Abstract
Synthetic peptides corresponding to the Gap 26 and Gap 27 domains of the first and second extracellular loops of the major vascular connexins (Cx37, Cx40 and Cx43), designated as 43Gap 26, 40Gap 27, 37,40Gap 26 and 37,43Gap 27 according to Cx homology, were used to investigate the role of gap junctions in the spread of endothelial hyperpolarizations evoked by cyclopiazonic acid (CPA) through the wall of the rabbit iliac artery.
Immunostaining and confocal microscopy demonstrated that gap junction plaques constructed from Cx37 and Cx40 were abundant in the endothelium, whereas Cx43 was the dominant Cx visualized in the media.
None of the Cx-mimetic peptides affected endothelial hyperpolarizations evoked by CPA directly.
When administered individually, 40Gap 27, 37,40Gap 26 and 37,43Gap 27, but not 43Gap 26, attenuated endothelium-dependent subintimal smooth muscle hyperpolarization. By contrast, only 43Gap 26 and 37,43Gap 27 reduced the spread of subintimal hyperpolarization through the media of the rabbit iliac artery. The site of action of the peptides therefore correlated closely with the expression of their target Cxs in detectable gap junction plaques.
The findings provide further evidence that the EDHF phenomenon is electrotonic in nature, and highlight the contribution of myoendothelial and homocellular smooth muscle communication via gap junctions to arterial function.
Keywords: Gap junctions, connexin, EDHF, hyperpolarization
Introduction
Synthetic peptides homologous to sequences present in the extracellular loops of connexins (Cxs) 37, 40 and 43 are capable of attenuating endothelium-dependent hyperpolarizations and relaxations that are independent of nitric oxide and prostanoids in isolated arteries and in vivo (Chaytor et al., 1998; 2001; De Vriese et al., 2002; Griffith et al., 2002; Sandow et al., 2002). Since these agents impair intercellular communication via gap junctions, endothelium-dependent smooth muscle hyperpolarization is likely to involve direct cell–cell coupling, rather than extracellular transfer of a freely diffusible endothelium-derived hyperpolarizing factor (EDHF; for a review, see Griffith, 2004). Indeed, in the rabbit iliac artery, Cx-mimetic peptides have been shown to attenuate the transfer of fluorescent tracer dye from the endothelium into the media and transmission of endothelial hyperpolarization to subintimal smooth muscle cells via myoendothelial gap junctions (Griffith et al., 2002; Chaytor et al., 2003). Such peptides are also capable of uncoupling vascular smooth muscle cells, suggesting that a component of their action against the EDHF phenomenon might also reflect an ability to attenuate the electrotonic relay of endothelial hyperpolarization through successive layers of the media (Chaytor et al., 1997; Edwards et al., 2000). In the present study, we have therefore employed four peptides, 37,40Gap 26, 43Gap 26, 37,43Gap 27 and 40Gap 27, homologous to specific domains of the first (Gap 26) and second (Gap 27) extracellular loops of the dominant vascular connexins (Cx37, Cx40 and Cx43), to determine whether such agents can be used to dissociate electrical coupling via myoendothelial and homocellular smooth muscle gap junctions.
Cyclopiazonic acid (CPA), a SERCA inhibitor that activates endothelial KCa channels by promoting capacitative Ca2+ entry via store-operated Ca2+ channels, was used to evoke EDHF-type hyperpolarizations of subintimal and subadventitial smooth muscle cells in the rabbit iliac artery (Chaytor et al., 1998; Taylor et al., 1998; Griffith, 2004). Control experiments were performed to confirm that Cx-mimetic peptides do not inhibit endothelial hyperpolarization directly, and their effects against subintimal and subadventitial smooth muscle hyperpolarization correlated with the endothelial and medial localization of gap junction plaques constructed from Cx37, Cx40 and Cx43, as characterized by immunostaining and confocal microscopy. Previous studies have provided evidence that the action of these peptides is Cx-selective. In confluent COS fibroblasts expressing Cx43, for example, intercellular dye transfer of Lucifer yellow is impaired by 37,43Gap 27, but not by 40Gap 27, which differs by just three amino acids (Chaytor et al., 1999). Furthermore, in confluent rat aortic A7r5 myocytes, which are coupled by plaques constructed from Cx40 and Cx43, intercellular transfer of Lucifer yellow can be inhibited by 40Gap 27 and 43Gap 26 in combination, but is essentially unaffected by either peptide individually (Chaytor et al., 2001). This suggests that, in tissues that express multiple Cx subtypes, more than one peptide may be necessary to inhibit intercellular communication because of Cx specificity. It should also be noted that the first and second loop peptides (43Gap 26 and 37,43Gap 27) appear to be equally effective in attenuating dye transfer between confluent HeLa cells transfected to express Cx43 (Berman et al., 2002).
