Abstract
Peroxisome proliferator-activated receptor-γ (PPAR-γ) expression is very low in skeletal muscle cells, which is one of the most important target tissues for insulin and plays a predominant role in glucose homeostasis. It has recently been shown that muscle-specific PPAR-γ deletion in mouse causes insulin resistance. However, it is likely that the observed effects might be due to secondary interaction in whole animal.
The aim of the study was to explore the role of muscle PPAR-γ in insulin sensitivity. We stably transfected C2C12 skeletal muscle cells with plasmids containing sense or antisense constructs of PPAR-γ and examined the effect of modulation of PPAR-γ expression in terms of glucose uptake. Effect was also examined in insulin-resistant C2C12 skeletal muscle cells.
In transfected C2C12 cell line, the inhibition of PPAR-γ expression (23.0±0.005%) was observed to induce insulin resistance as determined by functional assessment of 2-deoxyglucose incorporation.
Overexpression of PPAR-γ (28.5±0.008%) produced an additional effect on insulin (100 nM) and Pioglitazone (50 μM), resulting in 42.7±3.5% increase in glucose uptake as against 29.2±2.8% in wild-type C2C12 skeletal muscle cells differentiated under normal (2% horse serum) condition. Under similar treatment, PPAR-γ overexpressing cells resistant to insulin exhibited enhanced glucose uptake upto 60.7±4.08%, as compared to 23.8±5.1% observed in wild-type C2C12 skeletal muscle cells.
These data demonstrate a direct involvement of PPAR-γ in insulin sensitization of TZD action on skeletal muscle cells, and suggest that pharmacological overexpression of muscle PPAR-γ gene in skeletal muscle might be a useful strategy for the treatment of insulin resistance.
Keywords: PPAR-γ, insulin resistance, skeletal muscle cell
Introduction
Peroxisome proliferator-activated receptor-γ (PPAR-γ) is a member of the nuclear hormone receptor superfamily of ligand-dependent transcription factors and regulates the expression of genes involved in insulin signaling and lipid metabolism (Barroso et al., 1999; Kubota et al., 1999; Jiang et al., 2002; Herzig et al., 2003; Evans et al., 2004). PPAR-γ is considered to have a specific role in the etiology of insulin resistance (Barroso et al., 1999; Miles et al., 2000; Kraegen et al., 2002; Rangwala et al., 2003; Evans et al., 2004). It is expressed at a high level in adipose tissue (Braissant et al., 1996; Loviscach et al., 2000). Although skeletal muscle is the primary organ for insulin-stimulated glucose disposal (Kraegen et al., 1985; DeFronzo et al., 1992; Saltiel & Olefsky, 1996), expression of PPAR-γ is very low in skeletal muscle cells, accounting for as little as 5–10% of the expression observed in fat cells (Braissant et al., 1996; Loviscach et al., 2000). It is not understood why despite muscle being the site of major glucose disposal, it has a small amount of PPAR-γ expression. The role of PPAR-γ in regulating insulin resistance in skeletal muscle is not fully known. It is not known whether a critical and optimum level of muscle PPAR-γ expression has any role to play in maintaining glucose disposal. Recently, it has been reported that muscle-specific PPAR-γ gene deletion in mice causes insulin resistance (Hevener et al., 2003; Norris et al., 2003). However, in the whole animal, skeletal muscle-specific PPAR-γ deletion leads to a number of secondary and adaptive changes to other tissues (Hevener et al., 2003). Crosstalk among these tissues in regulating insulin sensitivity cannot be ruled out to play a major role(s) (Hevener et al., 2003). Therefore, a direct demonstration of the effect of PPAR-γ expression in regulating glucose homeostasis in skeletal muscle is warranted.
Thiazolidinediones (TZDs) including Pioglitazone, are known to act as ligands of PPAR-γ (Lehmann et al., 1995; Hauner, 2002), resulting in its activation and upregulation of glucose disposal (Zierath et al., 1998). Insulin along with TZDs is known to further stimulate glucose uptake in vitro (Kumar & Dey, 2003) and in vivo (Olefsky, 2000). Pioglitazone is a known antidiabetic agent that improves hyperglycaemia and hyperlipidaemia in obese and diabetic animals via reduction in hepatic and peripheral insulin resistance, and has a major therapeutic impact as a class of insulin sensitizer (Saltiel & Olefsky, 1996; Olefsky, 2000). Previously, we have reported the effects of TZD in an insulin-resistant C2C12 skeletal muscle cell line on the insulin-stimulated activation and tyrosine phosphorylation of insulin receptor (IR) and insulin receptor substrate (IRS) (Kumar & Dey, 2003). The model was validated using several antidiabetic drugs including Metformin (Kumar & Dey, 2002a), Gliclazide (Kumar & Dey, 2002b) and Pioglitazone (Kumar & Dey, 2003). However, a direct action of TZD on skeletal muscle PPAR-γ, especially in the condition of up- or downregulation of PPAR-γ in insulin-sensitive and -resistant C2C12 skeletal muscle cells has not been demonstrated.
Therefore, the present study was performed to determine the effect of PPAR-γ expression on insulin sensitivity in insulin-sensitive and insulin-resistant C2C12 skeletal muscle cells. We explored whether up- or downregulation of PPAR-γ expression in insulin-sensitive or insulin-resistant skeletal muscle cells affect insulin-stimulated glucose uptake due to Pioglitazone treatment. Here, we report that skeletal muscle PPAR-γ expression has a crucial role in insulin sensitivity and/or TZD action.
