicle (0.1% ethanol) for 30 min and then stimulated with TNFα (1.0 ng ml−1) for 5 h. Luciferase activity was measured by the Dual-Luciferase reporter assay system (Promega, Madison, WI, U.S.A.) using Renilla luciferase driven by a thymidine kinase as a transfection control.
Statistical analysis
Results were expressed as the mean ± s.e.m. and Student's t-test was performed with P values <0.05 taken as statistically significant.
Results
ATL analog inhibits PMN infiltration in rat peritonitis
ATL analogs are potent inhibitors of PMN infiltration in murine dorsal air pouch and dermal inflammation (Serhan & Chiang, 2002). To test whether LXA4 and ATL also display anti-inflammatory action in rats, an ATL analog (ATLa) was evaluated for its ability to impact exudate formation and leukocyte trafficking in a casein-induced peritonitis model. When given intravenously (see experimental timeline in Figure 1), two consecutive doses of ATLa (∼60 μg kg−1 each injection) profoundly inhibited leukocyte trafficking into peritoneal cavity (∼32% for total leukocytes and ∼43% for PMN) compared to animals receiving vehicle alone (Figure 1). Also, ATLa inhibited protein extravasation (∼42%) as determined by total exudate protein levels. These results indicate that LXA4 and ATL can regulate PMN trafficking and vascular leakage in rats (Figure 1) as reported earlier in murine inflammation in vivo (Takano et al., 1997;1998).
Cloning of rat ALX: primary structure analysis
To extend our knowledge of the structure–function relation of ALX (murine vs human) and to evaluate whether ALX mediates the action of LXA4, ATL and their analogs in rats, we set out to clone rat ALX. Total RNA from rat peripheral blood leukocytes was isolated and initial RT–PCR product was obtained with primers 1 and 2 that were designed based on the sequence of mouse ALX (see Figure 2a). cDNA sequence analysis of this 670 bp fragment (Figure 2a, left gel) showed 81 and 74% homology to the mouse and human ALX, respectively, suggesting that rat leukocytes express an orthologue of ALX. Mice and rats are developmentally close and some extent of homology is often seen between the two species even in the 5′ or 3′-noncoding regions. Thus, we designed primers corresponding to the 5′ and 3′ ends of mouse ALX (primers 3 and 6) and paired them with the internal primers designed from the rat (primers 4 and 5). Primer pairs 3–4 and 5–6 yielded PCR products of ∼230 and ∼350 bp, respectively (Figure 2a, middle gel). Both fragments were sequenced and showed a high homology to the mouse receptor. To clone the full coding region, primers 7 and 8 were designed and a PCR reaction using these primers yielded the full-length rat orthologue of ALX (Figure 2a, right gel). It contains 1053 nucleotides and encodes a protein of 351 amino acids (Figure 2b). In addition, mRNA expression of this rat ALX was also found in casein-elicited peritoneal leukocytes (data not shown) and gave identical nucleotide sequences.
Figure 2.
Cloning of a rat orthologue of ALX. (a) Schematic presentation of PCR cloning of rat ALX using mouse ALX as a template. Primers designed based on cDNA sequences of mouse (….) and rat (–) ALX are indicated. (see Methods for details). PCR fragments were analyzed on agarose gels and molecular sizes of expected products are indicated by arrows. (b) Nucleotide and deduced amino-acid sequences of rat ALX.
Alignment of the deduced amino-acid sequences revealed that the rat orthologue of ALX shares 74 and 84% homology with human and mouse ALX, respectively (Figure 3a). The highest homology is found in their second intracellular loop (identical, 100%) followed by the sixth transmembrane segment (TM) (93%). A phylogenetic tree constructed with related GPCR demonstrated that this rat receptor is most closely related to mouse and human ALX, followed by formyl peptide receptors (FPR) (∼60% identity in amino-acid sequences) (Figure 3b and c). As a class, human, mouse and rat ALX is only distantly related to prostanoid receptors, and belongs to the cluster of chemoattractant peptide receptors exemplified by fMLP and C5a receptors and now also include leukotriene B4 receptors (BLT).
Figure 3.
