Abstract
Receptor-mediated calcium entry (RMCE) was examined in well-differentiated cultures of normal human bronchial epithelial cells (HBECs). Changes in intracellular free Ca2+ ([Ca2+]i) were quantified using fluorescence ratio imaging of Fura-2-loaded cells during perfusion with Ca2+ mobilizing agonists.
Initial studies revealed an agonist potency of ATP=uridine triphosphate (UTP) >ADP=uridine diphosphate, consistent with purinergic activation of an apical P2Y2-receptor mediating the increase in [Ca2+]i in HBECs.
Apical UTP (30 μM) induced a sustained period of elevated [Ca2+]i between 300 and 600 s following agonist stimulation that extracellular Ca2+ free studies indicated was dominated by Ca2+ influx.
RMCE was inhibited by 100 nM La3+ (83±3%) or Gd3+ (95±7%) (P<0.005, n=4–11) and was partially attenuated by Ni2+ (1 mM) (58.7±5.0%, P<0.005, n=9).
RMCE was also partially sensitive (< 25% inhibition, P<0.01) to the cation channel blockers SKF96365 (30 μM) and econazole (30 μM), but was insensitive to both verapamil (1 μM) and ruthenium red (10 μM).
Using either a sided Ca2+ readdition protocol or unilateral La3+, established that the RMCE pathway was located exclusively on the basolateral membrane.
The pharmacological sensitivity of the P2Y2-receptor activated Ca2+ entry pathway in the human airway epithelium is inconsistent with the established profile of TRP channel families and is therefore likely to be of an as-yet uncharacterized molecular identity.
Keywords: Airway epithelium, cation channel, econazole, Gd3+, mucociliary clearance, receptor-mediated calcium entry, signal transduction, SKF96365
Introduction
Changes in the levels of intracellular free calcium [Ca2+]i regulate a multitude of biological processes in both excitable and nonexcitable cells (Clapham, 1995). These signals can arise from either the release of Ca2+ from intracellular stores or the influx of Ca2+ through the plasma membrane. The emptying of stored Ca2+ can activate plasma membrane Ca2+ channels resulting in the influx of extracellular Ca2+. This process is commonly known as ‘Capacitative Calcium Entry' (CCE), or ‘Store-operated calcium entry' (Putney & McKay, 1999). CCE serves to both refill depleted Ca2+ stores in the ER and to potentially prolong Ca2+-dependent signalling. Alternatively, receptor stimulation can directly open plasma membrane Ca2+ channels without necessarily depleting intracellular Ca2+ stores, a process termed ‘Receptor-Operated Calcium Entry' (Barritt, 1999).
Changes in [Ca2+]i have been demonstrated to regulate several functions of the airway epithelium. Mucus secretion (Davis et al., 1992; Conway et al., 2003), surfactant secretion (Haller et al., 1998), ciliary beat frequency (Lansley et al., 1992) and ion transport mechanisms (Ribeiro et al., 2001) are all modulated by a variety of stimuli that elevate [Ca2+]i. The nucleotide triphosphates ATP and UTP are present in the airway surface liquid that bathes the mucosal (apical) surface of the airway epithelium (Donaldson et al., 2000; Lazarowski et al., 2000) and have emerged as key regulators of many of these processes through the activation of apical P2Y-receptors (Ribeiro et al., 2001). P2Y2-receptor agonists are currently being tested in the clinic to enhance mucociliary clearance in cystic fibrosis (Kellerman, 2002).
Recent studies have investigated the Ca2+ influx pathways induced by purinergic agonists in the respiratory epithelium (Paradiso et al., 1995; Kerstan et al., 1999; Braiman and Priel, 2001; Zsembery et al., 2003). The reports are however inconsistent regarding the polarity of the RMCE pathway and to date there has been no description of the pharmacological sensitivity of Ca2+-influx. The aims of the present study were therefore to utilize well-differentiated cultures of normal human bronchial epithelial cells (HBECs) to investigate: (1) the nature of the dominant purinergic receptor responsible for elevations in [Ca2+]i, (2) whether purinergic stimulation could induce RMCE, (3) the pharmacological sensitivity of the RMCE pathway, and (4) to determine the polarity of the pathway(s) using both Ca2+-readdition and pharmacological approaches.
