Abstract
It is increasingly recognized that the compartmental organization of signaling processes has a profound influence on cellular behavior. However, our inability to influence these compartmental events in a spatially restricted and acute manner limits our understanding of causation. To determine whether local compartmental loss of a phosphoinositide disrupts the normal traffic of specific cargoes through endosomes, we developed the use of a regulated dimerization device, here designed to compartmentally modify the phosphoinositide content of Rab5-positive endosomes. This modification is effected through the specific regulated recruitment of the 3-phosphatase myotubularin to endosomal membranes in intact cells. The selective manipulation of endosomal phosphatidylinositols (PIs) demonstrates that it is the phosphatidylinositol 3-phosphate (PtdIns3P) or its metabolite PtdIns(3,5)P2 within this compartment that determines the normal maturation of the endosomal compartment and the flux of receptors through it. On local loss of PtdIns3P/PtdIns(3,5)P2, the endosomal compartment itself fails to continue its normal maturation process, leading to the microtubule-dependent tubularization of the endosomal network. Furthermore, it is shown that endosomal PtdIns3P/PtdIns(3,5)P2 is necessary for transferrin receptor traffic through this compartment while having an effect on EGF receptor (EGFR) entry into and sorting from this endosome compartment. The ability to acutely and selectively influence compartmental behavior as exemplified here for endomsomes clearly illustrates the power of the approach used to dissect the role of localized signals and events.
The endocytic sorting of receptors is intimately linked to their signal output as reflected in the finding that translocations of multiple components of the endocytic machinery are associated with cancers (1). Dissecting the role of these and other signals in specific cellular subcompartments is currently problematic. The use of pharmacological agents or mutant proteins provides tools for investigating the global effect of signals only in subcellular compartments. The former suffer from acting in a compartment-nonspecific manner, the latter from a combination of chronic exposure (i.e., the mutant protein of interest is present for many hours) and compartment delivery (the mutant protein influences the dynamic pathway of its own delivery to the compartment of interest). To circumvent these constraints in defining the localized, endocytic role for the phosphoinositide phosphatidylinositol 3-phosphate (PtdIns3P), we have developed the use of an acutely inducible compartment recruitment device and exemplified this approach to selectively modify PtdIns3P lipids specifically in Rab5-positive endosomes.
Results and Discussion
Regulated Compartmental Recruitment.
The approach to compartmental “interference” developed here exploits an acute recruitment process designed for dimerization studies (2), (3). To exemplify the approach, we have investigated the role of PtdIns3P in the specific endosomal compartment marked by the GTPase Rab5a. A fusion protein comprising Rab5a fused to GFP and the rapalogue-binding domain (2xFKBP) was constructed (GFP-Rab5a-target). This chimera was designed to permit the acute binding of rapalogue and hence the rapalogue-dependent recruitment to this compartment of fusion proteins containing the targeted domain from mTOR (Fig. 1a). To manipulate PtdIns3P levels in this compartment, the inositol lipid phosphatase myotubularin 1 (MTM1) was used. MTM1 was coexpressed in HeLa cells with the GFP-Rab5a-target as a fusion protein with RFP (for facile detection) and the mTOR-binding domain of the rapalogue complex for regulated recruitment (RFP-MTM-recruit). Both WT and catalytically inactive mutants of MTM1 were used (Fig. 1a).
Fig. 1.
In vitro and in vivo validation of the heterodimerization device. (a) Principle of the dimerization system. Based on Ariad's heterodimerization kit, the system exploits the natural rapamycin-induced dimerization of FKBP with a mutated portion of mTOR/FRAP (FRB*). To target the endosomal pool of PtdIns3P, the early endosomal marker hRab5a and the PtdIns3P-specific 3-phosphatase myotubularin (hMTM1wt) have been fused to 2xFKBP and FRB, respectively. The nonimmunosuppressive analogue of rapamycin (rapalogue) was used to induce heterodimerization and therefore recruitment of myotubularin to the Rab5a compartment. (b) Time course of FKBP-FRB complex formation. HeLa cells were transiently cotransfected with EGFP-2xFKBP-hRab5a (GFP-Rab5a-target) and mRFP-Flag-FRB-hMTM1wt (RFP-MTM-recruit) for 24 h, treated with or without 500 nM rapalogue for the indicated time, and lysed with Triton X-100. GFP-Rab5a-target was immunoprecipitated by using anti-GFP antibody. Coimmunoprecipitated RFP-MTM-recruit was detected by anti-Flag antibody. (c and d) The immunoprecipitated FKBP-FRB* complex specifically dephosphorylates PtdIns3P and PtdIns(3,5)P2 in a rapalogue-dependent manner. Cos7 cells transiently coexpressing GFP-Rab5a-target and RFP-MTM-recruit were treated for 20 min with or without rapalogue and lysed with Triton X-100. The FKBP/FRB* complex was immunoprecipitated by using anti-GFP antibody, and its 3-phosphatase activity was assessed by using 100 μM different C16-PI substrates during a 30-min reaction at 37°C. Released free phosphate was detected by using the malachite green assay (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site) (n = 3 experiments). (e) The 3-phosphatase activity of the FKBP/FRB complex depends on the presence and activity hMTM1wt. The 3-phosphatase activity of the WT complex was confirmed by the inhibitory effect of the vanadate–derived 3-phosphatase inhibitor bpV (OHpic) (100 μM). The assay was repeated for the FKBP/FRB complex containing the mRFP-Flag-FRB (RFP-FRB) mutant lacking hMTM1wt and the phosphatase inactive mRFP-Flag-FRB-MTM1C375S (RFP-MTM*-recruit) mutant (n = 3 experiments). Coimmunoprecipitation of all of the FRB-containing proteins was detected by anti-flag antibody. (f) MTM1 gets specifically recruited on the Rab5a-positive compartment in a rapalogue-dependent manner. HeLa cells were transiently transfected with GFP-Rab5a-target (green) and RFP-MTM-recruit (red) for 24 h and treated for 5 min with or without 500 nM rapalogue. The colocalization of Rab5a and MTM1 is shown in yellow. Confocal z-stack projections of representative cells are shown (n = 3 experiments). (Scale bar: 10 μm.)
