Abstract
Within the vascular system, the mucin-type transmembrane glycoprotein T1α/podoplanin is predominantly expressed by lymphatic endothelium, and recent studies have shown that it is regulated by the lymphatic-specific homeobox gene Prox1. In this study, we examined the role of T1α/podoplanin in vascular development and the effects of gene disruption in mice. T1α/podoplanin is first expressed at around E11.0 in Prox1-positive lymphatic progenitor cells, with predominant localization in the luminal plasma membrane of lymphatic endothelial cells during later development. T1α/podoplanin–/– mice die at birth due to respiratory failure and have defects in lymphatic, but not blood vessel pattern formation. These defects are associated with diminished lymphatic transport, congenital lymphedema and dilation of lymphatic vessels. T1α/podoplanin is also expressed in the basal epidermis of newborn wild-type mice, but gene disruption did not alter epidermal differentiation. Studies in cultured endothelial cells indicate that T1α/podoplanin promotes cell adhesion, migration and tube formation, whereas small interfering RNA-mediated inhibition of T1α/podoplanin expression decreased lymphatic endothelial cell adhesion. These data identify T1α/podoplanin as a novel critical player that regulates different key aspects of lymphatic vasculature formation.
Keywords: angiogenesis/lymphangiogenesis/podoplanin/Prox1/T1α
Introduction
The lymphatic vascular system maintains tissue fluid homeostasis and mediates the afferent immune response, but can also aid in the metastatic spread of malignant tumors (Detmar and Hirakawa, 2002). Dysfunction or abnormal development of cutaneous lymphatic vessels results in lymphedema, which is associated with defects in tissue repair and the immune response (Mallon and Ryan, 1994). Although the mechanisms that control the development of the blood vascular system have been well studied (Carmeliet, 2000), those of the lymphatic vessels are poorly understood.
Recent analyses of Prox1-deficient mice have shown that the lymphatic vascular system, as predicted by Sabin (1902), originates from the embryonic veins (Wigle and Oliver, 1999; Oliver and Detmar, 2002). Beginning at embryonic day (E) 9.5 of mouse development, the homeobox gene Prox1 is specifically expressed by a subpopulation of endothelial cells that are located on one side of the anterior cardinal vein. This is followed by polarized budding and migration of these Prox1-positive lymphatic progenitor cells, which eventually form lymphatic sacs and then the entire lymphatic vasculature. In Prox1-null mice, the budding and sprouting of lymphatic endothelial cells from the veins is arrested at ∼E11.5–E12.0, and these mice completely lack a lymphatic vascular system (Wigle and Oliver, 1999). We and others have shown recently that ectopic expression of Prox1 in primary human blood vessel endothelial cells represses the expression of several genes that are associated with the blood vascular phenotype (Hong et al., 2002; Petrova et al., 2002). Prox1 expression was also found to upregulate the expression of lymphatic-specific genes (Hong et al., 2002; Petrova et al., 2002), indicating its function as a master control gene that determines lymphatic endothelial cell fate (Oliver and Detmar, 2002). These studies identified the mucin-type transmembrane glycoprotein T1α/podoplanin as one of the primary Prox1-induced genes (Hong et al., 2002).
T1α/podoplanin is expressed by cultured human lymphatic endothelial cells, and is one of the most highly expressed lymphatic-specific genes (Petrova et al., 2002; Hirakawa et al., 2003). In vivo expression of T1α/podoplanin in lymphatic endothelium was first reported by Wetterwald et al. (1996), who named it ‘E11 antigen’. It was characterized further under the name ‘podoplanin’, because of its low level expression in kidney podocytes (Breiteneder-Geleff et al., 1997). Podoplanin is homologous to T1α, which was originally found to encode an antigen that is selectively expressed at the apical surface of alveolar type I cells in rat lung (Dobbs et al., 1988; Rishi et al., 1995). Expression of T1α has also been detected in the choroid plexus, ciliary epithelium of the eye, intestine, kidney, thyroid and esophagus of the fetal rat (Williams et al., 1996), and it has been shown to be homologous to the OTS-8 gene, a phorbol ester-induced gene in MC3T3-E1 mouse osteoblast cells (Nose et al., 1990). Other homologs include RTI40 (Gonzalez and Dobbs, 1998), murine gp38 (Farr et al., 1992), canine gp40 (Zimmer et al., 1997), human gp36 (Zimmer et al., 1999) and murine PA2.26 (Gandarillas et al., 1997).