Methods
Mechanical and microelectrode studies
Iliac arteries were obtained from male NZW rabbits (2–2.5 kg) killed with sodium pentobarbitone (120 mg kg−1; i.v.) and incubated in oxygenated (95% O2, 5% CO2) Holman's buffer (composition in mM: 120 NaCl, 5 KCl, 2.5 CaCl2, 1.3 NaH2PO4, 25 NaHCO3, 11 glucose, and 10 sucrose). In preliminary mechanical studies, rings 1.5–2 mm wide were cut and mounted in a Mulvany Multi Myograph (Danish Myo Technology, Aarhus, Denmark) containing oxygenated buffer at 37°C at pH 7.4. Tension was set at 2 mN and during an equilibrium period of 1 h the tissues were washed repeatedly with fresh buffer and tension readjusted following stress relaxation. Cumulative concentration–response curves to CPA were constructed both in resting rings and preparations constricted by phenylephrine (1 μM) in the presence of the NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 300 μM) and the cyclooxygenase inhibitor indomethacin (10 μM). In some experiments, rings were denuded of their endothelium by gentle abrasion.
For electrophysiological measurements, arterial strips with intact endothelium were held adventitia down for endothelial and subintimal smooth muscle measurements or intima down for smooth muscle impalements via the adventitia in an organ chamber superfused (2 ml min−1 at 37°C) with oxygenated Holmans solution containing L-NAME (300 μM) and indomethacin (10 μM). Subintimal and subadventitial smooth muscle membrane potentials were recorded with glass capillary microelectrodes using a sharp intracellular electrode and endothelial membrane potential measured in conventional whole-cell patch clamp mode, as described previously (Chaytor et al., 2003; Griffith et al., 2004). In preliminary experiments, subintimal smooth muscle recordings were also obtained in arterial strips denuded of their endothelium. To achieve subintimal impalements, the sharp electrode was placed on the luminal surface of the vessel and advanced through the internal elastic lamina until a sudden drop in potential was obtained and the electrode further advanced to impale a second cell layer from which measurements were recorded. To investigate electrical responses in smooth muscle cells in the outer media, a sharp electrode was advanced through the adventitia to a point of resistance corresponding to the external elastic lamina. On piercing this barrier, sharp negative deflections in the potential reflected impalement of smooth muscle cells and recordings were made from a second impalement after a further small advancement of the electrode. As required, the peptides 37,40Gap 26 (VCYDQAFPISHIR), 43Gap 26 (VCYDKSFPISHVR), 40Gap 27 (SRPTEKNVFIV) or 37,43Gap 27 (SRPTEKTIFII) were included in the buffer at a concentration of 600 μM for 40 min prior to addition of CPA (30 μM). A subset of experiments was also performed with the combination of 37,40Gap 26 and 43Gap 26 at 300 μM each. Markedly different effects of these peptides on hyperpolarizations measured via the intimal route by patch and sharp electrode techniques confirmed that the recordings were obtained from endothelial and smooth muscle cells (see below). Peptides were dissolved in distilled water before use and administered directly into the organ chamber under conditions of no flow; CPA was dissolved in DMSO. All reagents were obtained from Sigma, U.K.
Immunohistology
Iliac arteries were cryopreserved in OCT compound (Agar Scientific) cooled by liquid N2 and cryosections prepared for immunostaining as described previously (Ujiie et al., 2003). Sections were labelled with the following primary antibodies: for Cx37 and Cx40 rabbit polyclonal antibodies, respectively, prepared against specific sequences of 16 and 19 amino acids (Alpha Diagnostics, 5 μg ml−1); for Cx43 a mouse monoclonal antibody generated against amino acids 252–270 (Chemicon, 5 μg ml−1); for Cx45 a mouse monoclonal antibody generated against amino acids 354–367 (Chemicon, 10 μg ml−1). The secondary antibodies were goat anti-mouse-conjugated Alexa 488 or goat anti-rabbit-conjugated Alexa 546 (Molecular Probes, 1 : 500 dilution), according to the primary antibody. To allow visualization of different cell layers, on some sections smooth muscle cells were labelled with anti-α-smooth muscle actin mouse monoclonal primary antibody (Sigma, 1 : 400 dilution) plus goat anti-mouse-conjugated Alexa 488 secondary antibody, and cell nuclei were labelled with propidium iodide (Sigma, added to the mounting agent at 5 μg slide−1). Sections were mounted in Fluorsave (Calbiochem, U.K.) and imaged with a Leica TCS 4D confocal laser-scanning microscope equipped with an argon/krypton laser. Maximum projection images were derived from eight to 10 optical sections obtained at 0.5 μm intervals.