Methods
Cell culture
Nontransformed, primary HBECs (Biowhittaker, U.K.) (passage 1) were cultured as previously described (Danahay et al., 2002). At passage 3 (following expansion and freezing), cells were seeded (8.25 × 105 cells/insert) onto polycarbonate Snapwell ™ inserts (Costar, U.K.). Cells were maintained submerged for the first 7 days in culture after which time the apical media was removed and the cells exposed to an air–liquid interface for the remainder of the culture period. Cells were used between days 14 and 21 after establishment of the air–liquid interface. At all stages of culture, cells were maintained at 37°C in 5% CO2 in an air incubator. HBECs from three donors were used for these studies. These culture conditions provide a well-differentiated epithelium composed primarily of ciliated, goblet and basal cells (Atherton et al., 2003) with an ion transport phenotype consistent with the native epithelium (Danahay et al., 2002). Although not specifically measured during the present studies, these cultures typically develop a transepithelial resistance of 400–1000Ω. cm2.
Measurement of intracellular free calcium concentration [Ca2+]i
The apical surface of the HBECs was rinsed 3 times with warmed (37°C) HBSS (containing Ca2+, Mg2+, 20 mM HEPES, pH 7.4) (normal HBSS) to remove any accumulated mucus. Cells were then loaded (apical side only) with 5 μM Fura-2-AM in 200 μl of normal HBSS (containing pluronic acid 0.2%) for 60 min (37°C). The Fura-2-AM loading solution was then aspirated and the apical surface was again rinsed 3 times with normal HBSS. At this time, the culture media bathing the basolateral surface was replaced with 2 ml normal HBSS. Cells were then incubated for a further 30 min (37°C) and were placed into a custom-made, plastic chamber with normal HBSS bathing both apical and basolateral membranes (room temperature). The chamber enabled independent perfusion of both apical and basolateral surfaces with test solutions. The chamber was mounted on an upright Leica DMLM microscope and cells were observed with a × 20 fluid immersion lens. Emitted fluorescence was observed after excitation was alternated at 340 and 380 nM by a 75 W xenon lamp linked to a Delta Ram illuminator (Photon Technology International (PTI) U.K.). Images of emitted fluorescence above 510 nM were recorded by an intensified CCD camera (PTI) and displayed on a monitor. The ratio of the emitted fluorescence following excitation at 340 and 380 nM was used as a surrogate measure of [Ca2+]i. The imaging system was under the control of ImageMaster software (PTI). At the completion of each experiment, background fluorescence was quantified following permeabilization with ionomycin (∼50 μM) and quenching with MnCl2.
Experimental protocols
At the start of all studies, normal HBSS was perfused over both apical and basolateral surfaces (2.5 ml min−1) to establish a stable [Ca2+]i baseline. For concentration–response experiments, cells were apically perfused with normal HBSS while the basolateral path was static. Following a 300 s stabilization period, normal HBSS solution was switched to the agonist-containing solution for 30 s, before reverting back to agonist-free normal HBSS. Pilot studies indicated that a 30 s perfusion was sufficient for the change in 340 : 380 ratio to reach the peak of the response. Perfusion with agonist-free normal HBSS continued until the 340 : 380 ratio had returned to baseline at which time the solutions were switched to deliver the next concentration of agonist. For experiments designed to test for UTP ‘leak' from apical to basolateral membranes, apyrase (10 U ml−1) was added to the basolateral perfusion solution. For low Ca2+ experiments, normal HBSS was exchanged for an HBSS (containing 100 μM Ca2+, 1 mM Mg2+, 200 μM EGTA, pH 7.4) (nominal Ca2+ solution) and was perfused over the appropriate epithelial surface(s). In the studies indicated, Ca2+ (1 mM) was added back to the nominal Ca2+ solution.
For pharmacological profiling of the RMCE pathway sensitivity, HBECs were perfused both apically and basolaterally with normal HBSS (containing vehicle or test inhibitor) for 300 s. The apical perfusion path was then switched to contain UTP (30 μM) (in the continued presence of vehicle or inhibitor). After 60 s of UTP perfusion apical and basolateral perfusion paths were stopped. Data acquisition was continued for a further 540 s resulting in a total UTP exposure of 600 s. In the La3+ and Gd3+ studies, normal HBSS was replaced with a phosphate-free salt solution containing (in mM): 140 NaCl, 5.4 KCl, 4.2 NaHCO3, 5.6 glucose, 1.0 CaCl2, 1.0 MgCl2, 20 HEPES (pH 7.4).