Methods
Cell culture and treatments
The C2C12 skeletal muscle cell lines (wild type and transfectants) were cultured as described previously (Kumar & Dey, 2002a). Briefly, they were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 15% foetal calf serum (FCS) and antibiotics (penicillin 100 IU ml−1, streptomycin 100 μg ml−1) in 5% CO2 at 37°C. When the cells achieved 70% confluency, they were differentiated in 2% horse serum for 3 days. For undertaking experiments on insulin resistance, cells were prepared as described previously (Kumar & Dey, 2002a, 2003). Briefly, C2C12 cells were differentiated in an equal mixture of two serum-free media (MCDB 201 and Ham's F-12 medium) in the absence (MF) and chronic presence of 100 nM insulin (MFI) for 3 days. Fully differentiated myotubes under both the conditions of differentiation were stimulated with different concentrations of insulin as indicated in the respective experiments for 15 min. Under both conditions, myotubes were treated with Pioglitazone during the last 24 h of differentiation at concentration as indicated. Pioglitazone was dissolved in dimethyl sulphoxide and a final concentration of 0.1% dimethyl sulphoxide was used. The control samples received an equal amount of the same solvent.
Plasmid constructs
For sense and antisense constructs of PPAR-γ, a copy of ∼1.5 kb mouse PPAR-γ gene was isolated from pCMX-mPPARg cDNA clone (kind gift from Ronald M. Evans, The Salk Institute for Biological Studies, San Diego, CA, U.S.A.) and inserted into the cloning site of the plasmid pCDNA3.1neor (Invitrogen) in sense and antisense orientation with respect to CMV promoter (sense, pCDNA3.1-mPPARγ/+ and antisense, pCDNA3.1-mPPARγ/−). Plasmids were expanded in Escherichia coli (strain DH5α) and isolated with Wizard Plus Midiprep DNA isolation kit (Promega, Madison, WI, U.S.A.).
Transfection
C2C12 skeletal muscle cells in the exponential growth phase were transfected with PPAR-γ sense or antisense plasmid construct using TransFast transfection reagent (Promega, Madison, WI, U.S.A.) as described previously (Khurana & Dey, 2002). Briefly, the transfection reagent was incubated with plasmid DNA constructs in serum-free DMEM at room temperature for 15 min. This transfection mixture was applied to the proliferating cells and incubated for 1 h at 37°C. Following incubation, DMEM with 15% FCS was overlaid to the plate and further incubated at 37°C. Selection drug (G-418) was applied to a final concentration of 400 μg ml−1 after 24 h incubation, and was maintained in a medium containing G-418 until cells in the control plate had died.
Preparation of cellular extracts of C1C12 cells for immunoblotting
The cellular extracts were prepared as described previously (Kumar & Dey, 2002a). Briefly, cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in lysis buffer (mM: HEPES 50 (pH 7.4), NaCl 150, MgCl2 1.5, EGTA 1, sodium pyrophosphate 10, sodium fluoride 50, β-glycerophosphate 50, Na3VO4 1, 1% Triton X-100, phenylmethylsulphonyl fluoride 2, 10 μg ml−1 each of leupeptin, aprotonin and soyabean trypsin inhibitor). Lysis was carried out at 4°C for 30 min. Cell lysates were centrifuged at 16,000 × g for 15 min at 4°C. Protein estimation was performed by the bicinchoninic acid (BCA) method (Smith et al., 1985) using bovine serum albumin (BSA) as a standard. Cell lysates were boiled with Laemmli sample buffer (final concentration: Tris–HCl 62.5 mM (pH 6.7), Glycerol 10% (v/v), sodium dodecyl sulphate (SDS) 2% (w/v), bromophenol blue 0.002% (w/v) containing β-mercaptoethenol 143 mM) (Laemmli, 1970) for 5 min, resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) under reducing conditions and transferred to nitrocellulose membranes. The membranes were blocked with 5% BSA solution and incubated with the indicated primary antibodies for 12–16 h, followed by 1 h incubation with alkaline phosphatase-conjugated secondary antibody. The protein bands of approximately 55 kDa size were visualized with BCIP/NBT as substrate.
Immunofluorescence microscopy
Immunofluorescence studies with PPAR-γ were carried out as described previously (Khurana & Dey, 2002). Briefly, cells were grown on coverslips and allowed to differentiate for 3 days. Medium was removed and coverslips were rinsed with PBS. The cells were fixed by incubating with 2% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.3 for 30 min. Cells were washed in PBS containing 1% BSA and blocked using blocking buffer (BSA 1%, goat serum 2% in PBS), for 30 min, followed by washing with PBS/BSA solution. Cells were permeabilized by incubating with 0.2% Triton X-100 for 10 min, followed by washing with PBS/BSA solution. Cells were incubated with anti-PPAR-γ antibody for 2 h at room temperature, followed by washing with PBS/BSA. Bound antibody was visualized under microscope (Nikon, Tokyo, Japan) by incubating with secondary antibodies labelled with fluorescein isothiocyanate (FITC). Digital micrographs were taken using a Nikon camera mounted on Nikon E600 microscope using × 10 phase contrast objective. Images were processed using Image-Pro Express (Media Cybernetics, Madison, U.S.A.) and Adobe Photoshop 5.5 (Adobe Systems Inc., Mountain View, CA, U.S.A.) softwares. At least 20 microscopic fields were observed for each sample. The relative quantitative value of the fluorescent signal intensity of immunoflorescent images was determined by QuantityOne analysis software (Bio-Rad, U.S.A.). Control sample in each experiment was assigned an arbitrary value of 1.0 and the background as 0.