Statistics
Maximal hyperpolarizations evoked by CPA under the different experimental conditions were compared by ANOVA followed by Dunnett's multiple comparison test (GraphPad Prism 3.03). The decrement of endothelial hyperpolarization across the media was calculated by expressing the difference between subintimal and subadventitial hyperpolarization as a percentage of subintimal hyperpolarization. EC50 values for mechanical responses are given with 95% confidence intervals (CI); other results are given as mean±s.e.m., with n denoting the number of animals studied for each data point. P<0.05 was considered significant.
Results
Preliminary studies
In rings with intact endothelium, relaxations to CPA exhibited an Rmax of 90.9±2.9% with an EC50 of 15.0 μM (CI 12.4–18.2, n=16), and no significant relaxation was evident in rings from which the endothelium had been removed (n=4; Figure 1). Under resting conditions, CPA did not significantly affect tension in preparations with intact endothelium (n=3), whereas endothelial denudation unmasked a small concentration-dependent constrictor response exhibiting an Rmax of 25.5±5.2% and an EC50 of 16.1 μM (CI 6.7–38.8, n=7; Figure 1). In subsequent electrophysiological experiments with preparations possessing an intact endothelium, CPA was therefore employed at 30 μM, as this concentration was found to evoke near-maximal relaxation. Resting smooth muscle membrane potential was −40.6±4.8 mV in arteries denuded of endothelium and not significantly affected by application of 30 μM CPA (n=4; Figure 1).
Figure 1.
(a) Representative traces confirming that EDHF-type relaxations evoked by CPA exhibit an obligatory requirement for the presence of an intact endothelium (endo) in rabbit iliac arteries constricted by 1 μM phenylephrine (PE). (b) Representative traces showing that, in the absence of phenylephrine, CPA caused small contractions in rings denuded of their endothelium, which were not evident in endothelium-intact preparations. (c) Concentration–response curves for relaxation and constriction to CPA. Constriction was normalized as a percentage of the tension response to 1 μM phenylephrine in endothelium-denuded preparations. (d) Original recording showing that 30 μM CPA did not affect resting membrane potential in an endothelium-denuded strip of rabbit iliac artery impaled via its luminal surface.
Endothelial membrane potential in arteries with intact endothelium
In patch-clamp experiments, resting endothelial membrane potential was −42.2±4.4 mV and exposure to 30 μM CPA evoked a hyperpolarizing response of 12.1±0.8 mV (n=12). Incubation with 37,40Gap26 (600 μM), 37,43Gap27 (600 μM), 40Gap27 (600 μM) or 43Gap26 (600 μM) did not significantly affect the resting membrane potential or hyperpolarizations evoked by 30 μM CPA (n=3 for each; Figure 2).
Figure 2.
(a) Original whole-cell patch-clamp recordings of endothelial membrane potential showing that 37,40Gap 26, 40Gap 27, 37,43Gap 27 and 43Gap 26 (at 600 μM each) did not affect CPA-evoked hyperpolarizations, thus excluding nonspecific endothelial effects of these peptides. (b) Histograms confirming lack of effect of the peptides on peak endothelial hyperpolarization.