Rat ALX sharing high homology with human and mouse ALX. (a) Alignment of deduced amino-acid sequence of ALX from human, mouse and rat. The identical amino-acid residues in three species are boxed. The proximate positions of the putative transmembrane segment (TM) are indicated and the conserved residues/motifs are marked (*). (b) Phylogenetic tree of ALX and related human GPCRs. This tree is constructed using the ‘All All Program' at the Computational Biochemistry Server at ETHZ (http://cbrg.inf.ethz.ch/Server/AllAll.html (c) The percent identity (in amino-acid sequences) and divergence is listed for each pairwise comparison using the MegAlign program (DNASTAR).
Rat tissue distribution of ALX
RNase protection assay with total RNAs from various tissues of the rat revealed the highest expression in leukocytes and spleen, followed by lung and with lesser amounts in heart, liver, kidney and intestine (Figure 4). This expression pattern is very similar to those of human and mouse ALX (Fiore et al., 1994; Takano et al., 1997). It is of interest to note that this rat ALX is expressed in tissues/cells where LXA4 and/or ATL is generated and displays potent biological roles (Badr et al., 1987; Katoh et al., 1992; Papayianni et al., 1995; Scalia et al., 1997; Munger et al., 1999; Bandeira-Melo et al., 2000), such as kidney, intestine, leukocytes and lungs (Table 1), suggesting that ALX play an important role in modulating inflammatory responses at these sites.
Figure 4.
Rat ALX tissue distribution: RNAse protection analysis. The PCR product of primers 3 and 4 was used as template for the probe. RNase protection assay was performed as described in the ‘Methods'. The protected fragment is indicated by an arrow.
Interactions with lipid and peptide ligands: radioligand binding and function
To examine whether the rat receptor is a functional ALX, it was stably expressed in HEK293 cells. Specific radioligand binding with rat receptor was determined and compared to that with recombinant human ALX. Rat ALX gave specific [3H]LXA4 binding with an apparent Kd of ∼5 nM (Figure 5a), which is comparable to that of human ALX (Chiang et al., 2000). In addition, with an iodinated annexin 1-derived peptide (e.g. [125I-Tyr]Ac2-26), rat ALX displayed specific binding (Figure 5b) with an apparent Kd of ∼820 nM and B max of ∼3200 sites cell−1 as determined by Scatchard analysis (Figure 5c), which are at comparable levels of those obtained with human ALX (Perretti et al., 2002). In contrast, fMLP at 0.01–10 μM did not compete for specific [125I-Tyr]Ac2-26 binding with either human or rat ALX (Figure 5b, inset). Also, [3H]fMLP did not give specific binding to recombinant rat ALX (data not shown). These results indicate that this rat orthologue of ALX directly interacts with selective lipid and peptide ligands in a rank order essentially identical to that demonstrated for human ALX (Chiang et al., 2000; Perretti et al., 2002).
Figure 5.
LXs and peptides directly interact with rat ALX: [3H]-LXA4 and [125I-Tyr]Ac2-26 binding with recombinant ALX. Human or rat ALX-transfected HEK293 cells (0.5 × 106 cells ml−1) were incubated with (a) [3H]LXA4 or (b) [125I-Tyr]Ac2-26 for 40 min at 4°C in the presence of an increasing concentration of homoligands. (inset) Recombinant human or rat ALX were incubated with [125I-Tyr]Ac2-26 together with fMLP at indicated concentrations. Bound and unbound radioligands were separated by filtration and specific binding was determined. (c) Scatchard analysis of [125I-Tyr]Ac2-26 binding (representative from n=3). Data represent (a, b) the mean±s.e.m. from duplicates of n=3 and (inset) the mean from duplicates of n=2. The three-dimensional illustrations for [3H]LXA4 and [125I-Tyr]Ac2-26 were prepared with ‘CS Chem3D' software.
Since LXA4 directly interacts with the rat orthologue of ALX, it was of interest to determine whether LXA4 could activate endogenous anti-inflammatory signals with the recombinant rat ALX. To this end, we evaluated NF-κB activation utilizing a luciferase reporter gene system as in an earlier report (Gewirtz et al., 2002). 15-epi-LXA4 (ATL) clearly inhibited TNFα-stimulated luciferase activity at concentrations as low as 0.1 nM and peaked at 10 nM with cells expressing recombinant rat ALX (Figure 6). ATL alone did not evoke significant luciferase activity (data not shown). This inhibition proved to be ALX-dependent since mock-transfected cells did not give significant inhibition (Figure 6). Together, these results indicated that ATL can transmit signal with recombinant rat ALX expressed in HEK293 cells.