There have been many studies of T1α/podoplanin expression in the lymphatic vascular system (Kriehuber et al., 2001; Maekinen et al., 2001; Hong et al., 2002; Petrova et al., 2002; Hirakawa et al., 2003). In spite of the large number of descriptive studies, little is understood about T1α/podoplanin’s biological function. We examined its role in lymphatic and blood vessel development by examining mice that have targeted deletions in the T1α/podoplanin gene (Ramirez et al., 2003).
Here, we show that within the vascular system, T1α/podoplanin is first expressed between E10.5 and E11.5 in endothelial cells of the cardinal vein and in budding, Prox1-positive lymphatic progenitor cells. T1α/podoplanin expression becomes specifically restricted to lymphatic endothelium during later development. Ultrastructural analysis revealed its predominant localization to the luminal plasma membrane of lymphatic vessels. We found that T1α/podoplanin–/– mice have defects in lymphatic vessel, but not blood vessel, pattern formation. These defects lead to diminished lymphatic transport, congenital lymphedema and dilation of cutaneous and intestinal lymphatic vessels. Overexpression of T1α/podoplanin in cultured vascular endothelial cells promoted the formation of elongated cell extensions and significantly increased endothelial cell adhesion, migration and tube formation. Together, these findings suggest that the transmembrane glycoprotein T1α/podoplanin is required to regulate key aspects of lymphatic vascular formation.
Results
T1α/podoplanin is expressed by budding Prox1-positive lymphatic progenitor cells
In agreement with previous observations showing T1α/podoplanin expression in the central nervous system and the foregut at around E9 (Rishi et al., 1995; Williams, 2003), we detected expression of T1α/podoplanin in the neural tube of wild-type mice at E10.5 (Figure 1A). However, no vascular expression of T1α/podoplanin was detected yet at this time point, whereas the homeobox gene Prox1 was already expressed by a subset of endothelial cells of the anterior cardinal vein (Figure 1A and B). By E11.5 (data not shown) and E12.5, T1α/podoplanin was expressed by all endothelial cells of the cardinal vein and by the Prox1-positive lymphatic progenitor cells that had already budded off from the embryonic veins (Figure 1C and D). Two days later, T1α/podoplanin expression was restricted to the budded Prox1-positive lymphatic endothelial progenitor cells and to the Prox1-positive lymphatic endothelial cells that lined the developing primitive lymph sacs (Figure 1C and F). After birth, vascular T1α/podoplanin expression was almost exclusively detected in lymphatic vessels (see below).
Fig. 1. T1α/podoplanin is expressed by Prox1-positive lymphatic progenitor cells during embryogenesis. (A and B) At day 10.5 of embryonic mouse development, Prox1 (green) is already expressed by endothelial cells predominantly located on one side of the anterior cardinal vein (CV), whereas the expression of T1α/podoplanin (red) is still restricted to the neural tube (NT). (C and D) At E12.5, T1α/podoplanin-positive endothelial cells are present throughout the anterior cardinal vein. Budding Prox1-positive lymphatic progenitor cells also express T1α/podoplanin. (E and F) At E14.5, the expression of T1α/podoplanin becomes restricted to Prox1-positive lymphatic endothelial cells of the lymph sac (LS), whereas no or only low-level expression is detected on endothelial cells of the jugular vein (JV). Bars = 50 µm.
Impaired lymphatic transport and formation of lymphedema in neonatal T1α/podoplanin-deficient mice
Mice with heterozygous and homozygous disruptions of the T1α/podoplanin gene (Ramirez et al., 2003) were compared with their wild-type littermates for all investigations. Whereas T1α/podoplanin+/– mice were healthy and fertile, and were macroscopically indistinguishable from their wild-type littermates, T1α/podoplanin–/– mice died immediately after birth, due to respiratory failure caused by impaired formation of alveolar airspace, associated with reduced numbers of differentiated type I alveolar epithelial cells in the lung (Ramirez et al., 2003). The skin of these mice was cyanotic and its texture was smoothened (Figure 2A). The lower limbs were markedly swollen, and thickened skin folds were clearly detectable in the neck area, indicative of cutaneous lymphedema (Figure 2A).