Smooth muscle membrane potential in arteries with intact endothelium
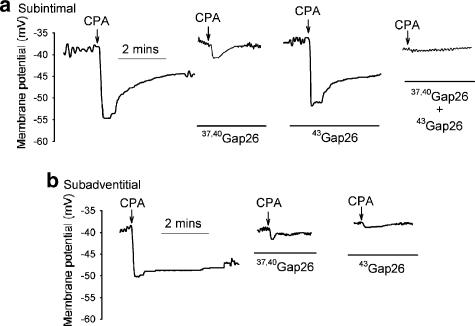
Subintimal and subadventitial smooth muscle cells had similar resting membrane potentials of −42.5±1.5 and −40.2± 1.8 mV (n=24 and 12, respectively), which were statistically similar and not affected by incubation with 37,40Gap 26, 40Gap 27, 37,43Gap 27 or 43Gap 26 at 600 μM each, or the combination of 37,40Gap 26 and 43Gap 26 at 300 μM each. Addition of 30 μM CPA evoked a peak subintimal hyperpolarization of 12.6±0.5 mV (n=24) that was, respectively, reduced to 3.3±1.1, 8.0±0.7 and 4.9±2.3 mV by 37,40Gap 26, 40Gap 27 and 37,43Gap 27 (P<0.001 for each), but unaffected by 43Gap 26 (Figures 3a and 4; n=3–10). Incubation with the combination of 37,40Gap 26 and 43Gap 26 abolished changes in subintimal membrane potential (Figures 3a and 4; n=3, P<0.001).
Figure 3.
Recordings of CPA-evoked hyperpolarizations in (a) subintimal and (b) subadventitial smooth muscle cells of endothelium-intact arterial strips. 37,40Gap 26 (600 μM) attenuated changes in membrane potential in both locations, whereas 43Gap 26 (600 μM) was ineffective subintimally but significantly attenuated the subadventitial response. Note that subintimal hyperpolarization was abolished by combined administration of 37,40Gap 26 and 43Gap 26 at 300 μM each.
Figure 4.
Histograms showing the effects of 37,40Gap 26, 40Gap 27, 37,43Gap 27 and 43Gap 26 (at 600 μM each) against peak CPA-evoked hyperpolarizations of subintimal and subadventitial smooth muscle cells. Also shown is the effect of 37,40Gap 26 plus 43Gap 26 (at 300 μM each) on peak subintimal hyperpolarization. *P<0.05 compared to control; ***P<0.001 compared to control; †P<0.01 compared to control subintimal responses.
CPA-evoked hyperpolarization of subadventitial smooth muscle cells was 9.8±0.5 mV, reflecting a 15.1% decrement in the electrotonically conducted signal across the media (Figures 3b and 4, Table 1; n=12, P<0.01). Incubation with 37,40Gap 26 or 40Gap 27 reduced subadventitial hyperpolarization to 3.0±0.6 and 6.8±0.5 mV, respectively (Figures 3b and 4; n=3 and 4, P<0.001 and 0.05), although the normalized decrement of hyperpolarization across the media in the presence of these peptides did not differ from control (Table 1). Subadventitial hyperpolarization was reduced by 37,43Gap 27 and 43Gap 26 to 1.3±1.3 and 1.7±0.9 mV, respectively (Figures 3b and 4; n=3 and 7, P<0.001 for both), with the decrement of hyperpolarization across the media being five- to six-fold greater for each peptide compared to control, 37,40Gap 26 or 40Gap 27 (Table 1; P<0.001).
Table 1.
Decrements in CPA-evoked hyperpolarization across the vessel wall derived from paired subintimal and subadventitial measurements under the different experimental conditions
| Control | 37,40Gap 26 | 40Gap 27 | 37,43Gap 27 | 43Gap 26 | |
|---|---|---|---|---|---|
| % Decrement±s.e.m. | 15.1±3.2 | 12.6±4.2 | 15.0±3.8 | 73.5±6.5*** | 85.0±6.0*** |
All peptides were administered at 600 μM. n=3–12.
P<0.001 compared to control.
Confocal microscopy
The combination of labelling with anti-α-smooth muscle actin antibody and staining of cell nuclei with propidium iodide clearly delineated the endothelium, media and adventitia of the rabbit iliac artery and demonstrated that the media of this vessel contained ∼10 layers of smooth muscle cells (Figure 5). Immunostaining revealed marked spatial heterogeneity in the distribution of Cx protein present in gap junction plaques in the endothelium and media (Figure 6). Abundant punctate staining of Cx37 and Cx40 was evident in the endothelium, whereas in the media no labelling of Cx37 was evident and only a small number of plaques containing Cx40 were identified. No consistent staining for Cx45 was seen in either cell layer. By contrast, Cx43 was not detectable in the endothelium, but plaques containing Cx43 were widely distributed throughout the media. Strong autofluorescence from the internal elastic lamina was evident in the absence of antibody at the excitation wavelength of Alexa 488. No staining was evident in sections incubated with either Alexa 488 or Alexa 546 in the absence of primary antibodies.