Expression of results and statistical analysis
Control inserts were run alongside all experiments for paired comparisons to be made owing to the potential day-to-day, interbatch and interdonor variability of responsiveness. Data are expressed as mean (±s.e.m.) absolute changes in the peak 340 : 380 ratio fluorescence units (FU) or as area under the curve for the 300–600 s period after UTP stimulation (AUC300−600). Data are presented as FU rather than absolute values of [Ca2+]i because of the difficulties involved in ensuring a robust and stable permeabilization of the HBECs that would be required to accurately calibrate the system. The entire field that was visualized by the microscope was used as the region of interest for all calculations. A Student's t-test, with Bonferonni correction for multiple comparisons, was used to compare between control and test groups with statistical significance assumed when P<0.05.
Reagents
Unless otherwise stated, cell culture reagents and HBSS solutions were purchased from Life Technologies (U.K.). Fura-2-AM and pluronic acid were purchased from Molecular Probes (U.S.A.). All other reagents were purchased from Sigma (U.K.) unless otherwise stated.
Results
Apical UTP activates P2Y2-receptors
Concentration–response studies using apical perfusions of nucleotide di- and triphosphates revealed a rank-order agonist potency profile for the peak increase in [Ca2+]i of (mean pEC50±s.e.m.): ATP (5.54±0.13)=UTP (5.72±0.06)>ADP (3.94±0.09)=UDP (3.95±0.11) (Figure 1a), indicative of a P2Y2-receptor-mediated response (Barnard et al., 1994). It should be noted that the nucleotide diphosphate solutions were not hexokinase treated to ensure depletion of any potential triphosphate contamination. It cannot therefore be excluded that the responses observed to UDP and ADP are as a result of a 1% contamination with UTP or ATP, respectively.
Figure 1.
An apical P2Y2-receptor mediates UTP-stimulated increase in [Ca2+]i in HBECs. Potency order for purine nucleotide-evoked increase in [Ca2+]i in HBECs (a). Each data point represents a mean (±s.e.m.) Δ 340 : 380 ratio normalized to the response achieved with a supramaximal concentration of ionomycin (n=6–15/group). Mean (±s.e.m.) kinetic data (b) illustrating the responses to UTP (30 μM) in either normal HBSS or nominal Ca2+ solution. Following a 300 s stabilization period before UTP-stimulation the peak increases in 340 : 380 ratio were not different between the two groups. In contrast, the 340 : 380 ratio had returned to baseline levels by 300 s after stimulation in the nominal Ca2+ solution while remaining elevated under normal Ca2+ conditions (n=6/group). Sample raw data trace illustrating the effect of basolateral UTP (30 μM) on the 340 : 380 ratio (c). Apical UTP (30 μM) was added at the end of the experiment to illustrate the responsiveness of the cells.
Apical P2Y2-receptor activation stimulates Ca2+ influx
In normal HBSS buffer, apical UTP (30 μM) induced a rapid rise in [Ca2+]i peaking at 1.45±0.10 FU (n=6) that remained elevated above the starting baseline for the duration of the study (600 s following stimulation). When performed in a nominal Ca2+ solution, the peak increase was unchanged (1.42±0.10 FU; n=6; P>0.05). However, under nominal Ca2+ conditions the increase in [Ca2+]i had returned to baseline levels within 300 s of stimulation (Figure 1b). The period between 300 and 600 s after apical UTP stimulation was therefore considered to be dominated by RMCE (AUC300−600).
In normal Ca2+ solution, UTP (30 μM) added to the basolateral perfusion solution induced an increase in [Ca2+]i that peaked at 0.06±0.01 FU (n=5) in contrast to the paired apical control which peaked at 1.22±0.07 FU (n=5) (Figure 1c). The area under the curve for the basolateral UTP response accounted for <5% of the equivalent response to apical UTP in paired cells. In the presence of basolateral apyrase (10 U ml−1), the AUC300−600 in response to apical UTP (94.2±9.5) was not different from the paired apyrase-free control (94.9±5.3; P>0.05). This concentration of apyrase abolished the response to apical UTP (data not shown). These observations indicate that the changes in [Ca2+]i observed following apical stimulation with UTP are indeed mediated via a direct effect at this membrane, rather than as a consequence of any putative paracellular leakage of exogenous UTP to enable basolateral membrane stimulation.
Pharmacological sensitivity of the RMCE in HBECs
The lanthanide cations, La3+ (Figure 2) and Gd3+ (Figure 3) blocked the Ca2+ influx response in UTP-stimulated HBECs in a concentration-dependent manner. The peak responses to UTP were unaffected by the presence of either La3+ (1–100 nM) (Figure 2b) or Gd3+ (10–100 nM) (Figure 3b) indicating that Ca2+ release from intracellular stores was unaffected by the trivalent cations. In contrast, the lanthanides significantly attenuated the UTP-stimulated AUC300−600 (Ca2+ influx) (Figures 2c and 3c). At 100 nM, La3+ and Gd3+ significantly inhibited AUC300−600 by 83.2±2.8% (P<0.005; n=4) and 94.5±7.5% (P<0.005; n=4), respectively.