To test the ability of the fusion proteins to dimerize in a rapalogue-inducible manner, Cos7 and HeLa cells were cotransfected with these two constructs, and coimmunoprecipitation was tested for both candidate fusion proteins. Only after rapalogue treatment were complexes of the two proteins observed (Fig. 6, which is published as supporting information on the PNAS web site). The rapalogue-induced dimerization was rapid, with complex formation being observed within 2 min of rapalogue treatment of cells (Fig. 1b). The maximum extent of complex formation was seen by 30 min. To ensure that the MTM1 fusion protein was active in the recovered complex, we immunoprecipitated the complexed MTM1 via GFP-Rab5a-target using anti-GFP. PtdIns3P phosphatase activity was associated with the immunoprecipitated GFP-Rab5a-target only when recovered from cells treated with rapalogue (Fig. 1c). The specificity of the complexed phosphatase toward phosphoinositides was investigated, and the observed selectivity for PtdIns3P and PtdIns(3,5)P2 was as described previously for MTM1 (Fig. 1d) (4–7). Furthermore, the phosphoinositide phosphatase activity was entirely due to the active MTM1, being inhibited by the vanadate-derived 3-phosphatase inhibitor bpV (OHpic) (8) and absent from coimmunoprecipitations of RFP-FRB (protein containing the mTOR-binding domain) or a catalytic site point mutant of MTM1 (RFP-MTM*-recruit) (Fig. 1e). To establish the appropriate compartmental behavior of these fusion proteins in cells, their localization was monitored before and after rapalogue treatment (Fig. 1f). The GFP-Rab5a-target fusion protein displayed a typical Rab5a distribution, with small punctate structures widely distributed in the cytoplasm and in a perinuclear compartment (9). By contrast, RFP-MTM-recruit was diffusely distributed in the basal state (10). However, on acute rapalogue treatment, RFP-MTM-recruit was recruited to the GFP-Rab5a-target compartment, yielding an almost complete overlap of the GFP-Rab5a-target and the RFP-MTM-recruit.
Recruitment of MTM1 Leads to Loss of PtdIns3P.
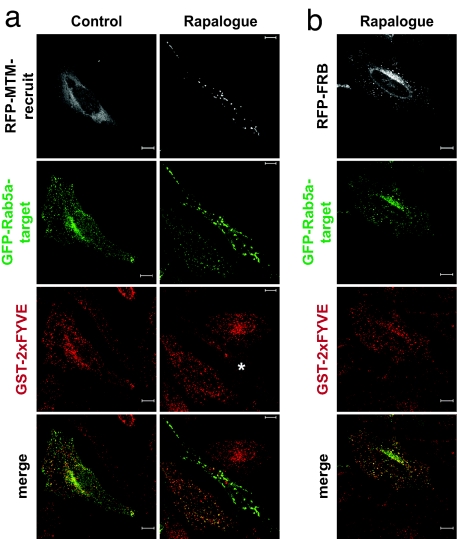
To investigate the expected loss of PtdIns3P within the Rab5-positive compartment, the local presence of PtdIns3P was assessed by employing a GST-2xFYVE domain construct that binds PtdIns3P and has been shown to report on PtdIns3P in cells (11, 12). Monitoring the localization of the FYVE domain fusion protein showed that, under normal growth conditions, it localized to the GFP-Rab5a-target compartment, indicative of the expected PtdIns3P content (Fig. 2a). In response to rapalogue, the GST-2xFYVE protein was displaced from the Rab5a-positive structures only in those cells coexpressing GFP-Rab5a-target and RFP-MTM-recruit; surrounding GFP-positive cells not expressing RFP-MTM-recruit displayed an unaltered colocalization between GFP-Rab5a-target and GST-2xFYVE. Of note, the displacement of the FYVE domain from the Rab5a-positive compartment revealed a less abundant, Rab5a negative, GST-2xFYVE-positive compartment suggestive of a minor PtdIns3P-rich compartment quite distinct from the Rab5a-positive endosomal one. The fusion construct without MTM1 (RFP-FRB) had no effect on GST-2xFYVE colocalization with GFP-Rab5a-target (Fig. 2b). The targeting construct is thus appropriately localized. It recruits RFP-MTM-recruit in response to rapalogue, and the ensuing complex retains normal MTM1 phosphatase activity, causing the localized loss of PtdIns3P.