Figure 5.
Low-magnification image stained for α-smooth muscle actin (green) and cell nuclei (red). E, endothelium; M, media; A, adventitia. Bar=25 μm.
Figure 6.
(a–c) High-magnification transverse images delineating the presence or absence of Cx37, Cx40 and Cx45 in endothelial and subintimal smooth muscle gap junction plaques in the rabbit iliac artery. Arrows indicate small medial gap junction plaques containing Cx40. Punctate staining for Cx45 could not be consistently identified. (d) Low-magnification image showing the extent of Cx43 expression in the media. (e) High-magnification image stained for Cx43. (f) High-magnification image obtained in the absence of primary or secondary antibodies showing autofluorescence of the internal elastic lamina (IEL) at the excitation wavelength of the Alexa 488 secondary antibody used to stain for Cx43 and Cx45. Note the absence of punctate staining compared to (e). L, lumen; E, endothelium; M, media; A, adventitia. Bars=25 μm.
Discussion
The present study has provided evidence that differences in the amino-acid sequences of Cx-mimetic peptides can be exploited to dissociate electrotonic signalling through the wall of the rabbit iliac artery via myoendothelial and homocellular smooth muscle gap junctions in a selective fashion. Immunostaining demonstrated that endothelial gap junction plaques were constructed solely from Cx37 and Cx40, whereas the dominant Cx protein expressed by smooth muscle cells was Cx43. Correspondingly, subintimal smooth muscle hyperpolarizations evoked by CPA were attenuated ∼35% by 40Gap 27, which targets Cx40, and by ∼75% with 37,40Gap 26, which simultaneously targets Cxs 37 and 40, whereas the spread of endothelial hyperpolarization through the media was unaffected by either peptide. By contrast, the exclusive localization of plaques containing Cx43 to the media of the rabbit iliac artery and observations that the 43Gap 26 peptide targeted to this Cx subtype significantly attenuated the spread of subintimal hyperpolarization to the outer media indicate that gap junctions constructed from Cx43 underpin electrotonic signalling between smooth muscle cells in this artery. Consistent with these findings, simultaneous targeting of Cxs 37 and 43 with 37,43Gap 27 led to a reduction in the magnitude of both subintimal and subadventitial smooth muscle hyperpolarizations, with the inhibitory effect of this peptide against electrical transmission across the media being equivalent to that of 43Gap 26 on a percentage basis. Taken together, the data suggest that the ability of peptides possessing homology with Cx37, Cx40 and Cx43 to inhibit the spread of EDHF-type hyperpolarizations through successive layers of the vascular wall correlates closely with the incidence and anatomical location of gap junction plaques containing the corresponding Cx subtypes. Nonspecific effects were excluded by the demonstration that none of the peptides affected the initiating endothelial hyperpolarization evoked by CPA.
Small residual subintimal hyperpolarizations observed in the presence of 37,40Gap 26 (∼3 mV) were effectively abolished by 43Gap 26 and subintimal hyperpolarizations to CPA were slightly reduced (∼10%) by 43Gap 26 alone, although this action of the peptide did not achieve statistical significance. It nevertheless remains unclear if Cx43 contributes directly to myoendothelial communication in the rabbit iliac artery as measurements of subintimal smooth muscle membrane potential were generally made after two successive impalements so that the effects of 43Gap 26 might in part reflect an inhibitory action against smooth muscle coupling in the near-intima. Delineation of the composition of myoendothelial gap junctions is therefore an area for further research, as immunostaining cannot provide unambiguous visualization of these structures because of their small size and close proximity to large interendothelial plaques, and functional studies with Cx-mimetic peptides are unlikely to permit their classification as homotypic (composed from a single Cx subtype) or heterotypic/heteromeric (in which each component hemichannel is constructed from a different Cx subtype or mixtures of Cx subtypes, respectively) (Sandow & Hill, 2000; Sandow et al., 2002; Chaytor et al., 2001). In this context, it should be noted that dual-electrode current recordings from coupled endothelial and smooth muscle cells in rat cerebral arterioles have demonstrated an asymmetric transjunctional conductance–voltage relationship that could reflect the presence of myoendothelial gap junctions constructed from more than one Cx subtype (Yamazaki & Kitamura, 2003). It also remains to be determined whether a component of the ability of 40Gap 27 and 37,40Gap 26 to impair the spread of endothelial hyperpolarization into the media reflects an action against direct communication between endothelial cells. Pharmacological uncoupling of cells in this monolayer can increase its input electrical resistance by as much as ∼150-fold, thus raising the possibility that the presence of numerous large inter-endothelial gap junction plaques, as visualized in the present study, indirectly facilitates the spread of hyperpolarization into the media by allowing the endothelium to serve as an ultralow-resistance current source that feeds high-resistance myoendothelial gap junctions (Yamamoto et al., 1998; 2001; Sandow & Hill, 2000; Chaytor et al., 2001).