Fig. 2. Congenital skin lymphedema and impaired lymphatic transport in homozygous T1α/podoplanin knockout mice. (A) Newborn wild-type (+/+) and heterozygous mice (+/–) showed no phenotypic abnormalities, whereas T1α/podoplanin-null mice (–/–) died immediately after birth due to respiratory failure. Examination of T1α/podoplanin–/– mice revealed smoothened skin texture, thickened wrinkles, particularly in the neck area, and swelling of the lower extremities (inset). (B–D) Intradermal lymphatic capillaries and larger collecting lymphatic vessels of wild-type (B) and T1α/podoplanin+/– (C) mice were filled with dye after injection of Evan’s blue dye into the dorsum of the paws. In contrast, only enlarged subcutaneous lymphatic collectors (D, arrowheads) were detected in T1α/podoplanin–/– mice. After intradermal injection of Evan’s blue dye into the paws, retroperitoneal lymphatic vessels (arrowheads) and lymph nodes (arrows) were stained blue in wild-type (E) and T1α/podoplanin+/– (F) mice, but no dye was detected in the retroperitoneal lymphatics of T1α/podoplanin–/– mice (G). IVC = inferior vena cava; A = aorta.
To investigate lymphatic transport, we intradermally injected Evans blue dye into the dorsum of the footpads of newborn mice. In both wild-type and T1α/podoplanin+/– mice, the dye was immediately transported through a dense network of interconnected dermal lymphatic capillaries and larger collecting lymphatic vessels towards the popliteal lymph nodes (Figure 2B and C). In T1α/podoplanin–/– mice, in contrast, only dilated lymphatic vessels were visible, and small dermal capillaries were not detectable (Figure 2D). Immediately after injection of Evans blue into all four extremities, retroperitoneal para-aortic lymph nodes and lymphatic ducts were clearly stained blue in wild-type and in T1α/podoplanin+/– mice, indicating efficient centripetal lymphatic transport (Figure 2E and F). Although immunofluorescence stainings revealed the presence of retroperitoneal lymphatic ducts in all of the investigated mice (data not shown), no staining of para-aortic lymphatic structures was detected in T1α/podoplanin–/– mice (Figure 2G), demonstrating that lymphatic transport was impaired.
Dilation of intestinal and cutaneous lymphatic vessels, but not of blood vessels, in T1α/podoplanin-null mice
Both the skin and intestine are characterized by their rich lymphatic vascularization, and these tissues are highly sensitive to impairment of lymphatic network formation. Using differential immunostains for the lymphatic-specific hyaluronan receptor LYVE-1 (Prevo et al., 2001) and the endothelial plasma membrane molecule CD31, we found several slightly enlarged submucosal lymphatic vessels in the small intestine of T1α/podoplanin+/– mice as compared with wild-type mice (Figure 3A and E). In T1α/podoplanin–/– mice, an increased number of severely dilated submucosal lymphatic vessels was found (Figure 3I), whereas no major alterations of subserosal lymphatic capillaries were detected. LYVE-1-positive lacteals were completely absent in T1α/podoplanin–/– mice (Figure 3I), whereas these formed normally in wild-type and T1α/podoplanin+/– mice (Figure 3A and E, asterisks). The number and size of CD31-positive/LYVE-1-negative intestinal blood vessels, in contrast, were comparable in all genotypes (Figure 3A, B, E, F, I and J).