Fig. 2.
Tubularization is due to the localized decrease of the endosomal PtdIns3P. A recombinant GST-2xFYVEHrs PtdIns3P-specific probe was used to detect the status of endogenous PtdIns3P. HeLa cells expressing GFP-Rab5a-target (green) and RFP-MTM-recruit (white) (a) or RFP-FRB (b) were treated for 90 min with or without the rapalogue, fixed with 4% paraformaldehyde, and permeabilized with 50 μg/ml digitonin. Localization of the GST-2xFYVEHrs probe was assessed by indirect immunofluorescence confocal microscopy by using mouse anti-GST antibody combined with Cy5-conjugated (red) anti-mouse antibody. The asterisk indicates where the GST-2xFYVEHrs staining is significantly decreased as a result of the rapalogue-induced tubularization of the Rab5a-positive compartment. Confocal z-stack projections of representative cells are shown. (Scale bar: 10 μm.)
Loss of Local PtdIns3P Leads to Microtubule-Dependent Tubularization of the Rab5a Compartment.
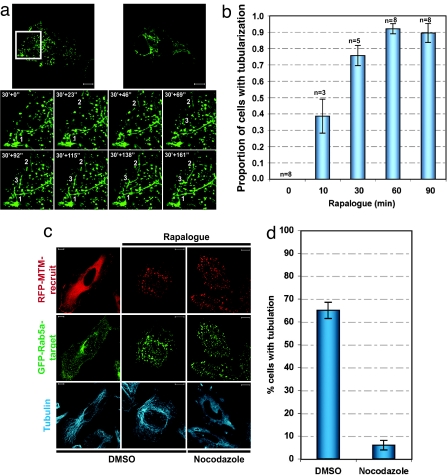
To determine the influence of the local PtdIns3P on this Rab5a-positive endosomal compartment, the effect of rapalogue on the behavior of the compartment was monitored. Rapalogue treatment led to the rapid tubularization of the Rab5a-positive compartment (Fig. 3a and Movie 1, which is published as supporting information on the PNAS web site). The formation of these tubules was very dynamic extended over long distances and did not display any polarity. Initiated mainly at the cell periphery, tubularization was observed to expand progressively in the perinuclear region. Over time, endosome tubularization resulted in the formation of an extensive, less dynamic, perinuclear tubular network in which interconnected endosomes seem to be trapped. Quantitation of the extent of tubularization (Fig. 3b) showed a time course of events that closely followed the time course of MTM1 recruitment (Fig. 1b), being near maximal at 30 min. To confirm that this effect was due to PtdIns3P loss, we tested the vector without MTM1 and the MTM1 mutant; no such response was observed with either the vector void of MTM1 itself or with the inactive RFP-MTM-recruit mutant (Fig. 7 a and b, which is published as supporting information on the PNAS web site). The directed recruitment approach clearly demonstrates that this is a local PtdIns3P depletion response. Previously (as shown in Fig. 8, which is published as supporting information on the PNAS web site), the tubularization of endosomal compartments in response to pan-phosphatidylinositol 3-kinase (pan-PI3-kinase) inhibitors could not distinguish local and distal requirements for any of the 3-phosphoinostides in this process (all are influenced by currently available inhibitors). The localized depletion effected here clearly shows that the lack of endosomal PtdIns3P is itself all that is necessary to elicit this morphological change.
Fig. 3.
hMTM1wt recruitment induces tubularization of the Rab5a compartment in a microtubule-dependent manner. (a) HeLa cells expressing GFP-Rab5a-target (green) and RFP-MTM-recruit were stimulated with rapalogue and imaged live. Frames were captured every 23 s for 1 h 23 min. Frames from the Inset correspond to 30 min after rapalogue stimulation and depict the formation of the tubularized Rab5a-positive endosomes. The numbers indicate regions where tubularization events occur. (b) HeLa cells expressing GFP-Rab5a-target and RFP-MTM-recruit were treated with rapalogue as indicated. To assess the occurrence of tubularization over time, the percentage of cells (out of 50 cells per condition) was calculated and normalized to the maximum percent tubularization. The average value from the indicated number of experiments is represented. (c and d) HeLa cells expressing GFP-Rab5a-target (green) and RFP-MTM-recruit (red) were pretreated for 30 min with the microtubule-depolymerizing drug nocodazole (20 μM) and incubated for 90 min with or without rapalogue. (c) The microtubule network (blue) was detected by indirect immunofluorescence by using Cy5-conjugated anti-mouse antibody combined with anti-tubulin antibody. Confocal z-stack projections of representative cells are presented. (Scale bar: 10 μm.) (d) The inhibitory effect of nocodazole on tubularization was quantified and represented as the percentage of cells containing tubularized structures (n = 3, 150 cells per experiment). The endosomal compartment was considered to be tubularized when there was a minimum of two tubules of ≈50 μm.