Figure 6.
ATL inhibits NF-κB-dependent luciferase gene expression via rat ALX in a ligand- and receptor-dependent fashion. HEK293 cells were transiently transfected with NF-κB-luciferase reporter plasmid together with pcDNA3 containing rat ALX or pcDNA3 alone. Cells were treated with indicated concentrations of 15-epi-LXA4 or vehicle for 30 min and then stimulated with TNFα (1.0 ng ml−1) for 5 h and luciferase activity was measured. Typical TNFα response resulted in a 600-fold induction in luciferase activity with either rat ALX or mock-transfected cells. Results represent the mean±s.e.m. from triplicates of n=4 (*P<0.05).
Discussion
Eicosanoids generated by both 5-lipoxygenase (LO) and cyclooxygenase (COX) pathways display well-appreciated roles in acute inflammation (Brink et al., in press). Inhibitors for 5-LO and COX as well as receptor antagonists for leukotriene B4 (BLT) and cysteinyl leukotrienes (CysLTs) have been developed and examined for their potential anti-inflammatory properties in acute inflammation models including rats. Among them, BLT antagonists were reported to be the most effective as a class (Souza et al., 2000). For example, CP 105,696 at 3 mg kg−1, s.c. and LY 255283 at 3 mg kg−1, i.v. give >50% inhibition in vascular permeability and PMN accumulation in the intestine, mesentery and lung ischemia reperfusion. In contrast, inhibitors for COX and 5-LO pathways are not effective in the rat models tested (Wallace et al., 1999; Holma et al., 2001). In the present study, ATLa proved to be very potent since two doses of ATLa (60 μg kg−1 each injection) given intravenously significantly inhibited both PMN infiltration and vascular leakage in peritonitis. By comparison, ATLa is at least one log order magnitude more potent than BLT antagonists as a class. This is likely because that ATLa represents endogenously generated local mediators in inflammation.
Human ALX interacts directly with its endogenous anti-inflammatory and ‘pro-resolving' lipid ligands (i.e. LXA4 and ATL) as well as their bioactive stable analogs (e.g. ATLa) to downregulate or ‘stop' PMN recruitment to inflammatory loci. In addition, it interacts with selective peptides that are revealed during necrotaxis (Chiang et al., 2000) as well as ANXA1-derived peptides (Perretti et al., 2002). To further understand the molecular mechanism of ATL's actions in vivo, the rat ALX was cloned because of the many actions discovered earlier for LX and ATL and also many rat models were widely used for pharmacological purposes. Our present results indicated that this rat ALX also directly interacts with both lipid and peptide ligands and suggested that the functional redundancies in lipid and peptide anti-inflammatory circuits are conserved across species.
ALX is closely related at the nucleotide sequence level to N-formyl peptide receptor (FPR). In this regard, we (Fiore et al., 1994) and others (Quehenberger et al., 1993) found that the ALX does not effectively bind and respond to formyl peptide fMLP, unless higher pharmacological doses (i.e., >1–10 μM) were used, suggesting that these are not physiologically relevant ligands. Along these lines, our present results showed that fMLP at 0.01–10 μM did not compete for specific [125I-Tyr]Ac2-26 binding with rat ALX (Figure 5b, inset). Moreover, phylogenetic tree and sequence distances analysis demonstrated that this rat receptor is closer to ALX than FPR in nucleotide sequences (Figure 3b and c), further indicating that this rat receptor is a functional orthologue of ALX.
When the open reading frame of this rat ALX was submitted to BLAST search with rat genome, several related sequences were found on two genomic clones (CH230-304M12 and CH230-276M12, accession #AC108651 and #AC136668, respectively) sharing 86–93% homology in nucleotide sequence with our rat ALX. Since sequencing of the rat genome is in progress and these genomic clones were deposited into GenBank as ‘working drafts', it is not clear at present whether these related rat sequences represent functional genes. Along these lines, there are six reported sequences related to the mouse FPR setting a gene cluster denoted Fpr1 and Fpr-rs1 through Fpr-rs5 that were cloned from B6/CBA mouse (Gao et al., 1998). Among them, Fpr-rs1 is 97% identical in deduced amino-acid sequence to the ALX that we cloned earlier from Balb/c mouse (Takano et al., 1997). Fpr-rs4 and Fpr-rs5 RNA were not detected in any tissues tested. In comparison, three sequences present in humans are related to FPR, also referred to as the FPR gene cluster, namely FPR, FPRL1/ALX and FPRL2. FPRL1/ALX was originally cloned as an orphan receptor with high-sequence homology to FPR (Perez et al., 1992; for a recent review, see Serhan & Chiang, 2002) and was identified afterwards as a high-affinity receptor that is activated by LXA4 (Fiore et al., 1994). The function of human FPRL2 per se remains to be established.