It has been argued that conducted endothelial hyperpolarizations are unlikely to contribute to smooth muscle relaxation in conduit arteries, on the basis that the large relative mass of the media will readily attenuate passive electrotonic signals by acting as a current sink (Bény, 1999). Indeed, this consideration might in part explain why EDHF-type relaxations that are sensitive to Cx-mimetic peptides are smaller in the rabbit central ear artery (diameter ∼800 μm) than its second branch generation (diameter ∼100 μm) (Berman et al., 2002). The present findings indicate, however, that the decay of endothelium-dependent subintimal hyperpolarization within the media of the rabbit iliac artery is relatively limited since hyperpolarizing responses measured via the adventitial route were only ∼15% smaller than those in subintimal smooth muscle cells, even though staining for smooth muscle actin revealed the presence of ∼10 layers of smooth muscle cells. Observations that the degree of electrical and dye coupling between adjacent cells may correlate with plaque size, a property thought to reflect cooperative interactions between the individual gap junction channels that aggregate to form such structures in the cell membrane (Bukauskas et al., 2000), are consistent with the existence of numerous smooth muscle plaques containing Cx43 in the rabbit iliac artery and the finding that only peptides targeted to this Cx subtype attenuated electrotonic signalling in the media of this vessel. Further studies are nevertheless necessary to delineate how the incidence and size of medial gap junction plaques influence the electrotonic spread of endothelial hyperpolarization and associated relaxation. In small vessels from the rat mesenteric bed, for example, medial plaques may be impossible to identify on immunostaining (Gustafsson et al., 2003), although it is well recognized that there is a progressive increase in the magnitude of EDHF-type relaxations in arteries isolated from successive branch generations of this bed (Hwa et al., 1994; Shimokawa et al., 1996).
In conclusion, we have demonstrated that specific Cx-mimetic peptides can be employed to inhibit electrotonic signalling via myoendothelial and homocellular smooth muscle gap junctions in a selective fashion. Functional studies have previously shown that heterogeneity in the patterns of endothelial and medial Cx expression present in rabbit ear, middle cerebral and iliac arteries and rat hepatic arteries lead to wide differences in the ability of individual peptides and peptide combinations to inhibit EDHF-type responses (Chaytor et al., 2001; 2003; Berman et al., 2002; Ujiie et al., 2003). The present findings may therefore only be generalized with reference to the patterns of Cx expression that are actually present in specific artery types.
Acknowledgments
ATC was supported by the British Heart Foundation.