2-Deoxyglucose (2-DOG) uptake assay
Glucose uptake assay was conducted with or without Pioglitazone and/or insulin stimulation, as described previously (Kumar & Dey, 2002a). Briefly, differentiated cells were washed with Kreb's Ringer phosphate (KRP) buffer (mM: phosphate 10 (pH 7.2), NaCl 136, KCl 4.7, CaCl2 1.25, MgSO4 1.25) containing 0.05% BSA and incubated at 37°C for 30 min in KRP buffer. They were then stimulated with insulin in KRP buffer for 15 min at 37°C or left unstimulated. Cells were further incubated in KRP buffer containing [3H]2-DOG (0.2 μCi ml−1 in 1 μM of unlabelled 2-DOG) for 10 min. Cells were washed in ice-cold PBS three times and solubilized in 0.1 N NaOH. Protein concentration of the samples was measured by BCA method (Smith et al., 1985) and 40 μg of protein of each sample was subjected to liquid scintillation counting (Wallac, Finland). The uptake was measured in duplicates. Non-specific 2-DOG uptake was determined in the presence of 10 μM of cytochalasin B and the value obtained was subtracted from all the experimental values. Nonspecific uptake was always less than 10% of total uptake. Data were presented in terms of pmol mg−1 min−1.
Materials
Mouse skeletal muscle cell line C2C12 was kindly provided by Dr H. Blau, Stanford University, School of Medicine, Stanford, U.S.A. and Dr J. Dhawan, CCMB, India. DMEM, horse serum, trypsin-EDTA were from Gibco BRL (Grand Island, NY, U.S.A.). FCS was purchased from Biological Industries (Kibbutz Beit, Haemek, Israel). Nutrient mixture F-12 Ham, MCDB 201 medium, bovine albumin (cell culture grade) were from Sigma (St Louis, MO, U.S.A.). Rabbit polyclonal anti-PPAR-γ antibody, anti-IgG FITC conjugate and anti-rabbit IgG alkaline phosphatase were purchased from Santa Cruz Biotechnology (CA, U.S.A.). Antibiotic G-418 sulphate, TransFast Transfection Reagent and Wizard Plus plasmid DNA isolation kit were obtained from Promega (Madison, WI, U.S.A.). Nitrocellulose membranes, TEMED, acrylamide, bisacrylamide and glycine were purchased from Bio-Rad (Hercules, U.S.A.). All the reagents, unless attributed specifically, were from Sigma (St Louis, MO, U.S.A.). All the plasticwares were purchased from Tarsons (India).
Densitometric analysis
Densitometric analyses of the Western immunoblots were performed by using Gel Doc 2000 (Bio-Rad, U.S.A.) equipped with QuantityOne 1-D analysis software. The relative values of the samples were determined by giving an arbitrary value of 1.0 to the respective control samples of each experiment, keeping the background value as 0.
Statistical analysis
The data are expressed as mean±s.e.m. For comparison of two groups, P-values were calculated by two-tailed unpaired Student's t-test. In all cases, P<0.05 was considered to be statistically significant.
Results
Modulation of PPAR-γ expression in C2C12 skeletal muscle cells
To study the function of PPAR-γ in skeletal muscle, we modulated its expression in C2C12 skeletal muscle cells by transfecting them stably with sense or antisense plasmid constructs of PPAR-γ cDNA. C2C12 cell line was selected because of its ability to differentiate in serum-free medium, which was utilized for developing in vitro insulin-resistant skeletal muscle model (Kumar & Dey, 2002a, 2002b, 2003). For quantifying changes in PPAR-γ expression, wild-type C2C12 (C2C12wt) and transgenic skeletal muscle cells were subjected to Western immunoblot analysis with anti-PPAR-γ antibody (Figure 1a). Stable transfection of C2C12 skeletal muscle cells with sense and antisense plasmid constructs of PPAR-γ significantly modulated the level of PPAR-γ expression with 28.5±0.008% increase in overexpressing (C2PPARγ/+) cells as compared to C2C12wt (Figure 1b, lane 2 compared to lane 1; P < 0.01) and 23.0±0.005% decrease in underexpressing (C2PPARγ/−) cells as compared to C2C12wt (Figure 1b, lane 3 compared to lane 1; P<0.01). Observed differences in the level of PPAR-γ expression were also reflected in their immunofluorescence image when probed with anti-PPAR-γ antibody and detected by FITC labelled secondary antibody (Figure 1c). Fluorescence signal intensity was 27.0±0.09% higher in C2PPARγ/+ (Figure 1d, lane 2 compared to lane 1; P<0.01) and 48.0±0.3% lower in C2PPARγ/− as compared to C2C12wt cells (Figure 1d, lane 3 compared to lane 1; P<0.01).
Figure 1.
PPAR-γ expression profile of C2C12wt, C2PPARγ/+ and C2PPARγ/−. Cell lysates (100 μg each) of C2C12wt (lane 1), C2PPARγ/+ (lane 2) and C2PPARγ/− (lane 3) were Western immunoblotted with anti-PPAR-γ antibody (a). Expression levels of PPAR-γ were quantified by densitometry and values are expressed relative to C2C12wt (control) sample (b). PPAR-γ expression by immunofluorescence microscopy using anti-PPAR-γ antibody as detected by FITC-labelled secondary antibody (c). Intensity of immunofluorescent images was quantified by QuantityOne analysis software and values are expressed relative to C2C12wt (control) sample (d). Experiments were repeated thrice and a representative result is shown. Error bars represent s.e.m. of three independent experiments. **P<0.01 compared with C2C12wt.