Several conserved motifs and amino-acid residues important for receptor topology and signaling were found in rat orthologue of ALX. For example, this rat ALX contains consensus residues for GPCR including Cys-98 and Cys-176 in the extracellular loops 1 and 2, which may form disulfide linkage (Dohlman et al., 1990), NPXXY motif in the TM7, which plays a role in receptor desensitization and/or resensitization (Barak et al., 1995), and DRY motif in the TM3 that is essential for β-arrestin binding and G-protein activation (Bennett et al., 2000). In addition, conserved N-glycosylation sites, which are important for ligand specificity of human ALX (Chiang et al., 2000), were also present in rat receptor at Asn-4, Asn-10 and Asn-179 (Figure 3a). Serine and tyrosine residues that are essential for human ALX phosphorylation and signaling (Kang et al., 2000) were also found in rat receptor, suggesting a conserved mechanism in ALX regulation. Of interest, ATLa upregulates a subset of genes upon short exposure to PMN including a transcriptional corepressor NAB1 identified previously as a glucocorticoid-responsive gene in hamster smooth muscle cells and also found to be upregulated by ATLa in murine lung vascular smooth muscle in vivo (Qiu et al., 2001). These findings provide evidence for rapid transcriptional induction of a cassette of genes via an ATLa-stimulated GPCR pathway.
Phagocytosis of apoptotic PMN is a critical event in the resolution of acute inflammation and tissue injury. We and others have demonstrated that LXA4 and ATL inhibit release of select ‘proinflammatory cytokines' (Hachicha et al., 1999) and promote resolution (Godson et al., 2000; Levy et al., 2001). In this regard, NF-κB activation is held to play a central role in proinflammatory cytokine-activated gene expression that may lead to the delay of PMN apoptosis (McDonald et al., 1997). In addition, recent report demonstrated that LXA4 analogs downregulate intestinal epithelial proinflammatory gene expression via the inhibition of NF-κB pathway and also reduce the severity of dextran sodium sulfate-induced colitis (Gewirtz et al., 2002). Further support for this role of LXA4 and ATL came from our present results that clearly demonstrate 15-epi-LXA4 (ATL) inhibition of NF-κB-dependent luciferase activity using an isolated recombinant receptor system. Thus, modulation of NF-κB activity might be a relevant component in lipoxin's anti-inflammatory and proresolution action in several in vivo models of rat inflammation.
In summary, we demonstrated potent actions of LXA4 and ATL in inhibiting rat PMN in vivo and reported the cloning and characterization of a novel rat ALX that is conserved among human, mouse and rat. This rat receptor serves as a functional ALX that recognizes distinct structures (e.g. both lipid and peptide ligands) and transduces signals with LXA4. Together, our results indicate that ALX is structurally and functionally conserved in rats and may play a role in anti-inflammation and pro-resolution in rats. In addition, analysis of the primary structures of ALX from various species may gain new insights in identifying essential domains of ALX in ligand recognition and signal transduction involved in ‘downregulatory' lipid mediator circuits in inflammation and resolution.
Acknowledgments
This work was supported in part by Grant nos. GM38765 and DE013499 (C.N.S.) from the National Institutes of Health and by a grant from the Kidney Foundation of Canada (T.T). T.T is a recipient of a scholarship from the Canadian Institute of Health Research. We thank Mary Halm Small for assistance in manuscript preparation, Daniel Kim for technical assistance and Dr Greg Stahl for helpful discussion of rat in vivo experiments.