Previous studies have established a role for cytoskeletal networks in the spatial distribution, motility and morphology of endocytic compartments. The actin cytoskeleton has been reported to mediate early endocytic events (13) and to interfere with endosome dynamics and distribution (9, 14), whereas the microtubule network has been shown to be required for both plus- and minus-end motility of early endosomes (9), (15). Actin disruption was found to have no effect on the rapalogue-induced tubularization (Fig. 9, which is published as supporting information on the PNAS web site). However, disruption of the microtubule network with nocodazole blocked rapalogue-induced tubularization of the Rab5a-positive compartment (Fig. 3 c and d). Furthermore, counterstaining for tubulin revealed that the pattern of tubularization was consistent with association between this extended endosomal network and the microtubule network. This behavior indicates that normal budding, but not movement along the microtubule network, is defective in the local absence of this lipid, leading to the accumulation of an extended compartment in association with microtubules.
Endosomal Tubularization Displaces Sorting Nexin 1 (SNX1) and Prevents Transferrin Receptor (TfnR) Recycling, but Only Slows EGF Receptor (EGFR) Traffic.
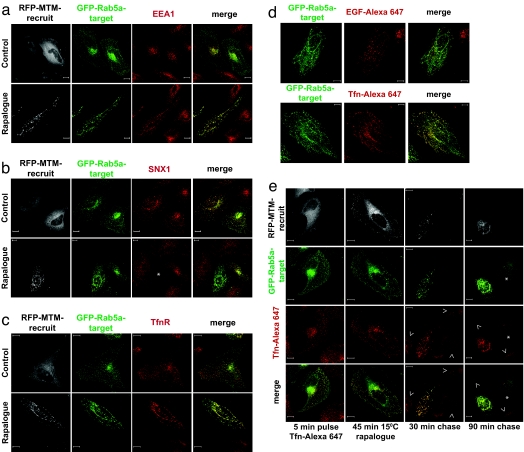
The Rab5a endosomal compartment typically retains the early endosomal antigen 1 protein (EEA1) (16–18), SNX1 (19, 20), and endocytosed receptors en route to recycling and/or degradative compartments (21). To assess how the localized loss of endosomal PtdIns3P influences these markers and receptor traffic, we monitored their behavior in response to rapalogue treatment. EEA1 colocalized with the perinuclear Rab5a-positive compartment in the absence of rapalogue (Fig. 4a). On treatment, EEA1 remained associated with the tubularized Rab5-positive compartment, and this colocalization became virtually complete. This colocalization is indicative of the coalescence of the heterogeneous Rab5a endosomes (i.e., both EEA1-positive and -negative) in the interconnected tubularized structures. The endosomal retention of EEA1, postdestruction of PtdIns3P, was unexpected in view of its reported recruitment via its PtdIns3P-binding FYVE domain (22). This retention indicates that, at least post-recruitment of EEA1, continued generation of PtdIns3P is not required for its maintenance within these endosomes. Of note, EEA1 was retained on tubularized endosomes, both in Rab5a-transfected and nontransfected cells (Fig. 10, which is published as supporting information on the PNAS web site). This pattern of retention for EEA1 was in stark contrast to another PtdIns3P-binding protein, SNX1 (23). On rapalogue treatment of cells expressing RFP-MTM-recruit, the endosomal localization of SNX1 was abolished (Fig. 4b). SNX1 clearly behaves in a more dynamic fashion than EEA1, requiring maintenance of local PtdIns3P for retention.
Fig. 4.
Tubularized endosomes displace SNX1 and prevent recycling of transferrin. (a–c) HeLa cells expressing GFP-Rab5a-target (green) and RFP-MTM-recruit (white) were treated for 90 min with or without rapalogue, fixed, and stained with antibodies against EEA1 (a), SNX1 (b), or TfnR (c) combined with the Cy5-conjugated (red) secondary antibody. The asterisk in the SNX1 panel indicates a cell containing the rapalogue-induced tubularized endosomes. EEA1 and TfnR strongly stain the structures; SNX1 is completely displaced. Confocal z-stack projections of representative cells are shown. (Scale bar: 10 μm.) (d and e) Early endosomes in transfected HeLa cells were loaded with Tfn or EGF by incubating for 5 min with 30 μg/ml Tfn-Alexa Fluor 647 (red) or 10 min with 1 μg/ml EGF-Alexa Fluor 647 (red) at 37°C, respectively. Excess ligand was washed off, and dimerization was induced at 15°C as described in Materials and Methods. Tfn or EGF were chased at 37°C for different times. (d) After a 30-min chase, we compared the localization of endocytosed Tfn- and EGF-Alexa Fluor 647. Although EGF was retained on the main body of tubularized endosomes, Tfn was found on both the tubular and vesicular regions of the network. (e) To assess the effect of tubularization of Tfn traffic, Tfn-Alexa Fluor 647 was chased for longer time periods. The intensity of the Tfn signal was assessed by using the same parameters for image acquisition. Arrowheads indicate nontransfected cells, and the asterisk identifies a cell expressing only GFP-Rab5a-target. (Scale bar: 10 μm.)