Effects of different concentrations of insulin on 2-DOG uptake due to modulation of PPAR-γ expression in C2C12 skeletal muscle cells
To determine whether up- or downregulation of PPAR-γ expression in skeletal muscle cells had any effect on insulin sensitivity in terms of glucose uptake, C2C12wt, C2PPARγ/+, and C2PPARγ/− cells differentiated in 2% horse serum were stimulated with different concentrations of insulin ranging between 10 and 300 nM and subjected to 2-DOG uptake assay as described in the ‘Methods' section. No significant change in basal 2-DOG uptake was observed in C2PPARγ/+ cells although C2PPARγ/− cells showed 14.8±1.6% less 2-DOG uptake as compared to C2C12wt cells (Figure 2, bar 3 compared to bar 1; P<0.05). Insulin stimulation caused an increase in 2-DOG uptake in a dose-dependent manner in C2C12wt as well as in C2PPARγ/+ cells (Figure 2). At 100 nM insulin concentration, C2PPARγ/+ cells showed maximum stimulation, resulting in 35.7±2.9% increase in 2-DOG above basal level (Figure 2, bar 11 compared to bar 1; P<0.01), whereas C2C12wt cells showed only 20.0±1.8% increase in 2-DOG uptake at the same concentration of insulin (Figure 2, bar 10 compared to bar 1; P<0.01). C2PPARγ/− did not show insulin-stimulated glucose uptake at the insulin concentrations tested (Figure 2, bars 6, 9, 12 and 15 compared to bar 3). We have previously shown that the exposure of C2C12 muscle cells to 100 nM insulin was optimal for glucose uptake in which insulin could upregulate 2-DOG uptake in the range of 20–25% (Kumar & Dey, 2002a, 2003).
Figure 2.
Effects of insulin on 2-DOG uptake in C2C12wt, C2PPARγ/+ and C2PPARγ/− cells. Cultured cells were differentiated in 2% horse serum for 3 days, washed with KRP buffer followed by stimulation with various concentrations of insulin (10, 50, 100 and 300 nM) for 15 min. 2-DOG uptake was measured as described in ‘Methods'. Nonspecific uptake was determined in the presence of 10 μM cytochalasin B and subtracted from all the experimental values. Error bars represent s.e.m. of three independent experiments. *P<0.05 compared with untreated C2C12wt, **P<0.01 compared with untreated C2C12wt, #P<0.05 compared with untreated C2PPARγ/+.
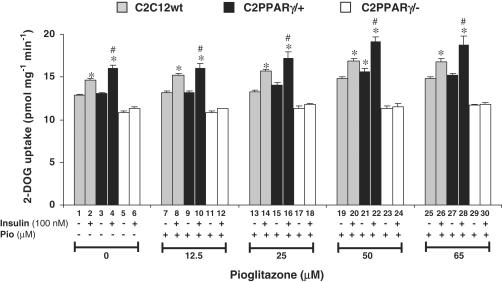
Effects of Pioglitazone on 2-DOG uptake due to modulation of PPAR-γ expression in C2C12 skeletal muscle cells
We examined the effects of different concentrations of Pioglitazone ranging between 12.5 and 65 μM (cell lifting was observed beyond 65 μM concentration of Pioglitazone) on the 2-DOG uptake in all the three cell lines differentiated in 2% horse serum as described in the ‘Methods' section. Data show insulin-stimulated dose-dependent increase in 2-DOG uptake in the C2C12wt and C2PPARγ/+ by Pioglitazone (Figure 3). However, the maximum stimulation of 2-DOG uptake was observed at 50 μM concentration of Pioglitazone in the presence of 100 nM insulin in C2C12wt (Figure 3, bar 20 compared to bar 1; P<0.05) as well as in C2PPARγ/+ cells (Figure 3, bar 22 compared to bar 1; P<0.05). We have previously shown that the exposure of C2C12 muscle cells to 50 μM Pioglitazone was optimal for insulin-stimulated glucose uptake and tyrosine phosphorylation of insulin receptor (IR) and insulin receptor substrate (IRS) (Kumar & Dey, 2003). C2C12wt treated with 50 μM Pioglitazone showed 30.4±2.1% increase in insulin-stimulated 2-DOG uptake (Figure 3, bar 20 compared to bar 1; P<0.05). C2PPARγ/+ cells treated with 50 μM Pioglitazone alone were able to increase 2-DOG uptake by 21.1±2.4% above basal level (Figure 3, bar 21 compared to bar 1; P<0.05). However, when the C2PPARγ/+ cells were treated with 50 μM Pioglitazone and stimulated with 100 nM insulin, a significant increase in 2-DOG uptake upto 48.9±3.5% above the basal level was observed (Figure 3, bar 22 compared to bar 1; P<0.05). Data show that, under PPAR-γ overexpressed condition, C2C12 skeletal muscle exhibits significant increase in glucose uptake, which was not achievable by C2C12wt cells. In contrast, when C2PPARγ/− cells were treated with or without different concentrations of Pioglitazone (10–65 μM) and were subjected to similar experimental conditions as described above, no insulin-stimulated increase in glucose uptake was observed. C2PPARγ/− cells thus became insulin resistant. Pioglitazone-mediated increase in glucose uptake in C2PPARγ/+ cells and the corresponding no effect in C2PPARγ/− cells indicate a direct interaction of Pioglitazone with PPAR-γ in skeletal muscle cells affecting glucose uptake. As a whole, these data suggest that modulation in PPAR-γ expression significantly affects insulin sensitivity and hence glucose disposal ability of skeletal muscle cells.
Figure 3.
Effects of Pioglitazone on 2-DOG uptake in C2C12wt, C2PPARγ/+ and C2PPARγ/− cells. Cultured skeletal muscle cells were differentiated in 2% horse serum for 3 days, and were treated with various concentrations of Pioglitazone (12.5, 25, 50 and 65 μM) as indicated during the last 24 h of differentiation. Differentiated cells were washed with KRP buffer and incubated for 1 h in KRP buffer at 37°C followed by insulin stimulation (100 nM) for 15 min. 2-DOG uptake was measured as described in ‘Methods'. Nonspecific uptake was determined in the presence of 10 μM cytochalasin B and subtracted from all the experimental values. Error bars represent s.e.m. of three independent experiments. *P<0.05 compared with untreated C2C12wt, #P<0.05 compared with untreated C2PPARγ/+.