Figure 2.
Sample raw data traces (a) illustrating the effect of La3+ (100 nM, apical and basolateral) on the profile of the apical UTP-(30 μM) stimulated elevation of [Ca2+]i (absolute 340 : 380 ratio; FU). La3+ was without effect on the initial peak increase in [Ca2+]i (b) but significantly attenuated the AUC300−600 (c). Mean (±s.e.m.) data are shown (n=4–11/group). **Indicates P<0.005.
Figure 3.
Sample raw data traces (a) illustrating the effect of Gd3+ (10 and 100 nM, apical and basolateral) on the profile of the apical UTP- (30 μM) stimulated elevation of [Ca2+]i (Δ 340 : 380 ratio; FU). Gd3+ was without effect on the initial peak increase in [Ca2+]i (b) but significantly attenuated the AUC300−600 following stimulation with UTP (c). Mean (±s.e.m.) data are shown (n=4–11/group). **Indicates P<0.005.
The non-selective cation channel blockers: Ni2+, SKF96365 and econazole, all partially inhibited the AUC300−600 Ca2+ influx response while having no effect on the initial peak increase induced by UTP (Table 1). In contrast verapamil, at a supra-maximal concentration to that required to inhibit L-VOCC channels (Glossmann & Striessnig, 1990), was without effect on either the peak or the AUC300−600 influx responses. Ruthenium red, at a concentration previously demonstrated to block vanilloid receptors TRPV-1 and TRPV-5 (Tominaga et al., 1998; Nilius et al., 2001), was also without effect on either phase of the UTP-stimulated Ca2+ response. It was impractical to use higher concentrations of ruthenium red because of interference with the Fura-2 fluorescence.
Table 1.
Effects of cation channel blockers on UTP-induced Δ[Ca2+]i
| Compound | Conc (μM) | n | Peak (% inhibition) | AUC300-600 (% inhibition) |
|---|---|---|---|---|
| Ni2+ | 100 | 4 | −1.3±13.1 | −2.1±1.9 |
| 300 | 4 | −7.7±9.3 | 14.4±10.4 | |
| 1000 | 9 | 0.4±7.7 | 58.7±5.0** | |
| Verapamil | 1 | 4 | −1.2±7.2 | 5.7±8.6 |
| SKF96365 | 10 | 6 | 13.6±6.4 | 3.1±4.8 |
| 30 | 5 | 9.6±6.8 | 20.6±2.1** | |
| Econazole | 3 | 4 | −1.7±2.2 | −13.7±6.2 |
| 10 | 5 | −3.6±4.5 | 9.6±4.6 | |
| 30 | 11 | −5.1±5.2 | 26.7±3.9** | |
| Ruthenium red | 10 | 7 | −3.6±5.5 | 3.0±6.1 |
Data represent the mean (±s.e.m.) % Inhibition of the initial peak increase in [Ca2+]i (Peak) and the phase of RMCE (AUC300−600) following stimulation of HBECs with UTP.
Indicates P<0.005 (test compound vs paired vehicle control).
2-Aminoethoxydiphenyl borate (2-APB) has been used as an inhibitor of Ca2+ mobilisation via the blockade of IP3 receptors (Maruyama et al., 1997). At 50 μM the AUC300−600 was significantly inhibited by 92.5±3.6% (P<0.005; n=5) (Figure 4b); however, the peak response was also significantly reduced by 17.0±3.5% (P<0.05) (Figure 4a). A similar, although more pronounced profile was observed for 2-APB (75 μM). 2-APB (10 μM) was without significant effect on either component of the response.
Figure 4.
2-APB significantly attenuated both the initial peak increase in [Ca2+]i (Δ 340 : 380 ratio; FU) (a) and the AUC300−600 following stimulation with UTP (b). Mean (±s.e.m.) data are shown (n=5–15/group). *Indicates P<0.05. **Indicates P<0.005.