The movement of receptors into/through these endosomal compartments displayed distinctive behaviors after local PtdIns3P loss and tubularization. The TfnR, which transits through endosomes and is recycled back to the plasma membrane, displays a broad colocalization with Rab5a both before and after rapalogue treatment (Fig. 4c). This colocaliztion demonstrates that the access of TfnR to the Rab5a-positive network is not impaired by PtdIns3P depletion within it. However, the receptor-positive, Rab5a-negative compartment is lost on rapalogue treatment, indicative of the trapping of the transferrin receptor within the tubularized endosomal network (Fig. 4c), i.e., it does not escape the Rab5a-positive compartment. To formally establish this inhibited traffic of the TfnR (24), cells were pulsed with Tfn-Alexa Fluor 647 and subsequently treated with rapalogue before a chase (Fig. 4 d and e). For GFP-Rab5a-target-positive cells, after 30 min and most strikingly 90 min of chase, only cells with a tubularized endosomal network retained Tfn-Alexa Fluor 647, with nontubularized cells (RFP-MTM-recruit low/negative cells) having released their internalized Tfn-Alexa Fluor 647 (Fig. 4e). Thus, the entry of the TfnR into the PtdIns3P-depleted tubular network leads to its being trapped there. It is evident that the recycling of the TfnR from the endosomal compartment requires the presence of PtdIns3P within the compartment itself, to effect sorting and/or budding of the sorted receptor.
Although it has been shown that PtdIns3P can play a role in receptor down-regulation, its precise regulatory function remains controversial (25–27). Monitoring EGFR traffic with EGF-Alexa Fluor 647 shows that this receptor marker can enter and exit the tubularized network (Fig. 5). Tubularization does not prevent entry of EGF-Alexa Fluor 647 into the tubularized endosomal compartment (Fig. 5a). Similarly, tubularization does not prevent exit of the EGFR ligand from tubularized endosomes preloaded with EGF-Alexa Fluor 647 (Fig. 5b). After a 30-min chase, EGF-Alexa Fluor 647-positive vesicular structures were both associated with the Rab5a-positive tubular network and distinct from it (Fig. 5a, panel 3). However, by 90 min of chase, much of the EGF-Alexa Fluor 647 was degraded, as evidenced by the loss of fluorescence (Fig. 5b). Of note, in cells with a tubularized network, the loss of EGF-Alexa Fluor 647 was delayed (Fig. 5b, 90 min chase). In the same manner, biochemical analysis of EGFR degradation shows that rapalogue-induced dimerization does not prevent receptor degradation (Fig. 11, which is published as supporting information on the PNAS web site). In rapalogue-treated cells expressing the active phosphatase, EGFR is eventually degraded, although with a minor delay (Fig. 11 Middle). Thus, the traffic of EGF is not blocked by local PtdIns3P depletion, but it is slightly less efficient.
Fig. 5.
Endosome tubularization delays but does not block EGF traffic. (a) HeLa cells coexpressing GFP-Rab5a-target (green) and RFP-MTM-recruit were treated as above with the rapalogue and incubated for 2.5 min with 1 μg/ml EGF-Alexa Fluor 647 (red). Unbound and surface bound ligand was washed off with ice-cold low-pH buffer (see Materials and Methods). The labeled EGF was chased at 37°C as indicated. Localization of the ligand was assessed by triple channel confocal fluorescence microscopy. (b) To label early endosomes with EGF, HeLa cells coexpressing GFP-Rab5a-target (green) and RFP-MTM-recruit (white) were incubated for 10 min at 37°C with 1 μg/ml EGF-Alexa Fluor 647 (red). Tubularization was induced as in Fig. 4e. EGF-Alexa Fluor 647 was then chased at 37°C for the indicated periods of time in the continuous presence of the rapalogue. Cells were analyzed as described for b. All images were captured by using the same imaging parameters. The asterisks indicate nontubularized cells expressing only GFP-Rab5a-target, where the EGF signal is significantly lower than in the adjacent tubularized cell. (Scale bar: 10 μm.)
Conclusion.