Abbreviations
- CPA
cyclopiazonic acid
- Cx
connexin
- DMSO
dimethylsulphoxide
- EDHF
endothelium-derived hyperpolarizing factor
- KCa
Ca2+-activated K+ channels
- SERCA
sarcoplasmic-endoplasmic reticulum Ca2+-ATPase
References
- BÉNY J.L. Information networks in the arterial wall. NIPS. 1999;14:68–73. doi: 10.1152/physiologyonline.1999.14.2.68. [DOI] [PubMed] [Google Scholar]
- BERMAN R.S., MARTIN P.E.M., EVANS W.H., GRIFFITH T.M. Relative contributions of NO and gap junctional communication to endothelium-dependent relaxations of rabbit resistance arteries vary with vessel size. Microvasc. Res. 2002;63:115–128. doi: 10.1006/mvre.2001.2352. [DOI] [PubMed] [Google Scholar]
- BUKAUSKAS F.F., JORDAN K., BUKAUSKIENE A., BENNETT M.V., LAMPE P.D., LAIRD D.W., VERSELIS V.K. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T, EDWARDS D.H., BAKKER L.M., GRIFFITH T.M. Distinct hyperpolarizing and relaxant roles for gap junctions and endothelium-derived H2O2 in NO-independent relaxations of rabbit arteries. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15212–15217. doi: 10.1073/pnas.2435030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J. Physiol. 1997;503:99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J. Physiol. 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., MARTIN P.E., EDWARDS D.H., GRIFFITH T.M. Gap junctional communication underpins EDHF-type relaxations evoked by ACh in the rat hepatic artery. Am. J. Physiol. 2001;280:H2441–H2450. doi: 10.1152/ajpheart.2001.280.6.H2441. [DOI] [PubMed] [Google Scholar]
- CHAYTOR A.T., MARTIN P.E.M., EVANS W.H., RANDALL M.D., GRIFFITH T.M. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J. Physiol. 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE VRIESE A.S., VAN DE VOORDE J., LAMEIRE N.H. Effects of connexin-mimetic peptides on nitric oxide synthase- and cyclooxygenase-independent renal vasodilation. Kidney Int. 2002;61:177–185. doi: 10.1046/j.1523-1755.2002.00122.x. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., THOLLON C., GARDENER M.J., FELETOU M., VILAINE J., VANHOUTTE P.M., WESTON A.H. Role of gap junctions and EETs in endothelium-dependent hyperpolarization of porcine coronary artery. Br. J. Pharmacol. 2000;129:1145–1154. doi: 10.1038/sj.bjp.0703188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFITH T.M. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis. Br. J. Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFITH T.M., CHAYTOR A.T., EDWARDS D.H., DAVERIO F., MCGUIGAN C. Enhanced inhibition of the EDHF phenomenon by a phenyl methoxyalaninyl phosphoramidate derivative of dideoxyadenosine. Br. J. Pharmacol. 2004;142:27–30. doi: 10.1038/sj.bjp.0705782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFITH T.M., CHAYTOR A.T., TAYLOR H.J., GIDDINGS B.D., EDWARDS D.H. cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction via gap junctions. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6392–6397. doi: 10.1073/pnas.092089799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSSON F., MIKKELSEN H.B., ARENSBAK B., THUNEBERG L., NEVE S., JENSEN L.J., HOLSTEIN-RATHLOU N.H. Expression of connexin 37, 40 and 43 in rat mesenteric arterioles and resistance arteries. Histochem. Cell. Biol. 2003;119:139–148. doi: 10.1007/s00418-002-0493-0. [DOI] [PubMed] [Google Scholar]
- HWA J.J., GHIBAUDI L., WILLIAMS P., CHATERJEE M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am. J. Physiol. 1994;266:H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- SANDOW S.L., HILL C.E. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ. Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- SANDOW S.L., TARE M., COLEMAN H.A., HILL C.E., PARKINGTON H.C. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ. Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- SHIMOKAWA H., YASUTAKE H., FUIJI K., OWADA M.K., NAKAIKE R., FUKUMOTO Y., TAKAYANAGI T., NAGAO T., EGASHIRA K., FUJISHIMA M., TAKESHITA A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J. Cardiovasc. Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- TAYLOR H.J., CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Inhibition of the gap junctional component of endothelium-dependent relaxation in rabbit iliac artery by 18α-glycyrrhetinic acid. Br. J. Pharmacol. 1998;125:1–4. doi: 10.1038/sj.bjp.0702078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UJIIE H., CHAYTOR A.T., BAKKER L.M., GRIFFITH T.M. Essential role of gap junctions in NO- and prostanoid-independent relaxations evoked by acetylcholine in rabbit intracerebral arteries. Stroke. 2003;34:544–550. doi: 10.1161/01.str.0000054158.72610.ec. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO Y., FUKUTA H., NAKAHIRA Y., SUZUKI H. Blockade by 18β-glycyrrhetinic acid of intercellular electrical coupling in guinea-pig arterioles. J. Physiol. 1998;511:501–508. doi: 10.1111/j.1469-7793.1998.501bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO Y., KLEMM M.F., EDWARDS F.R., SUZUKI H. Intercellular electrical communication among smooth muscle and endothelial cells in guinea-pig mesenteric arterioles. J. Physiol. 2001;535:181–195. doi: 10.1111/j.1469-7793.2001.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAZAKI J., KITAMURA K. Intercellular electrical coupling in vascular cells present in rat intact cerebral arterioles. J. Vasc. Res. 2003;40:11–27. doi: 10.1159/000068934. [DOI] [PubMed] [Google Scholar]