Fig. 3. T1α/podoplanin deficiency leads to dilation of lymphatics, but not of blood vessels, in the skin and intestine. (A–L) Immunofluorescence stains of the ileum (A, B, E, F, I and J; L = lumen; AC = abdominal cavity) and the skin (C, D, G, H, K and L) for CD31 (green) and LYVE-1 (red, C, G and K) or T1α/podoplanin (red, B, F, J, D, H and L) revealed slightly enlarged lymphatic vessels of the submucosal plexus of T1α/podoplanin+/– mice (E), whereas T1α/podoplanin–/– mice have greatly enlarged lymphatics (I). Lacteals of wild-type and T1α/podoplanin+/– mice were LYVE-1 positive (asterisks, A and E), whereas no LYVE-1-positive lacteals were detected in T1α/podoplanin–/– mice (I). Lymphatic expression of T1α/podoplanin was confirmed in the ileum and the skin of wild-type (B and D) and T1α/podoplanin+/– (F and H) mice (asterisks = lacteals), but was not detected in T1α/podoplanin–/– mice (J and L). Staining for CD31 revealed no differences of the size of blood vessels in all of the mice. These findings were confirmed by computer-assisted image analysis which revealed a significant increase in the size of lymphatics (N), but not of blood vessels (M), in T1α/podoplanin–/– mice. Because some T1α/podoplanin-expressing basal keratinocytes were found in the epidermis of wild-type and T1α/podoplanin+/– mice (D and H; arrowheads), the comparative expression of the epidermal differentiation markers K14, K10 and loricrin was investigated (O–W). No differences in the expression of these markers were seen in the three genotypes. Bars = 50 µm.
The T1α/podoplanin–/– mice also had enlarged lymphatics in the skin, as compared with wild-type and T1α/podoplanin+/– mice (Figure 3C, G and K). Computer-assisted morphometric image analyses confirmed that the average area of dermal LYVE-1-positive lymphatic vessels was significantly increased in T1α/podoplanin–/– mice (Figure 3N), whereas no differences in the size of blood vessels were found (Figure 3M). Immunofluorescence analysis of the intestine and the skin confirmed that lymphatic vessels in both wild-type and T1α/podoplanin+/– mice strongly expressed T1α/podoplanin. In contrast, no immunoreactivity was detected in T1α/podoplanin–/– mice, confirming the complete disruption of this gene and the specificity of the 8.1.1 antibody, that was originally raised against the gp38 antigen (Farr et al., 1992), for T1α/podoplanin (Figure 3B, D, F, H, J and L).
T1α/podoplanin deficiency results in impaired patterning of lymphatic capillary networks
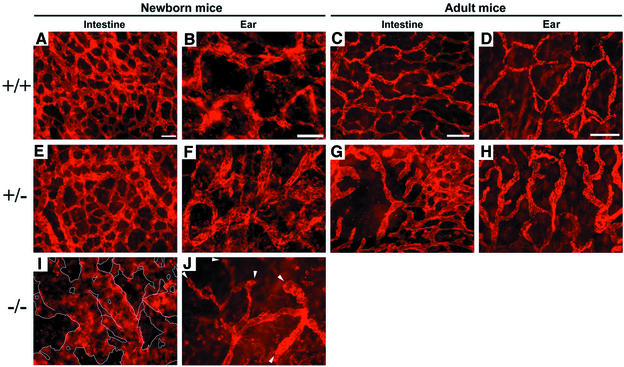
We investigated whether T1α/podoplanin deficiency, in addition to causing the enlargement of intestinal and cutaneous lymphatic vessels, might also affect the patterning of the lymphatic network in the same anatomical regions. To this end, we performed immunostain analysis of LYVE-1 expression on tissue samples from the intestine (ileum) and from the ear skin of all newborn genotypes. We observed a dense and well-organized network of intestinal lymphatic capillaries in wild-type and T1α/podoplanin+/– newborn mice (Figure 4A and E). T1α/podoplanin–/– newborn mice, in contrast, developed areas of extremely enlarged lymphatic vessels, and the pattern of these vessels was completely disorganized (Figure 4I). Analysis of the ear skin of wild-type and T1α/podoplanin+/– mice revealed well-organized networks of LYVE-1-positive lymphatic capillaries (Figure 4B and F). Most of these capillaries were interconnected, and only a few blind beginning lymphatic capillaries were detected (Figure 4B and F). In contrast, the formation of lymphatic capillary networks was impaired in the ear skin of T1α/podoplanin–/– mice. These mice possessed an increased number of non-anastomozing, blind beginning cutaneous lymphatic capillaries (Figure 4J, arrowheads), indicative of impaired lymphatic network patterning.