We have illustrated how the local, as opposed to the global, depletion of endosomal-PtdIns3P affects receptor trafficking through a specific subcellular compartment. To achieve this compartmental modulation, we have developed the use of an adaptable, modular rapalogue-regulated dimerization system, here designed for the purpose of the acute local degradation of PtdIns3P. Through this approach, we have shown that local PtdIns3P depletion alters the morphology of endosomes, the elaborated tubular network requiring an intact microtubule network and seeming to associate with it. We exploit this system to demonstrate that endosomal PtdIns3P is itself a key regulator of endosomal morphology and plays a selective role in determining the flux of receptors from the early endosomes toward the degradation and recycling pathways. Traffic of the TfnR through this network is severely impaired, with little or no exit of TfnR from the tubularized compartment. This evidence thus shows that the resident PtdIns3P or its metabolite PtdIns(3,5)P2 is required for normal Tfn exit from the Rab5a-positive endosomal compartment. It is suggested that this defective exit is reflected in the microtubule-associated, tubularized endosomal network, which accumulates. The localized depletion of PtdIns3P displays specificity because it does not lead to a complete arrest of all Rab5a-associated receptor traffic; EGF/EGFR is at most delayed in its exit and degradation. This delay is contrary to the effects of global inhibition of PI3-kinases on EGFR traffic and degradation where the latter has been reported to be promoted (27). This different response demonstrates the power of being able to manipulate these signals in a compartmentally restricted manner, providing in this instance a clearer view of the requirements for PtdIns3P in EGFR traffic and degradation. Finally, it should be noted that the localized manipulation of signals through this approach of regulated recruitment is amenable to the recruitment of essentially any signal-generating or -destroying system and therefore adds substantially to the armory available to tackle spatially restricted events in vivo.
Materials and Methods
The plasmid containing the human MTM1 (hMTM1) was a gift from J. Dixon (Department of Biological Chemistry, University of Michigan Medical School, Ann Arbor); the human Rab5a (hRab5a) was a gift from M. Seabra (Biochemical Sciences, Imperial College London); and the 2xFYVEHrs motif was a gift from H. Stenmark (Department of Biochemistry, Institute for Cancer Research, Norwegian Radium Hospital, Oslo, Norway). The plasmids containing the tandem FK506-binding protein (FKBP) domain (2xFKBP) and the mutant FRB domain (FRB*), as well as the AP21967 (rapalogue), were components of the ARGENT Regulated Heterodimerization Kit provided by Ariad (www.ariad.com/regulationkits). The pcDNA3.1(+)-hyg and pIRESpuro2 plasmids were obtained from Invitrogen (Paisley, U.K.) and Clontech, (Palo Alto, CA), respectively. The anti-GFP and anti-EGFR monoclonal antibodies were provided by Cancer Research U.K., Research Services. Other antibodies and reagents were obtained from the following sources: rabbit anti-GFP antibody (BD Biosciences, Palo Alto, CA); anti-Flag M2 monoclonal antibody (Stratagene, Amsterdam, Netherlands); anti-tubulin monoclonal antibody (Sigma, Poole, Dorset U.K.); goat anti-EEA1 and anti-EGFR antibodies (Santa Cruz Biotechnology, Santa Cruz, CA); GST-tag monoclonal antibody (Novagen, Madison, WI); anti-human transferrin receptor monoclonal antibody (Molecular Probes, Leiden, The Netherlands); anti-SNX1 (BD Transduction Laboratories, Palo Alto, CA); Alexa Fluor 647-conjugated EGF, transferrin, and phalloidin (Molecular Probes); Cy3- and Cy5-conjugated secondary antibodies Jackson ImmunoResearch, West Grove, PA; and nocodazole, cytochalasin D, wortmannin, and LY294002 (Calbiochem, San Diego, CA).
Construction of the Dimerization Device.
To construct EGFP-2xFKBP-hRab5a (GFP-Rab5a-target), EGFP-hRab5a from pEGFP-C3-hRab5a plasmid was subcloned into AgeI/BamHI sites of pIRESpuro2 vector. The 2xFKBP fragment from pC4M–F2E plasmid (ARIAD) was isolated by standard PCR, by using primers that correct for N- and C-terminal frame shifts. The fragment was then subcloned between EGFP and hRab5a, into the unique BrsGI site of the previously generated pIRESpuro2-EGFP-hRab5a plasmid.
For the FRB-containing chimera, the following cloning strategy allowed the generation of fusion proteins where the Flag, FRB, hMTM1 fragments were combined in different orders. hMTM1 was isolated from pcDNA3.1(+)-NF-hMTM1 plasmid by PCR. The PCR product was a Kozak-ATG-AgeI-hMTM1-XmaI-STOP cassette, in which the compatible restriction sites AgeI and XmaI were present within the coding sequence of hMTM1. Such a design allowed the subcloning of Flag/FRB fragments at the N- or C-terminal of hMTM1 using the same cassette. The cassette was then subcloned into the BamHI site of pcDNA3.1(+)-hyg vector (Invitrogen). In the case of mRFP-Flag-FRB-hMTM1wt fusion (RFP-MTM-recruit), Flag-tagged FRB fragment was isolated by PCR from the pC4-R4E plasmid (ARIAD) and inserted into the AgeI site of the cassette. Finally, the PCR-amplified mRFP fragment was subcloned into the AgeI site of the pcDNA3.1(+)-Flag-FRB-hMTM1-generated plasmid. Similarly, the mRFP-Flag-FRB-negative control construct (RFP-FRB) was generated by sequential subcloning of Flag-FRB into the KpnI site of pcDNA3.1(+)-hyg vector and then of mRFP into the AgeI site of pcDNA3.1(+)-Flag-FRB plasmid. The mRFP-Flag-FRB-MTM1C375S (RFP-MTM*-recruit) construct was generated by site-directed mutagenesis by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene).