Effect of insulin and/or Pioglitazone on 2-DOG uptake due to modulation of PPAR-γ expression in insulin-resistant C2C12 skeletal muscle cells
To test the response of modulation of PPAR-γ expression on 2-DOG uptake under insulin-resistant conditions, wild-type and transfected skeletal muscle cell lines were differentiated in serum-free medium in the absence (MF) and or chronic presence of 100 nM insulin (MFI). These cells were then treated with or without 50 μM Pioglitazone and/or stimulated with 100 nM insulin and were subjected to 2-DOG uptake as described in the ‘Methods' section. When all the three types of muscle cells (C2C12wt, C2PPARγ/+ and C2PPARγ/−) were differentiated in MF medium, insulin (100 nM)-stimulated increase in 2-DOG uptake up to 22±3.2 and 27±3.5% was observed in C2C12wt and C2PPARγ/+ cells, respectively (Figure 4, bars 4 and 5 compared to bar 1; P<0.05), whereas no significant insulin-stimulated 2-DOG uptake was observed in C2PPARγ/− cells (Figure 4, bar 6 compared to bar 1). When all the three cell types differentiated in MF medium were treated with 50 μM Pioglitazone, and insulin (100 nM)-stimulated 2-DOG uptake was measured, a significant increase in 2-DOG uptake upto 43.7±3.8% above basal level was observed in C2PPARγ/+ cells (Figure 4, bar 11 compared to bar 1; P<0.05) as against only 22±4.1% increase in C2C12wt cells (Figure 4, bar 10 compared to bar 1; P<0.05). Insulin-stimulated glucose uptake was not observed in C2PPARγ/− cells even after 50 μM Pioglitazone treatment (Figure 4, bar 12 compared to bar 3). When all three cell types were differentiated in MFI medium under the chronic presence of 100 nM insulin (insulin-resistant condition), no significant insulin-stimulated 2-DOG uptake was observed in C2C12wt (Figure 4, bar 16 compared to bar 13), whereas C2PPARγ/+ cells showed a marginal increase of 10±2.3% above basal level (Figure 4, bar 17 compared to bar 13). Under these insulin-resistant conditions, when treated with 50 μM Pioglitazone, a significant 33±3.9% increase in 2-DOG uptake was observed in C2PPARγ/+ cells (Figure 4, bar 20 compared to bar 13; P<0.05), whereas no significant increase in 2-DOG uptake was observed in C2C12wt or C2PPARγ/− cells (Figure 4, bar 19 and 21 compared to bar 13). Surprisingly, when insulin-resistant C2PPARγ/+ cells were treated with 50 μM Pioglitazone and stimulated with 100 nM insulin, a significant increase of 60.7±4.08% 2-DOG uptake was achieved (Figure 4, bar 23 compared to bar 13; P<0.05), whereas only 23.8±5.1% increase was observed in case of C2C12 wt cells (Figure 4, bar 22 compared to bar 13; P<0.05). No effect of Pioglitazone and/or insulin on 2-DOG uptake was observed in C2PPARγ/− cells.
Figure 4.
Effect of Pioglitazone and/or insulin on 2-DOG uptake in C2C12wt, C2PPARγ/+ and C2PPARγ/− cells under insulin-resistant condition. Cells were differentiated in serum-free medium in the absence (MF) or chronic presence of 100 nM insulin (MFI) for 3 days. Pioglitazone (50 μM) was added to the medium during the last 24 h of differentiation. Differentiated cells were washed in KRP buffer and incubated for 30 min in KRP buffer at 37°C, followed by insulin stimulation (100 nM) for 15 min. 2-DOG uptake was determined as described in ‘Methods'. Nonspecific uptake was determined in the presence of 10 μM cytochalasin B and subtracted from all the experimental values. Error bars represent s.e.m. of three independent experiments. *P<0.05 compared with untreated C2C12wt differentiated in MF medium, #P<0.05 compared with untreated C2PPARγ/+ differentiated in MF medium, .P<0.05 compared with untreated C2C12wt differentiated in MFI medium, ..P<0.01 compared with untreated C2C12wt differentiated in MFI medium, ΦP<0.05 compared with untreated C2PPARγ/+ differentiated in MFI medium.
Discussion
The importance of PPAR-γ in the regulation of glucose disposal and insulin sensitivity in the skeletal muscle has become increasingly apparent. Insulin-stimulated glucose uptake in the skeletal muscle of non-insulin-dependent diabetes mellitus (NIDDM) has been shown to be downregulated (Zierath et al., 1998) and Pioglitazone has been reported to improve insulin sensitivity in vivo as well as in vitro in type II diabetic subjects by activating PPAR-γ (Jiang, 2002). In addition, genetic studies have provided compelling evidences for a direct link between PPAR-γ and systemic insulin action. Dominant-negative mutations in human PPAR-γ have been reported to be associated with severe insulin resistance and type II diabetes (Barroso et al., 1999; Agarwal & Garg, 2002; Hegele et al., 2002; Savage et al., 2003). Unfortunately, PPAR-γ gene knockout studies in mice were unable to substantiate this result due to embryonic lethality (Miles et al., 2000). Surprisingly, PPAR-γ heterozygote knockout mice with a 50% reduction in PPAR-γ expression exhibited improved basal insulin sensitivity (Miles et al., 2000) and protected from high-fat diet-induced insulin resistance (Kubota et al., 1999; Kadoeaki, 2000). The reason for this is unclear, but adipose tissue is believed to have a major role (Evans et al., 2004). Working with our insulin resistant in vitro skeletal muscle cell model (Kumar & Dey, 2002a) with altered PPAR-γ expression, we found marked reduction in the parameters of differentiation (our unpublished data), which suggests that optimum expression of PPAR-γ is necessary and critical for the muscle cell survival and functionality.