Abbreviations
- ALX
lipoxin A4 receptor
- ANXA1
annexin 1
- ATL
aspirin-triggered 15-epi-LXA4 [5(S), 6(R), 15(R)-trihydroxy-7,9,13-trans-11-cis eicosatetraenoic acid]
- ATLa
ATL analog [15-epi-16-(para-fluoro)-phenoxy-LXA4 methyl ester]
- GPCR
G protein-coupled receptor
- LX
lipoxin
- LXA4
lipoxin A4 [5(S), 6(R), 15(S)-trihydroxy-7,9,13-trans-11-cis eicosatetraenoic acid]
- NF-κB
nuclear factor kappaB
- RT–PCR
reverse transcriptase–polymerase chain reaction
- TM
transmembrane segment
- TNFα
tumor necrosis factor alpha
References
- BADR K.F., SERHAN C.N., NICOLAOU K.C., SAMUELSSON B. The action of lipoxin-A on glomerular microcirculatory dynamics in the rat. Biochem. Biophys. Res. Commun. 1987;145:408–414. doi: 10.1016/0006-291x(87)91337-4. [DOI] [PubMed] [Google Scholar]
- BANDEIRA-MELO C., SERRA M.F., DIAZ B.L., CORDEIRO R.S., SILVA P.M., LENZI H.L., BAKHLE Y.S., SERHAN C.N., MARTINS M.A. Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: relationship with concurrent eosinophilia. J. Immunol. 2000;164:1029–1036. doi: 10.4049/jimmunol.164.2.1029. [DOI] [PubMed] [Google Scholar]
- BARAK L.S., MENARD L., FERGUSON S.S., COLAPIETRO A.M., CARON M.G. The conserved seven-transmembrane sequence NP(X)2,3Y of the G-protein-coupled receptor superfamily regulates multiple properties of the beta 2-adrenergic receptor. Biochemistry. 1995;34:15407–15414. doi: 10.1021/bi00047a003. [DOI] [PubMed] [Google Scholar]
- BENNETT T.A., MAESTAS D.C., PROSSNITZ E.R. Arrestin binding to the G protein-coupled N-formyl peptide receptor is regulated by the conserved ‘DRY' sequence. J. Biol. Chem. 2000;275:24590–24594. doi: 10.1074/jbc.C000314200. [DOI] [PubMed] [Google Scholar]
- BRINK C., DAHLÉN S.-E., DRAZEN J., EVANS J.F., HAY D.W.P., NICOSIA S., SERHAN C.N., SHIMIZU T., YOKOMIZO T. International Union of pharmacology classification XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol. Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- CHIANG N., FIERRO I.M., GRONERT K., SERHAN C.N. Activation of lipoxin A4 receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J. Exp. Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOHLMAN H.G., CARON M.G., DEBLASI A., FRIELLE T., LEFKOWITZ R.J. Role of extracellular disulfide-bonded cysteines in the ligand binding function of the beta 2-adrenergic receptor. Biochemistry. 1990;29:2335–2342. doi: 10.1021/bi00461a018. [DOI] [PubMed] [Google Scholar]
- FIORE S., MADDOX J.F., PEREZ H.D., SERHAN C.N. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J. Exp. Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIORUCCI S., DE LIMA O.M., JR, MENCARELLI A., PALAZZETTI B., DISTRUTTI E., MCKNIGHT W., DICAY M., MA L., ROMANO M., MORELLI A., WALLACE J.L. Cyclooxygenase-2-derived lipoxin A4 increases gastric resistance to aspirin-induced damage. Gastroenterology. 2002;123:1598–1606. doi: 10.1053/gast.2002.36558. [DOI] [PubMed] [Google Scholar]
- FLOWER R.J., ROTHWELL N.J. Lipocortin-1: cellular mechanisms and clinical relevance. Trends Pharmacol. Sci. 1994;15:71–76. doi: 10.1016/0165-6147(94)90281-x. [DOI] [PubMed] [Google Scholar]
- GAO J.L., CHEN H., FILIE J.D., KOZAK C.A., MURPHY P.M. Differential expansion of the N-formylpeptide receptor gene cluster in human and mouse. Genomics. 1998;51:270–276. doi: 10.1006/geno.1998.5376. [DOI] [PubMed] [Google Scholar]
- GARRICK R., SHEN S.Y., OGUNC S., WONG P.Y. Transformation of leukotriene A4 to lipoxins by rat kidney mesangial cell. Biochem. Biophys. Res. Commun. 1989;162:626–633. doi: 10.1016/0006-291x(89)92356-5. [DOI] [PubMed] [Google Scholar]