The GST-2xFYVEHrs was constructed by subcloning the 2xFYVEHrs from pGEM1-EGFP-2xFYVEHrs into the EcoRI-SalI sites of pGEX-4T1 bacterial expression vector (Amersham Pharmacia).
Immunofluorescence Microscopy.
HeLa cells grown on glass coverslips were transfected as described. After treatment at 37°C, cells were fixed in 4% (wt/vol) paraformaldehyde (PFA) for 15 min at room temperature. All subsequent steps were performed at room temperature. Residual PFA was quenched for 15 min with 50 mM ammonium chloride. Cells were permeabilized and simultaneously blocked with 0.1% (vol/vol) Triton X-100 and 2% (wt/vol) BSA in PBS for 15 min. All antibody dilutions were prepared in 2% (wt/vol) BSA, PBS and incubation times were 60 min. When staining with recombinant GST-2xFYVEHrs domain, cells were permeabilized for 30 min with 40 μg/ml digitonin in 3% (wt/vol) fatty acid free BSA, PBS. To avoid aggregate formation, the GST-2xFYVEHrs was used at 0.5 μg/ml in 3% fatty acid free BSA, PBS. Coverslips were mounted on microscope slides with mowiol (10% (vol/vol), 25% glycerol, 100 mM Tris·HCl, pH 8.5) and examined on a confocal laser scanning microscope (LSM 510; Carl Zeis Inc) with a Plan-Apochromat 63x/1.4 NA oil immersion phase objective. The z-sections acquired had a depth of 400 nm. Images were analyzed by using the Zeis LSM software package.
Live Cell Imaging.
Transfected HeLa cells were grown on MatTeK dishes. After treatment, cells were analyzed live at 37°C on the same Zeis confocal microscope by using with a Plan-Apochromat 63x/1.4 NA oil immersion DIC III lens. Frames were captured as indicated in the figure legends.
EGFR Degradation Assay.
HeLa nontransfected or transiently cotransfected with GFP-Rab5a-target and RFP-MTM-recruit or mutants as described. Cells were preincubated with or without 500 nM rapalogue for 1 h at 37°C in a serum free medium and then incubated with 1 μg/ml EGF for different times. Cells were washed with ice cold PBS and lysed in sample buffer (125 mM Tris·HCl pH 6.8, 6% (wt/vol) SDS, 20% (vol/vol) glycerol, 0.02% bromophenol blue, 10% β-mercaptoethanol). Lysates were heated for 10 min at 95°C and analyzed by SDS/PAGE and immunoblotting as described previously by using a goat anti-EGFR antibody.
EGF and Transferrin Trafficking Assays.
HeLa cells transiently coexpressing GFP-Rab5a-target and RFP-MTM-recruit were grown on glass coverslips. To assess the EGF entry in early endosomes, cells were preincubated with or without 500 nM rapalogue for 1h at 37°C in a serum free medium and stimulated for 2.5 min with 1 μg/ml EGF-Alexa Fluor 647 (pulse). Unbound and surface bound EGF was removed by washing sequentially with ice-cold PBS, low pH buffer (2.5 mM KCl, 135 mM NaCl and 50 mM CH3COOH, pH 4.5) and again PBS. Cells were further incubated at 37°C in the absence of ligand (chase) for various periods of time. To label early endosomes with fluorescently labeled ligands, cells were incubated for 10 min with 1 μg/ml EGF-Alexa Fluor 647 or 5 min with 30 μg/ml Tfn-Alexa Fluor 647 at 37°C. Unbound and surface bound ligands were washed off as above. To induce dimerization of the fusion proteins without endosome tubularization and further receptor trafficking, cells were incubated for 45 min with 500 nM rapalogue at 15°C. The ligands were then chased at 37°C for various periods of time in the continuous presence of the rapalogue. After the pulse–chase, cells were fixed in 4% (wt/vol) PFA and analyzed by fluorescence microscopy as mentioned previously.
Supplementary Material
Acknowledgments
We thank Harold Stenmark (Department of Biochemistry, Institute for Cancer Research, Norwegian Radium Hospital, Norway) for providing pGEM1-EGFP-2xFYVEHrs plasmid, Migual Seabra (Biochemical Sciences, Imperial College London) for providing pEGFP-C3-hRab5a plasmids, J. E. Dixon (Department of Biological Chemistry, University of Michigan Medical School, Ann Arbor, MI) for providing pCDNA3.1(+)-NF-hMTM1 plasmid, and Ariad (www.ariad.com/regulationkits) for providing the ARGENT Regulated Heterodimerization Kit. We thank all members of the Light Microscopy Laboratory, Research Services and Equipment Park (Cancer Research U.K.) for help and Sharon Tooze for critically reading the paper. This work has been supported by the Hellenic Republic State Scholarship's Foundation and Cancer Research U.K.