Muscle-specific deletion of PPAR-γ in mice caused severe insulin resistance with milder defects observed in adipose tissue and liver (Hevener et al., 2003; Norris et al., 2003). However, in these studies, the contribution of adipocyte-derived signalling molecules to the net effect of PPAR-γ detected in samples of muscle tissues cannot be excluded (Hevener et al., 2003; Norris et al., 2003). Change in PPAR-γ status in muscle can lead to secondary adverse effects in liver and adipose tissue action of the animal (Hevener et al., 2003). Considering the fact that loss of muscle PPAR-γ in mice leads to excess adiposity (Norris et al., 2003), it is likely that the resistance phenomenan may be a secondary effect due to uncontrolled gene expression and other related and/or unrelated phenomena of whole animal physiology. Altered adipokine expression and release associated with increased adiposity (Norris et al., 2003), hyperinsulinaemia, glucose intolerance and hypertriglyceridaemia (Hevener et al., 2003) were observed in muscle-specific PPAR-γ knockout mice. Norris et al. (2003) had observed that the expression of ACRP30 was reduced upto 55%, whereas the expression of TNF-α was increased upto 63% in adipose tissue and induced insulin resistance using muscle-specific PPAR-γ knockout mice. Given that skeletal muscle is a major site of fuel oxidation (Kelley & Mandarino, 2000), it is possible that loss of muscle PPAR-γ could produce such an effect(s) and/or defect(s) in the utilization of fatty acids by skeletal muscle, which could contribute to the development of insulin resistance leading to type II diabetes. It could also reflect the paracrine influence of unknown PPAR-γ regulated secretory factor(s) of muscle (Hevener et al., 2003). Therefore, muscle-specific PPAR-γ knockout in mice leads to a variety of secondary and adaptive changes in other tissues.
Previously, we have reported several studies in C2C12 skeletal muscle cells that exhibited insulin-stimulated glucose uptake in the range of 20–25% (Kumar & Dey, 2002a, 2002b, 2003), which was low but significant. Low glucose uptake in C2C12 cells might be due to very low level of GLUT4 expression as compared to L6 cell lines. Although L6 myotubes were used to generate insulin-resistant model (Huang et al., 2002), it was not validated with clinically used insulin sensitizers to determine whether it is responsive to those drugs. C2C12 cells had been shown to have endogenous PPAR-γ (Cabrero et al., 2000; Hunter et al., 2001) as compared to L6 cells where the expression was not detectable (Nagase et al., 1999) or barely detectable (Yonemitsu et al., 2001). L6 could not be differentiated in serum-free medium, which is also evidenced by other reports (Pinset et al., 1982; Lawson & Purslow, 2000). C2C12 did differentiate properly under serum-free conditions (Goto et al., 1999; Conejo et al., 2001; Kumar & Dey, 2003), for which the morphological and biochemical parameters of appropriate differentiation have already been examined and reported (Kumar & Dey, 2003). Therefore, for the present study, it was pertinent to choose the C2C12 cell line to examine the effect of PPAR-γ expression under hyperinsulinaemic conditions in serum-free medium. C2C12 cells have earlier been used by various workers to study insulin resistance (Del Aguila et al., 1999; Schmitz-Peiffer et al., 1999).
Our in vitro studies clearly demonstrated the activity of sense and antisense PPAR-γ constructs, which effectively modulated the expression of PPAR-γ in C2PPARγ/+ and C2PPARγ/−. Data on skeletal muscle cell line directly show the effect of PPAR-γ expression on glucose homeostasis and suggest that overexpression of PPAR-γ in C2PPARγ/+ cells makes them more sensitive to insulin. This might be due to the fact that overexpression of PPAR-γ counteracted insulin resistance (generated by chronic presence of insulin) and utilized excess insulin for the glucose disposal. All these data prove a dominant role of PPAR-γ expression in insulin sensitivity of skeletal muscle cells.
In conclusion, our study shows that expression of PPAR-γ affects insulin sensitivity of skeletal muscle cells. Inhibition of PPAR-γ expression in C2C12 skeletal muscle cells creates insulin resistance. Overexpression of PPAR-γ produces an additive effect of insulin and/or Pioglitazone on insulin-sensitive and -resistant skeletal muscle cells. There is a possibility of crosstalk between insulin and PPAR-γ function in skeletal muscle cells. The ability of overexpressed PPAR-γ to upregulate glucose uptake under insulin-resistant conditions indicates its ability to overcome insulin resistance. Based on our observations, we are tempted to suggest that the development of drugs which trigger PPAR-γ expression in skeletal muscle cells may provide a useful therapy for type II diabetic patients by enhancing insulin sensitivity and glycaemic control.
Acknowledgments
We thank Dr C.L. Kaul, Director, N.I.P.E.R., for his constant encouragement in this work. Dr A. Venkateshwarlu and Dr R. Chakaraborty, Dr Reddy's Research Foundation, India are being acknowledged for providing pioglitazone. We greatly acknowledge Dr Ronald M. Evans, The Salk Institute for Biological Studies, San Diego, CA, U.S.A. for providing cDNA clone of PPAR-γ (pCMXmPPARg) as a kind gift. We are thankful to Dr N. Kumar, Dr A. Khurana, and A. Ishrath, for their constant support.