- GEWIRTZ A.T., COLLIER-HYAMS L.S., YOUNG A.N., KUCHARZIK T., GUILFORD W.J., PARKINSON J.F., WILLIAMS I.R., NEISH A.S., MADARA J.L. Lipoxin A4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- GODSON C., MITCHELL S., HARVEY K., PETASIS N.A., HOGG N., BRADY H.R. Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- HACHICHA M., POULIOT M., PETASIS N.A., SERHAN C.N. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1α-initiated neutrophil responses and trafficking: regulators of a cytokine–chemokine axis. J. Exp. Med. 1999;189:1923–1929. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMA R., SALMENPERA P., RIUTTA A., VIRTANEN I., KORPELA R., VAPAATALO H. Acute effects of the cys-leukotriene-1 receptor antagonist, montelukast, on experimental colitis in rats. Eur. J. Pharmacol. 2001;429:309–318. doi: 10.1016/s0014-2999(01)01330-9. [DOI] [PubMed] [Google Scholar]
- KANG Y., TADDEO B., VARAI G., VARGA J., FIORE S. Mutations of serine 236–237 and tyrosine 302 residues in the human lipoxin A4 receptor intracellular domains result in sustained signaling. Biochemistry. 2000;39:13551–13557. doi: 10.1021/bi001196i. [DOI] [PubMed] [Google Scholar]
- KATOH T., TAKAHASHI K., DEBOER D.K., SERHAN C.N., BADR K.F. Renal hemodynamic actions of lipoxins in rats: a comparative physiological study. Am J Physiol. 1992;263:F436–F442. doi: 10.1152/ajprenal.1992.263.3.F436. [DOI] [PubMed] [Google Scholar]
- KIM S.J. Elevated formation of lipoxins in viral antibody-positive rat alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1990;3:113–118. doi: 10.1165/ajrcmb/3.2.113. [DOI] [PubMed] [Google Scholar]
- KIM S.J., TOMINAGA T. Formation and lipoxins by the brain: ischemia enhances production of lipoxins. Ann. N.Y. Acad. Sci. 1989;559:461–464. [Google Scholar]
- LEVY B.D., CLISH C.B., SCHMIDT B., GRONERT K., SERHAN C.N. Lipid mediator class switching during acute inflammation: signals in resolution. Nature Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- LIM L.H., SOLITO E., RUSSO-MARIE F., FLOWER R.J., PERRETTI M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14535–14539. doi: 10.1073/pnas.95.24.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARIDONNEAU-PARINI I., ERRASFA M., RUSSO-MARIE F. Inhibition of O2- generation by dexamethasone is mimicked by lipocortin I in alveolar macrophages. J. Clin. Invest. 1989;83:1936–1940. doi: 10.1172/JCI114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD P.P., BALD A., CASSATELLA M.A. Activation of the NF-kappaB pathway by inflammatory stimuli in human neutrophils. Blood. 1997;89:3421–3433. [PubMed] [Google Scholar]
- MITCHELL S., THOMAS G., HARVEY K., COTTELL D., REVILLE K., BERLASCONI G., PETASIS N.A., ERWIG L., REES A.J., SAVILL J., BRADY HR., GODSON C. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J. Am. Soc. Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- MUNGER K.A., MONTERO A., FUKUNAGA M., UDA S., YURA T., IMAI E., KANEDA Y., VALDIVIELSO J.M., BADR K.F. Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13375–13380. doi: 10.1073/pnas.96.23.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NG C.F., LAM B.K, PRITCHARD K.A., JR, STEMERMAN M.B., HEJNY P., WONG P.Y. Agonist-dependent generation of lipoxins from rat basophilic leukemia cell (RBL-1) Biochim. Biophys. Acta. 1989;1004:332–336. doi: 10.1016/0005-2760(89)90081-7. [DOI] [PubMed] [Google Scholar]
- PAPAYIANNI A., SERHAN C.N., BRADY H.R. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J. Immunol. 1996;156:2264–2272. [PubMed] [Google Scholar]
- PAPAYIANNI A., SERHAN C.N., PHILLIPS M.L., RENNKE H.G., BRADY H.R. Transcellular biosynthesis of lipoxin A4 during adhesion of platelets and neutrophils in experimental immune complex glomerulonephritis. Kidney Int. 1995;47:1295–1302. doi: 10.1038/ki.1995.184. [DOI] [PubMed] [Google Scholar]