Fig. 4. Abnormal patterning of lymphatic capillaries in T1α/podoplanin-deficient mice. (A–H) LYVE-1 whole-mount stains of lymphatic capillaries in the ileum (A, C, E, G and I) and ear (B, D, F, H and J) of newborn (A, B, E, F, I and L) and adult mice (C, D, G and H) revealed a regular network of lymphatics (A and E) in the intestine of newborn wild-type and T1α/podoplanin+/– mice. The network patterning was completely irregular and the diameter of lymphatics was strikingly increased in T1α/podoplanin–/– mice (I). The lymphatic vessels in the ear of T1α/podoplanin–/– mice also developed an irregular network (J) with a higher number of blind beginnings of lymphatics (arrowheads) compared with the lymphatic networks in the ears of newborn wild-type and T1α/podoplanin+/– mice (B and F). In the intestine and ears of adult wild-type mice, regular networks of lymphatic vessels were found (C and D), whereas areas of enlarged lymphatics (G and H) and incomplete network patterning (G) were seen in adult T1α/podoplanin+/– mice. Bars for (A), (B), (E), (F) and (J) = 100 µm, (C) and (G) = 200 µm, (D) and (H) = 300 µm.
Because there were no clear-cut differences in lymphatic network patterns between newborn T1α/podoplanin+/– and wild-type mice, we performed immunohistochemical analyses on intestine (ileum) and ear skin tissue obtained from adult (4-month-old) T1α/podoplanin+/– mice and their littermates. Although the defects in lymphatic network patterning were not as striking as those seen in newborn T1α/podoplanin–/– mice, there were areas of dilated lymphatic vessels in the ear skin (Figure 4H) and in the intestine of T1α/podoplanin+/– mice, in addition to incomplete network formation (Figure 4G) that was not observed in wild-type littermates (Figure 4C and D).
Ultrastructural localization of T1α/podoplanin in intestinal lymphatic vessels, but not in blood vessels
To investigate the ultrastructural localization of T1α/podoplanin in the vascular system, we performed immuno-nanogold electron microscopy, using intestinal tissue samples obtained from newborn mice. In wild-type mice, high levels of membrane-bound T1α/podoplanin were detected at the luminal side of intestinal lymphatic vessels (Figure 5A). Fewer nanogold particles were observed on the abluminal plasma membrane, and no labeling was detected within the cytoplasm (Figure 5A). Occasionally, the lateral plasma membranes between adjacent cells were also labeled. T1α/podoplanin expression was completely absent from blood vessel endothelial cells in the intestine of newborn wild-type mice (Figure 5B). Lymphatic endothelial cells in the small intestine of T1α/podoplanin–/– mice were not immunolabeled by the anti-T1α/podoplanin antibody (Figure 5D), confirming the disruption of the T1α/podoplanin gene in these mice.
Fig. 5. Ultrastructural localization of T1α/podoplanin in murine intestinal lymphatic vessels, but not in blood vessels, by immuno-nanogold staining. (A) In newborn wild-type mice, membrane-bound T1α/podoplanin was detected at the luminal (L) side of the lymphatic endothelium in the intestine (ileum). Fewer immuno-nanogold particles were observed at the abluminal plasma membrane and no T1α/podoplanin was detected within lymphatic endothelial cells. (B) T1α/podoplanin expression was completely absent from blood vascular endothelial cells in the intestine of newborn wild-type mice. (C) Absence of specific labeling of wild-type lymphatic endothelium after omission of the primary anti-T1α/podoplanin antibody. (D) Lymphatic endothelial cells of a newborn T1α/podoplanin–/– mouse do not react with the anti-T1α/podoplanin antibody. Bars for (A) = 0.4 µm, (B) = 0.2 µm, (C) = 0.3 µm, (D) = 0.5 µm.
T1α/podoplanin deficiency does not alter the ultrastructural architecture of intestinal lymphatic vessels or the distribution of LYVE-1 expression
We next investigated whether loss of T1α/podoplanin might result in abnormal ultrastructural architecture of lymphatic vessels or in altered distribution of the LYVE-1. We detected high levels of immuno-nanogold labeling for LYVE-1 at both the luminal and the abluminal plasma membrane of lymphatic endothelium in the intestine of newborn wild-type mice (Figure 6A). Some labeling of the lateral plasma membranes was also observed, with the exception of contact areas between adjacent cells. LYVE-1 expression was completely absent from blood vessel endothelium (Figure 6B), confirming the specificity of LYVE-1 for lymphatic endothelium. Lymphatic endothelial cells of T1α/podoplanin–/– mice also showed strong LYVE-1 immunogold labeling at both the luminal and the abluminal plasma membranes at levels similar to those observed in wild-type mice (Figure 6C and D). No ultrastructural differences in lymphatic vessel structure were observed between the different genotypes.