Abbreviations
- BCIP
5-bromo-4-chloro-3-indolyl-phosphate
- DMEM
Dulbecco's modified Eagle's medium
- 2-DOG
2-deoxyglucose
- FCS
foetal calf serum
- KRP
Kreb's Ringer phosphate
- NBT
nitro blue tetrazolium
- PBS
phosphate-buffered saline
- PPAR
peroxisome proliferator activated receptor
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- TEMED, N,N,N′
N′-tetramethylethylenediamine
- TZD
thiazolidinedione
References
- AGARWAL A.K., GARG A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-γ gene in a patient with familial partial lipidystrophy. J. Clin. Endocrinol. Metab. 2002;87:408–411. doi: 10.1210/jcem.87.1.8290. [DOI] [PubMed] [Google Scholar]
- BARROSO I., GURNELL M., CROWLEY V.E., AGOSTINI M., SCHWABE J.W., SOOS M.A., MASLEN G.L., WILLIAMS T.D., LEWIS H., SCHAFER A.J., CHATTERJEE V.K., O'RAHILLY S. Dominant negative mutation in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- BRAISSANT O., FOUFELLE F., SCOTTO C., DAUCA M., WAHLI W. Differential expression of peroxisome proliferator-activated receptor (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- CABRERO A., ALEGRET M., SANCHEZ R.M., ADZET T., LAGUNA J.C., VAZQUEZ M. Down-regulation of uncoupling proteins-3 and -2 by thiazolidinediones in C2C12 myotubes. FEBS Lett. 2000;484:37–42. doi: 10.1016/s0014-5793(00)02125-6. [DOI] [PubMed] [Google Scholar]
- CONEJO R., VALVERDE A.V., BENITO M., LORENZO M. Insulin produces myogenesis in C2C12 myoblasts by induction of NF-κB and downregulation of AP-1 activities. J. Cell Physiol. 2001;186:82–94. doi: 10.1002/1097-4652(200101)186:1<82::AID-JCP1001>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- DEFRONZO R.A., BONADONNA R.C., FERRANNINI E. Pathogenesis of NIDDM: a balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- DEL AGUILA L.F., CLAFFEY K.P., KIRWAN J.P. TNF-α impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am. J. Physiol. 1999;276:E849–E855. doi: 10.1152/ajpendo.1999.276.5.E849. [DOI] [PubMed] [Google Scholar]
- EVANS R.M., BARISH G.D., WANG Y.U. PPARs and the complex journey of obesity. Nat. Med. 2004;10:1–7. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- GOTO S., MIYAZAKI K., FUNABIKI T., YASUMITSU H. Serum-free culture conditions for the analysis of secretory proteinases during myogenic differentiation of mouse C2C12 myoblasts. Anal. Biochem. 1999;272:135–142. doi: 10.1006/abio.1999.4163. [DOI] [PubMed] [Google Scholar]
- HAUNER H. The mode of action of thiazolidinediones. Diabetes Metab. Res. Rev. 2002;18:510–515. doi: 10.1002/dmrr.249. [DOI] [PubMed] [Google Scholar]
- HEGELE R.A., CAO H., FRANKOWSKI C., MATHEWS S.T., LEFF T. PPARG F3882, a transactivation-deficient mutant, in familial partial lipidystrophy. Diabetes. 2002;51:3586–3590. doi: 10.2337/diabetes.51.12.3586. [DOI] [PubMed] [Google Scholar]
- HERZIG S., HEDRICK S., MORANTTE I., KOO S.H., GALIMI F., MONTMINY M. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPARγ. Nature. 2003;426:190–193. doi: 10.1038/nature02110. [DOI] [PubMed] [Google Scholar]
- HEVENER A.L., HE W., BARAK Y., LE J., BANDYOPADHYAY G., OLSON P., WILKES J., EVANS R.M., OLEFSKY J. Muscle specific Pparg deletion causes insulin resistance. Nat. Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- HUANG C., SOMWAR R., PATEL N., NIU W., TOROK D., KLIP A. Sustained exposure of L6 myotubes to high glucose and insulin decreases insulin stimulated GLUT4 translocation but upregulates GLUT4 activity. Diabetes. 2002;51:2090–2098. doi: 10.2337/diabetes.51.7.2090. [DOI] [PubMed] [Google Scholar]
- HUNTER J.G., VAN DELFT M.F., RACHUBINSKI R.A., CAPONE J.P. Peroxisome proliferator-activated receptor ligands differentially modulates muscle cell differentiation and MyoD gene expression via peroxisome proliferator-activated receptor γ-dependent and -independent pathways. J. Biol. Chem. 2001;276:38297–38306. doi: 10.1074/jbc.M103594200. [DOI] [PubMed] [Google Scholar]
- JIANG G., DALLAS-YANG Q., LI Z., SZALKOWSKI D., LIU F., SHEN X., WU M., ZHOU G., DOEBBER T., BERGER J., MOLLER D.E., ZHANG B.B. Potentiation of insulin signaling in tissues of Zucker obese rats after acute and long term treatment with PPARγ agonists. Diabetes. 2002;51:2412–2419. doi: 10.2337/diabetes.51.8.2412. [DOI] [PubMed] [Google Scholar]
- KADOEAKI T. Insights into insulin resistance and type 2 diabetes from knockout mouse models. J. Clin. Invest. 2000;106:459–465. doi: 10.1172/JCI10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLEY D.E., MANDARINO L.J. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- KHURANA A., DEY C.S. Subtype specific roles of mitogen activated protein kinases in L6L9 skeletal muscle cell differentiation. Mol. Cell Biochem. 2002;238:27–39. doi: 10.1023/a:1019957602038. [DOI] [PubMed] [Google Scholar]
- KRAEGEN E., COONEY G., YE J.M., FURLER S. Peroxisome proliferator activated receptors, fatty acids and muscle insulin resistance. J. R. Soc. Med. 2002;95:14–22. [PMC free article] [PubMed] [Google Scholar]