Apical P2Y2-receptor stimulation activates a basolateral RMCE pathway in HBECs
Ca2+ readdition
Apical UTP (30 μM) stimulation of HBECs in nominal Ca2+ solution (apical and basolateral) resulted in a rapid Ca2+ transient which returned to baseline values within 300–400 s poststimulation. Subsequent switching of the apical perfusion path to normal HBSS (1 mM Ca2+) had no effect on [Ca2+]i (Figure 5a). In contrast, switching the basolateral perfusion solution to normal HBSS (1 mM Ca2+) resulted in a rise in [Ca2+]i. In the paired control experiment (Figure 5b), an identical UTP-stimulation was performed in nominal Ca2+ solution but this time the order of Ca2+ readdition was reversed. Again, the basolateral readdition of Ca2+ resulted in an increase in [Ca2+]i while subsequent apical readdition was without effect. The magnitude of the peak Ca2+ responses that occurred in response to the apical and then basolateral readdition of 1 mM Ca2+ are illustrated in Figure 5c.
Figure 5.
Sample raw data traces illustrating the effects of readdition of Ca2+ (normal HBSS) to the apical (AP) and then basolateral (BL) (a) or basolateral and then apical (b) membranes of HBECs previously stimulated with UTP (30 μM) in nominal Ca2+ solution. Mean (±s.e.m.) data showing peak responses Ca2+ readdition apical then basolateral (Δ 340 : 380 ratio; FU) (c). **Indicates significant difference (P<0.005) between AP and BL readdition (n=6/group).
Basolateral or apical La3+ on the RMCE pathway
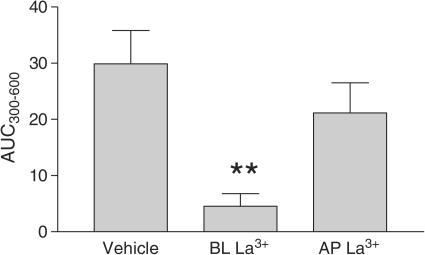
Pretreatment of the basolateral surface of HBECs with La3+ (100 nM, 300 s) in normal HBSS followed by stimulation of the apical surface with UTP (30 μM) significantly attenuated the AUC300−600 by 84.7±7.4% (P<0.005; n=6) compared to the vehicle control (Figure 6). Conversely, pretreatment of the apical surface with La3+ (100 nM, 300 s) was without significant effect on the UTP-stimulated AUC300−600.
Figure 6.
La3+ (100 nM) when included in the basolateral (BL) perfusion solution significantly attenuated the AUC300−600 following stimulation with UTP. Apical (AP) La3+ was without significant effect. Mean data (±s.e.m.) are shown. **Indicates significant difference (P<0.005) between vehicle control and basolateral (BL) La3+ (n=6/group).
Discussion and conclusions
In the present study, we have examined well-differentiated cultures of normal human bronchial epithelial cells to establish: (1) that an apical P2Y2-receptor is responsible for nucleotide triphosphate stimulated increases in [Ca2+]i, (2) apical UTP stimulates a biphasic elevation of [Ca2+]i, that from 300 s after stimulation is dominated by RMCE (AUC300−600), (3) a pharmacological profile inhibition of RMCE that is characterised by high sensitivity to the lanthanides La3+ and Gd3+, and (4) that this RMCE pathway is located exclusively on the basolateral membrane.
In view of recent literature reports, that have used a variety of airway epithelial preparations to study purinergic-receptor-mediated events (Communi et al., 1999; Ribeiro et al., 2001; Ito et al., 2003; Zsembery et al., 2003), it was important to confirm that an apical P2Y2-receptor was responsible for the nucleotide triphosphate-stimulated increase in [Ca2+]i in our HBEC cultures (Figure 1a). The inclusion of apyrase in the basolateral solution, and the relative lack of responsiveness to UTP applied to the basolateral membrane, confirmed that the effect of UTP was specific to the apical membrane and that any putative paracellular leakage of exogenous UTP had no significant impact on the increase in [Ca2+]i. The removal of extracellular Ca2+ confirmed that following apical stimulation with UTP, there followed a sustained phase of Ca2+ entry (Figure 1b) that was quantified as the AUC300−600.
Studies were then undertaken to establish the pharmacological sensitivity of the conductance(s) responsible for the passage of Ca2+ across the plasma membrane following apical P2Y2-receptor activation. The effects of test compounds on AUC300−600 was preferred to using a Ca2+ readdition protocol with subsequent addition of blocker (Clementi et al., 1992) as at no time was it necessary to expose the cells to a Ca2+-free solution that might influence the nature of a subsequent Ca2+ entry pathway.