Fig. 6. Comparable ultrastructural localization of LYVE-1 in intestinal lymphatic vessels of wild-type and T1α/podoplanin–/– mice. (A) High levels of immuno-nanogold labeling for LYVE-1 were detected at both the luminal (L) and the abluminal plasma membrane of lymphatic endothelium in the ileum of newborn wild-type mice. Some labeling of the lateral plasma membranes was also observed, whereas LYVE-1 was absent from the cytoplasm. (B) LYVE-1 expression was absent from blood vascular endothelium. (C and D) Lymphatic endothelial cells in the intestine of T1α/podoplanin–/– mice also had high levels of LYVE-1 immuno-nanogold labeling of the luminal and abluminal plasma membranes. The lateral plasma membranes were also labeled, with the exception of punctate contact areas between adjacent cells (A, C and D, arrows). (E and F) Replacement of the primary LYVE-1 antibody with an unrelated rabbit IgG control resulted in the absence of lymphatic endothelial cell labeling in the ileum of wild-type (E) and of T1α/podoplanin-null mice (F). Bars for (A), (C), (D) and (E) = 0.5 µm, (B) and (F) = 0.4 µm.
T1α/podoplanin deficiency does not impair epidermal differentiation
We occasionally detected T1α/podoplanin expression in basal keratinocytes of the epidermis in wild-type and T1α/podoplanin+/– mice (Figure 3D and H). To determine whether T1α/podoplanin deficiency might also affect epidermal structure or differentiation, we studied the expression of several markers of epidermal differentiation. A comparable expression pattern of keratin 14 (K14), which is expressed by proliferative basal keratinocytes (Fuchs and Byrne, 1994), was found in all three groups (Figure 3O, R and U). Expression patterns of the early and late epidermal terminal differentiation markers K10 (Figure 3P, S and V) and loricrin (Figure 3Q, T and W) were also similar in the skin in all three genotypes. Computer-assisted morphometric image analyses demonstrated comparable thickness of the epidermis in all genotypes (data not shown), and no major histological differences of epidermal structure were detected, indicating that T1α/podoplanin deficiency does not affect the formation or structure of the epidermis.
T1α/podoplanin promotes endothelial cell migration, adhesion and tube formation
To characterize further the biological function of T1α/podoplanin, we isolated lymphatic endothelial cells from the skin of newborn mice of all three genotypes. However, we were unable to expand lymphatic endothelial cell cultures obtained from all genotypes, even after addition of recombinant vascular endothelial growth factor (VEGF)-C to the growth medium (data not shown). We therefore decided to test the effects of T1α/podoplanin overexpression. Immortalized human microvascular endothelial cells (HMEC-1) were stably transfected with a pIRES2-rat T1α/podoplanin expression vector and pooled cells were used for subsequent in vitro studies. Immunostains revealed high levels of rat T1α/podoplanin protein in the stably transfected cells (Figure 7A) that projected extremely long and thin cell extensions, which were not seen in control cells that did not express rat T1α/podoplanin (Figure 7B and C). Some of the stably T1α/podoplanin-transfected cells also formed phalloidin-positive F-actin bundles at the periphery (Figure 7B and C), indicating that T1α/podoplanin expression might control endothelial cell cytoskeletal organization. We next studied whether overexpression of T1α/podoplanin could affect endothelial cell migration or adhesion. We found a >2-fold (P < 0.001) increase in haptotactic cell migration towards fibronectin in stably transfected cells, compared with controls (Figure 7D). T1α/podoplanin-overexpressing HMEC-1 cells also adhered more tightly to immobilized fibronectin (P < 0.01; Figure 7E).