- PEREZ H.D., HOLMES R., KELLY E., MCCLARY J., ANDREWS W.H. Cloning of a cDNA encoding a receptor related to the formyl peptide receptor of human neutrophils. Gene (Amst.) 1992;118:303–304. doi: 10.1016/0378-1119(92)90208-7. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., CHIANG N., LA M., FIERRO I.M., MARULLO S., GETTING S.J., SOLITO E., SERHAN C.N. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat. Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLANAGUMÀ A, TITOS E, LÓPEZ-PARRA M, GAYA J, PUEYO G, ARROYO V, CLÀRIA J. Aspirin (ASA) regulates 5-lipoxygenase activity and peroxisome proliferator-activated receptor α- mediated CINC-1 release in rat liver cells: novel actions of lipoxin A4 (LXA4) and ASA- triggered 15-epi-LXA4. FASEB J. 2002;16:1937–1939. doi: 10.1096/fj.02-0224fje. [DOI] [PubMed] [Google Scholar]
- QIU F.-H., DEVCHAND P.R., WADA K., SERHAN C.N. Aspirin-triggered lipoxin A4 and lipoxin A4 up-regulate transcriptional corepressor NAB1 in human neutrophils. FASEB J. 2001;15:2736–2738. doi: 10.1096/fj.01-0576fje. [DOI] [PubMed] [Google Scholar]
- QUEHENBERGER O., PROSSNITZ E.R., CAVANAGH S.L., COCHRANE C.G., YE R.D. Multiple domains of the N-formyl peptide receptor are required for high-affinity ligand binding Construction and analysis of chimeric N-formyl peptide receptors. J. Biol. Chem. 1993;268:18167–18175. [PubMed] [Google Scholar]
- SCALIA R., GEFEN J., PETASIS N.A., SERHAN C.N., LEFER A.M. Lipoxin A4 stable analogs inhibit leukocyte rolling and adherence in the rat mesenteric microvasculature: role of P-selectin. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9967–9972. doi: 10.1073/pnas.94.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERHAN C.N., CHIANG N. Lipid-derived mediators in endogenous anti-inflammation and resolution: lipoxins and aspirin-triggered 15-epi-lipoxins. The Scientific World J. 2002;2:169–204. doi: 10.1100/tsw.2002.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., COUTINHO S.F., SILVEIRA M.R., CARA D.C., TEIXEIRA M.M. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur. J. Pharmacol. 2000;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- TAGO K., FUNAKOSHI M., MANO H, YANAGISAWA K., HAYAKAWA M, KUROIWA K., IWAHANA H., KASAHARA T., TOMINAGA S. Presence of a genistein-responsive inhibitory mechanism on interleukin-1alpha-induced NF-kappaB activation. Eur. J. Biochem. 2001;268:6526–6533. doi: 10.1046/j.1432-1327.2001.02603.x. [DOI] [PubMed] [Google Scholar]
- TAKANO T., FIORE S., MADDOX J.F., BRADY H.R., PETASIS N.A., SERHAN C.N. Aspirin-triggered 15-epi-lipoxin A4 and LXA4 stable analogs are potent inhibitors of acute inflammation: Evidence for anti-inflammatory receptors. J. Exp. Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKANO T., CLISH C.B., GRONERT K., PETASIS N., SERHAN C.N. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J. Clin. Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKANO T., CYBULSKY A.V. Complement C5b-9-mediated arachidonic acid metabolism in glomerular epithelial cells: role of cyclooxygenase-1 and 2. Am. J. Pathol. 2000;156:2091–2101. doi: 10.1016/S0002-9440(10)65080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TITOS E., CHIANG N., SERHAN C.N., ROMANO M., GAYA J., PUEYO G., CLARIA J. Hepatocytes are a rich source of novel aspirin-triggered 15-epi-lipoxin A4. Am. J. Physiol. 1999;277:C870–C877. doi: 10.1152/ajpcell.1999.277.5.C870. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., CHAPMAN K., MCKNIGHT W. Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-airpouch inflammation. Br. J. Pharmacol. 1999;126:1200–1204. doi: 10.1038/sj.bjp.0702420. [DOI] [PMC free article] [PubMed] [Google Scholar]