Verapamil, a potent blocker of voltage-operated Ca2+ channels (Glossmann & Striessnig, 1990) was without effect on either the peak increase in [Ca2+]i or the RMCE phase indicating no role for this class of conductance in the P2Y2-receptor stimulated Ca2+-influx. The partial attenuation of the RMCE phase by the nonselective cation channel blockers: Ni2+ (1 mM), SKF96365 (30 μM) and econazole (30 μM) and the lack of effect of ruthenium red are consistent with a putative blocking effect on members of the TRPC (Li et al., 2002) rather than TRPV families of transient receptor potential cation conductances (Tominaga et al., 1998; Nilius et al., 2001; Peng et al., 2003). The effect of 2-APB was also consistent with inhibition of a CCE pathway, although at the concentrations used there were also significant effects on the release of stored Ca2+ potentially as a consequence of its well-established activity as an IP3-receptor antagonist (Maruyama et al., 1997). The intriguing aspect of the inhibitor profile was the low (100 nM) concentration of La3+ or Gd3+ that effectively inhibited the RMCE pathway. These concentrations are substantially lower than those widely used to demonstrate effects on TRPC and TRPV family members (5–50 μM). Interestingly, Gd3+ has been demonstrated to block CCE in both cultured (Broad et al., 1999) and primary vascular smooth muscle cells (Fellner & Arendshorst, 2002) at concentrations similar to those used to inhibit Ca2+ influx in the present study. The identity of the conductance(s) involved in these smooth muscle studies are unknown. Likewise, lanthanides inhibit CCE in both HL-60 cells and primary human neutrophils with IC50 values between 20 and 250 nM (P. Bahra et al., unpublished observations). This profile of sensitivity is consistent with a P2Y2-receptor stimulated capacitative Ca2+ entry pathway (i.e. as a result of store depletion) although inconsistent with a role for the TRP-family of cation conductances. It should however be considered that the published pharmacology of the TRP-channels has been elucidated in heterologous expression systems rather than in native tissues, a factor that could alter the profile of blocker sensitivities.
The observation that the apical P2Y2-receptor stimulated RMCE pathway is exclusively localized to the basolateral membrane was confirmed using both the classical Ca2+ readdition technique (Clementi et al., 1992) and by utilizing La3+ as a potent blocker of the HBEC Ca2+ entry conductance. The basolateral location of the RMCE pathway and that it is contralateral to the agonist-stimulated membrane, are in agreement with observations made using the human bronchial epithelial cell line 16HBE14o− (Kerstan et al., 1999). However, in contrast, a RMCE pathway ipsilateral to the ATP-stimulated membrane has been described in both human nasal (Paradiso et al., 1995; Braiman and Priel, 2001) and tracheobronchial epithelial cultures (Ribeiro et al., 2003). As discussed above, the apyrase studies confirmed that the stimulation of the RMCE was not due to the paracellular ‘leak' of exogenous UTP from the apical to basolateral membranes (Figure 1c). Furthermore, the response to direct stimulation of the basolateral membrane with UTP (30 μM) could not account for the magnitude of the observed phase of Ca2+-influx. This disparity between our observations and the study by Ribeiro et al. (2003) that used similar HBEC methodology to our own, are therefore difficult to reconcile. A number of studies support the concept that a P2Y-receptor-mediated stimulus can indeed traverse airway epithelial cells to induce functional effects at the contralateral membrane in terms of both basolateral to apical signal transduction (Davis et al., 1992; Paradiso et al., 1995) and apical to basolateral transduction (Devor & Pilewski, 1999). Furthermore, it has been demonstrated that a RMCE pathway on the basolateral membrane of pancreatic acinar cells is able to refill ER in the apical pole of the cell (Petersen et al., 1999).
The functional relevance of Ca2+-influx into the airway epithelium, beyond the refilling of depleted stores, remains largely unknown. Certainly, purinergic stimulation of the airway epithelium has been demonstrated to elevate [Ca2+]i, and to regulate a variety of functions including mucin and surfactant secretion, ciliary beat frequency and anion secretion, all of which influence the process of mucociliary clearance. RMCE has been demonstrated to regulate sustained anion secretion in 16HBE14o− cells (Kerstan et al., 1999). Recently, Bertrand et al. (2004) demonstrated that degranulation of the colonic goblet cell line HT29 was also partially dependent upon a rapid phase of La3+- and niflumic acid-sensitive Ca2+ influx following stimulation with ATP.
In conclusion, we have established that the apical stimulation of P2Y2-receptors results in the sustained activation of a basolateral RMCE pathway. This pathway is partially sensitive to blockers of the known TRPC cation conductance family but also to low (10–100 nM) concentrations of the trivalent cations, La3+ and Gd3+. The molecular identity of the conductance(s) responsible for the pathway and its functional relevance to the airway epithelium, beyond that of simply refilling ER Ca2+ stores, remains to be established. In view of the potential utility of P2Y2-receptor agonists as therapeutics to enhance mucociliary clearance, modulators of the RMCE pathway may represent novel drug targets.
Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- AUC
area under the curve
- AUC300−600
area under the curve between 300 and 600 s after UTP stimulation
- BEGM
bronchial epithelial growth media
- [Ca2+]i
intracellular free calcium
- CPA
cyclopiazonic acid
- DMEM
Dulbecco's modified Eagle's medium
- EGTA
ethylene glycol-O,O′-bis(2-aminoethyl)-N,N,N′^N′-tetra-acetic acid
- ER
endoplasmic reticulum
- FU
340 : 380 ratio fluorescence units
- HBEC
human bronchial epithelial cell
- HEPES
N-(hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid)
- HBSS
Hanks' balanced salt solution
- RMCE
receptor-mediated calcium entry
- SKF96365
1-[-[3-(4-methoxyphenyl) propoxy]-4-methoxyphenyl]-1H-imidazole hydrochloride
- TRP
transient receptor potential
- UTP
uridine triphosphate
References
- ATHERTON H.C., JONES G., DANAHAY H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;285:L730–L739. doi: 10.1152/ajplung.00089.2003. [DOI] [PubMed] [Google Scholar]
- BARNARD E.A., BURNSTOCK G., WEBB T.E. G protein coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol. Sci. 1994;8:280–281. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- BARRITT G.J. Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem. J. 1999;337:153–169. [PMC free article] [PubMed] [Google Scholar]
- BERTRAND C.A., DANAHAY H., POLL C.T., LABOISSE C., HOPFER U., BRIDGES R.J. Niflumic acid inhibits ATP-stimulated exocytosis in a mucin-secreting epithelial cell line. Am. J. Physiol. Cell Physiol. 2004;286:247–255. doi: 10.1152/ajpcell.00593.2002. [DOI] [PubMed] [Google Scholar]
- BRAIMAN A., PRIEL Z. Intracellular stores maintain stable cytosolic Ca2+ gradients in epithelial cells by active Ca2+ redistribution. Cell Calcium. 2001;30:361–371. doi: 10.1054/ceca.2001.0245. [DOI] [PubMed] [Google Scholar]
- BROAD L.M., CANNON T.R., TAYLOR C.W. A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin. J. Physiol. 1999;517:121–134. doi: 10.1111/j.1469-7793.1999.0121z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAPHAM D.E. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- CLEMENTI E., SCHEER H., ZACCHETTI D., FASOLATO C., POZZAN T., MELDOLESI J. Receptor-activated Ca2+ influx. Two independently regulated mechanisms of influx stimulation coexist in neurosecretory PC12 cells. J. Biol. Chem. 1992;267:2164–2172. [PubMed] [Google Scholar]
- COMMUNI D., PAINDAVOINE P., PLACE G.A., PARMENTIER M., BOEYNAEMS J.M. Expression of P2Y receptors in cell lines derived from the human lung. Br. J. Pharmacol. 1999;127:562–568. doi: 10.1038/sj.bjp.0702560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY J.D, BARTOLOTTA T., ABDULLAH L.H., DAVIS C.W. Regulation of mucin secretion from human bronchial epithelial cells grown in murine hosted xenografts. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284:945–954. doi: 10.1152/ajplung.00410.2002. [DOI] [PubMed] [Google Scholar]
- DANAHAY H., ATHERTON H., JONES G., BRIDGES R.J., POLL C.T. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L226–L236. doi: 10.1152/ajplung.00311.2001. [DOI] [PubMed] [Google Scholar]
- DAVIS C.W., DOWELL M.L., LETHEM M., VAN SCOTT M. Goblet cell degranulation in isolated canine tracheal epithelium: response to exogenous ATP, ADP, and adenosine. Am. J. Physiol. 1992;262:1313–1323. doi: 10.1152/ajpcell.1992.262.5.C1313. [DOI] [PubMed] [Google Scholar]
- DEVOR D.C., PILEWSKI J.M. UTP inhibits Na+ absorption in wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am. J. Physiol. 1999;276:827–837. doi: 10.1152/ajpcell.1999.276.4.C827. [DOI] [PubMed] [Google Scholar]
- DONALDSON S.H., LAZAROWSKI E.R., PICHER M., KNOWLES M.R., STUTTS M.J., BOUCHER R.C. Basal nucleotide levels, release, and metabolism in normal and cystic fibrosis airways. Mol. Meth. 2000;6:969–982. [PMC free article] [PubMed] [Google Scholar]