Abbreviations
- EEA1
early endosome autoantigen 1
- FKBP
FK506-binding protein
- GFP-Rab5a-target, EGFP-2xFKBP-hRab5a
- FRB, mTOR-binding domain for the rapalogue
- RFP-MTM-recruit, mRFP-Flag-FRB-hMTM1wt
- MTM1
myotubular myopathy protein 1
- PtdIns3P
phosphatidylinositol 3-phosphate
- SNX1
sorting nexin-1
- EGFR
EGF receptor
- Tfn
transferrin
- TfnR
Tfn receptor.
Footnotes
The authors declare no conflict of interest.
References
- 1.Bache KG, Slagsvold T, Stenmark H. EMBO J. 2004;23:2707–2712. doi: 10.1038/sj.emboj.7600292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera VM, Clackson T, Natesan S, Pollock R, Amara JF, Keenan T, Magari SR, Phillips T, Courage NL, Cerasoli F, Jr, et al. Nat Med. 1996;2:1028–1032. doi: 10.1038/nm0996-1028. [DOI] [PubMed] [Google Scholar]
- 3.Scheid MP, Marignani PA, Woodgett JR. Mol Cell Biol. 2002;22:6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor GS, Maehama T, Dixon JE. Proc Natl Acad Sci USA. 2000;97:8910–8915. doi: 10.1073/pnas.160255697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blondeau F, Laporte J, Bodin S, Superti-Furga G, Payrastre B, Mandel JL. Hum Mol Genet. 2000;9:2223–2229. doi: 10.1093/oxfordjournals.hmg.a018913. [DOI] [PubMed] [Google Scholar]
- 6.Schaletzky J, Dove SK, Short B, Lorenzo O, Clague MJ, Barr FA. Curr Biol. 2003;13:504–509. doi: 10.1016/s0960-9822(03)00132-5. [DOI] [PubMed] [Google Scholar]
- 7.Tronchere H, Laporte J, Pendaries C, Chaussade C, Liaubet L, Pirola L, Mandel JL, Payrastre B. J Biol Chem. 2004;279:7304–7312. doi: 10.1074/jbc.M311071200. [DOI] [PubMed] [Google Scholar]
- 8.Schmid AC, Byrne RD, Vilar R, Woscholski R. FEBS Lett. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M. Nat Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- 10.Laporte J, Liaubet L, Blondeau F, Tronchere H, Mandel JL, Payrastre B. Biochem Biophys Res Commun. 2002;291:305–312. doi: 10.1006/bbrc.2002.6445. [DOI] [PubMed] [Google Scholar]
- 11.Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, Parton RG, Stenmark H. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SA, Taylor GS, Torgersen KM, Dixon JE. J Biol Chem. 2002;277:4526–4531. doi: 10.1074/jbc.M111087200. [DOI] [PubMed] [Google Scholar]
- 13.Lamaze C, Fujimoto LM, Yin HL, Schmid SL. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- 14.Murphy C, Saffrich R, Grummt M, Gournier H, Rybin V, Rubino M, Auvinen P, Lutcke A, Parton RG, Zerial M. Nature. 1996;384:427–432. doi: 10.1038/384427a0. [DOI] [PubMed] [Google Scholar]
- 15.Hoepfner S, Severin F, Cabezas A, Habermann B, Runge A, Gillooly D, Stenmark H, Zerial M. Cell. 2005;121:437–450. doi: 10.1016/j.cell.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Christoforidis S, McBride HM, Burgoyne RD, Zerial M. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- 17.Rubino M, Miaczynska M, Lippe R, Zerial M. J Biol Chem. 2000;275:3745–3748. doi: 10.1074/jbc.275.6.3745. [DOI] [PubMed] [Google Scholar]
- 18.McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- 19.Zhong Q, Lazar CS, Tronchere H, Sato T, Meerloo T, Yeo M, Songyang Z, Emr SD, Gill GN. Proc Natl Acad Sci USA. 2002;99:6767–6772. doi: 10.1073/pnas.092142699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ. Curr Biol. 2004;14:1791–1800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 21.Gruenberg J. Nat Rev Mol Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- 22.Lawe DC, Chawla A, Merithew E, Dumas J, Carrington W, Fogarty K, Lifshitz L, Tuft R, Lambright D, Corvera S. J Biol Chem. 2002;277:8611–8617. doi: 10.1074/jbc.M109239200. [DOI] [PubMed] [Google Scholar]
- 23.Cozier GE, Carlton J, McGregor AH, Gleeson PA, Teasdale RD, Mellor H, Cullen PJ. J Biol Chem. 2002;277:48730–48736. doi: 10.1074/jbc.M206986200. [DOI] [PubMed] [Google Scholar]
- 24.Maxfield FR, McGraw TE. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 25.Petiot A, Faure J, Stenmark H, Gruenberg J. J Cell Biol. 2003;162:971–979. doi: 10.1083/jcb.200303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Futter CE, Collinson LM, Backer JM, Hopkins CR. J Cell Biol. 2001;155:1251–1264. doi: 10.1083/jcb.200108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Wang Z. EMBO Rep. 2001;2:842–849. doi: 10.1093/embo-reports/kve179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.