- KRAEGEN E.W., JAMES D.E., JENKINS A.B., CHISHOLM D.J. Dose response curves for in vivo insulin sensitivity in individual tissues in rats. Am. J. Physiol. 1985;248:E353–E362. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- KUBOTA N., TERAUCHI Y., MIKI H., TAMEMOTO H., YAMAUCHI T., KOMEDA K., SATOH S., NAKANO R., ISHII C., SUGIYAMA T., ETO K., TSUBAMOTO Y., OKUNO A., MURAKAMI K., SEKIHARA H., HASEGAWA G., NAITO M., TOYOSHIMA Y., TANAKA S., SHIOTA K., KITAMURA T., FUJITA T., EZAKI O., AIZAWA S., NAGAI R., TOBE K., KIMURA S., KADOWAKI T. PPARγ mediates high-fat diet – induces adipocyte hypertrophy and insulin resistance. Mol. Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- KUMAR N., DEY C.S. Metformin enhances insulin signalling in insulin-dependent and -independent pathways in insulin resistant muscle cells. Br. J. Pharmacol. 2002a;137:329–336. doi: 10.1038/sj.bjp.0704878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR N., DEY C.S. Gliclazide increases insulin receptor tyrosine phosphorylation but not p38 phosphorylation in insulin resistant skeletal muscle cells. J. Exp. Biol. 2002b;205:3739–3746. doi: 10.1242/jeb.205.23.3739. [DOI] [PubMed] [Google Scholar]
- KUMAR N., DEY C.S. Development of insulin resistance and reversal by thiazolidinediones in C2C12 skeletal muscle cells. Biochem. Pharm. 2003;65:249–257. doi: 10.1016/s0006-2952(02)01509-5. [DOI] [PubMed] [Google Scholar]
- LAEMMLI U.K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LAWSON M.A., PURSLOW P.P. Differentiation of myoblasts in serum free media: effects of modified media are cell line specific. Cells Tissue Organs. 2000;167:130–137. doi: 10.1159/000016776. [DOI] [PubMed] [Google Scholar]
- LEHMANN J.M., MOORE L.B., SMITH-OLIVER T.A., WILKISON W.O., WILLSON T.M., KLIEWER S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator activated receptor γ (PPARγ) J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- LOVISCACH M., REHMAN N., CARTER I., MUDALIAR S., MOHADEEN P., CIARALDI T.P., VEERKAMP J.H., HENRY R.R. Distribution of peroxisome proliferator activated receptor (PPARs) in human skeletal muscle and adipose tissue: relation to insulin action. Diabetologia. 2000;43:304–311. doi: 10.1007/s001250050048. [DOI] [PubMed] [Google Scholar]
- MILES P.D.G., BARAK Y., EVANS R.M., OLEFSKY J.M. Improved insulin sensitivity in mice heterozygous for PPARγ deficiency. J. Clin. Invest. 2000;105:287–292. doi: 10.1172/JCI8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGASE I., YOSHIDA S., CANAS X., IRIE Y., KIMURA K., YOSHIDA T., SAITO M. Up-regulation of uncoupling protein 3 by thyroid hormone, peroxisome proliferator-activated receptor ligands and 9-cis retionic acid in L6 myotubes. FEBS Lett. 1999;461:319–322. doi: 10.1016/s0014-5793(99)01477-5. [DOI] [PubMed] [Google Scholar]
- NORRIS A.W., CHEN L., FISHER S.J., SZANTO I., RISTOW M., JOZSI A.C., HIRSHMAN M.F., ROSEN E.D., GOODYEAR L.J., GONZALEZ F.J., SPIEGELMAN B.M., KAHN C.R. Muscle-specific PPARγ deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J. Clin. Invest. 2003;12:608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLEFSKY J.M. Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. J. Clin. Invest. 2000;106:462–467. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINSET C., GROS F., WHALEN R. Proliferation and differentiation of a myogenic line in synthetic media. C. R. Seances Acad. Sci. 1982;295:727–732. [PubMed] [Google Scholar]
- RANGWALA S.M., RHOADES B., SHAPIRO J.S., RICH S., KIM J.K., SHULMAN G.J., KAESTNER K.H., LAZAR M.A. Genetic modulation of PPARγ phosphorylation regulates insulin sensitivity. Dev. Cell. 2003;5:657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- SALTIEL A.R., OLEFSKY J.M. Thiazolidinediones in the treatment of insulin resistance and type 2 diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- SAVAGE D.B., TAN G.D., ACERINI C.L., JEBB S.A., AGOSTINI M., GURNELL M., WILLIAMS R.L., UMPLEBY A.M., THOMAS E.L., BELL J.D., DIXON A.K., DUNNE F., BOIANI R., CINTI S., VIDAL-PUIG A., KARPE F., CHATTERJEE V.K.K., O'RAHILLY S. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-γ. Diabetes. 2003;52:910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- SCHMITZ-PEIFFER C., CRAIG D.L., BIDEN T.J. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with plamitate. J. Biol. Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- SMITH P.K., KROHN R.I., HERMANSON G.T., MALLIA A.K., GARTNER F.H., PROVENZANO M.D., FUZIMOTO E.K., GOEKE N.M., OLSON B.J., KLENK D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- YONEMITSU S., NISHIMURA H., SHINTANI M., INOUE R., YAMAMOTO Y., MASUZAKI H., OGAWA Y., HOSODA K., INOUE G., HAYASHI T., NAKAO K. Troglitazone induces GLUT4 translocation in L6 myotubes. Diabetes. 2001;50:1093–1101. doi: 10.2337/diabetes.50.5.1093. [DOI] [PubMed] [Google Scholar]
- ZIERATH J.R., RYDER J.W., DOEBBER T., WOODS J., WU M., VENTRE J., LI Z., McCRARY C., BERGER J., ZHANG B., MOLLER D.E. Role of skeletal muscle in thiazolidinedione insulin sensitizer (PPARγ agonist) action. Endocrinology. 1998;139:5034–5041. doi: 10.1210/endo.139.12.6364. [DOI] [PubMed] [Google Scholar]