- FELLNER S.K., ARENDSHORST W.J. Store-operated Ca2+ entry is exaggerated in fresh preglomerular vascular smooth muscle cells of SHR. Kidney Int. 2002;61:2132–2141. doi: 10.1046/j.1523-1755.2002.00383.x. [DOI] [PubMed] [Google Scholar]
- GLOSSMANN H., STRIESSNIG J. Molecular properties of calcium channels. Rev. Physiol. Biochem. Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- HALLER T., ORTMAYR J., FRIEDRICH F., VOLKL H., DIETL P. Dynamics of surfactant release in alveolar type II cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1579–1584. doi: 10.1073/pnas.95.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO Y., SON M., SATO S., ISHIKAWA T., KONDO M., NAKAYAMA S., SHIMOKATA K., KUME H.ATP release triggered by activation of the Ca2+ -activated K+ channel in human airway Calu-3 cells Am. J. Respir. Cell Mol. Biol. 2003[Epub ahead of print] [DOI] [PubMed]
- KELLERMAN D.J. P2Y(2) receptor agonists: a new class of medication targeted at improved mucociliary clearance. Chest. 2002;121:201–205. doi: 10.1378/chest.121.5_suppl.201s. [DOI] [PubMed] [Google Scholar]
- KERSTAN D., THOMAS J., NITSCHKE J., LEIPZIGER J. Basolateral store-operated Ca2+-entry in polarized human bronchial and colonic epithelial cells. Cell Calcium. 1999;26:253–260. doi: 10.1054/ceca.1999.0088. [DOI] [PubMed] [Google Scholar]
- LANSLEY A.B., SANDERSON M.J., DIRKSEN E.R. Control of the beat cycle of respiratory tract cilia by Ca2+ and cAMP. Am. J. Physiol. 1992;263:232–242. doi: 10.1152/ajplung.1992.263.2.L232. [DOI] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., BOUCHER R.C., HARDEN T.K. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J. Biol. Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- LI S.W., WESTWICK J., POLL C.T. Receptor-operated Ca2+ influx channels in leukocytes: a therapeutic target. Trends Pharmacol. Sci. 2002;23:63–70. doi: 10.1016/s0165-6147(00)01897-6. [DOI] [PubMed] [Google Scholar]
- MARUYAMA T., KANAJI T., NAKADE S., KANNO T., MIKOSHIBA K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., VENNEKENS R., HOENDEROP J.G., BINDELS R.J., DROOGMANS G. Pharmacological modulation of monovalent cation currents through the epithelial Ca2+ channel ECaC1. Br. J. Pharmacol. 2001;134:453–462. doi: 10.1038/sj.bjp.0704272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARADISO A.M., MASON S.J., LAZAROWSKII E.R., BOUCHER R.C. Membrane-restricted regulation of Ca2+ release and influx in polarized epithelia. Nature. 1995;377:643–646. doi: 10.1038/377643a0. [DOI] [PubMed] [Google Scholar]
- PENG J.B., BROWN E.M., HEDIGER M.A. Epithelial Ca2+ entry channels: transcellular Ca2+ transport and beyond. J. Physiol. 2003;551:729–740. doi: 10.1113/jphysiol.2003.043349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSEN O.H., BURDAKOV D., TEPIKIN A.V. Polarity in intracellular calcium signaling. Bioessays. 1999;21:851–860. doi: 10.1002/(SICI)1521-1878(199910)21:10<851::AID-BIES7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W., JR, MCKAY R.R. Capacitative calcium entry channels. Bioessays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- RIBEIRO C.M., PARADISO A.M., LAZAROWSKI E., BOUCHER R.C.P2Y2 receptors and Ca2+-dependent Cl− secretion in normal and cystic fibrosis human airway epithelia Cilia and Mucus: From Development to Respiratory Defense 2001303–314.ed. Salathe M. pp
- RIBEIRO C.M., PARADISO A.M., LIVRAGHI A., BOUCHER R.C. The mitochondrial barriers segregate agonist-induced calcium-dependent functions in human airway epithelia. J. Gen. Physiol. 2003;122:377–387. doi: 10.1085/jgp.200308893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- ZSEMBERY A., BOYCE A.T., LIANG L., PETI-PETERDI J., BELL P.D., SCHWIEBERT E.M. Sustained calcium entry through P2X nucleotide receptor channels in human airway epithelial cells. J. Biol. Chem. 2003;278:13398–13408. doi: 10.1074/jbc.M212277200. [DOI] [PubMed] [Google Scholar]