Fig. 7. Overexpression of T1α/podoplanin in HMEC-1 cells enhances endothelial cell migration and adhesion in vitro. (A–C) Stable transfection of HMEC-1 cells with rat T1α/podoplanin cDNA resulted in the production of the rat T1α/podoplanin protein (green; A and C) and in the formation of long filopodia, which were not seen in cells that did not overexpress T1α/podoplanin. Some of the rat T1α/podoplanin-expressing cells showed a marginal accumulation of F-actin bundles (red, B and C). Bar = 150 µm. (D and E) Overexpression of T1α/podoplanin significantly stimulated cell migration (D) and adhesion (E) of HMEC-1 cells. **P < 0.01; ***P < 0.001.
To confirm these results further in a second, independent cell line, murine hemangioendothelioma-derived EOMA cells were stably transfected with the same pIRES2-rat T1α/podoplanin–enhanced green fluorescent protein (EGFP) construct or with the control vector. Three clones with high expression of the transfected T1α/podoplanin, along with three control clones, were used for subsequent experiments. Overexpression of T1α/podoplanin significantly enhanced EOMA cell migration towards type I collagen and also promoted cell adhesion in all three clones tested, as compared with the control clones (Figure 8A and B). T1α/podoplanin-overexpressing EOMA cell clones also showed a significantly increased ability to form tube-like structures after plating onto Matrigel (Figure 8C–E).
Fig. 8. Overexpression of T1α/podoplanin in EOMA cells enhances cell migration, adhesion and tube formation in vitro. (A and B) Stable transfection of EOMA cells with rat T1α/podoplanin cDNA resulted in significantly enhanced haptotactic cell migration and adhesion to type I collagen in all three clones tested (P1–P3), as compared with control clones (C1–C3). (C and D) Enhanced formation of tube-like structures by T1α/podoplanin-overexpressing EOMA cells after seeding onto Matrigel (24 h; D), as compared with control vector-transfected cells (C). Bar = 50 µm. (E) Overexpression of T1α/podoplanin significantly enhanced the formation of tube-like structures in all three T1α/podoplanin-overexpressing clones (P1–P3) as compared with control clones (C1–C3).
Inhibition of T1α/podoplanin expression by siRNA transfection reduces cell adhesion of human lymphatic endothelial cells
To investigate further the role of T1α/podoplanin in cell adhesion, we studied the effects of small interfering RNA (siRNA)-mediated inhibition of endogenous T1α/podoplanin expression in cultured human dermal lymphatic endothelial cells that are characterized by high expression levels of T1α/podoplanin (Hirakawa et al., 2003). Two out of three siRNAs tested efficiently reduced endogenous T1α/podoplanin protein expression by 49 and 34%, respectively, at 4 days after transfection, whereas the expression of another lymphatic marker, LYVE-1, remained unchanged (Figure 9A). Accordingly, lymphatic endothelial cell adhesion to type I collagen, which is closely associated with lymphatic vessels in vivo (Skobe and Detmar, 2000), was significantly inhibited by both of these siRNAs, as compared with control or sham-transfected control cells, at 4 days after transfection (Figure 9B).
Fig. 9. Inhibition of T1α/podoplanin expression by siRNA transfection reduces human lymphatic endothelial cell adhesion to type I collagen. (A) Four days after siRNA transfection of human primary lymphatic endothelial cells, endogenous T1α/podoplanin protein levels, but not LYVE-1 levels, were decreased by two different siRNA oligonucleotides (R1 and R2), as compared with control cells (C1, control vector cDNA; C2, sham-transfected cells). (B) Human dermal lymphatic endothelial cells showed significantly reduced adhesion to type I collagen 4 days after siRNA transfection (R1 and R2), as compared with control cells (C1 and C2). *P < 0.05; **P < 0.01.
Discussion
Previous studies have identified T1α/podoplanin as a Prox1-induced gene (Hong et al., 2002) that is predominantly expressed, within the vascular system, in lymphatic endothelium (Wetterwald et al., 1996; Kriehuber et al., 2001; Maekinen et al., 2001; Hirakawa et al., 2003). We show that similarly to VEGF receptor-3 (Wigle et al., 2002), another Prox1 target gene (Hong et al., 2002; Petrova et al., 2002), T1α/podoplanin is expressed throughout the endothelium of the anterior cardinal vein at E12.5 of embryonic mouse development and later becomes restricted to the budding Prox1-positive lymphatic progenitor endothelial cells and to lymphatic endothelial cells of the embryonic lymph sacs and of lymphatic vessels. In contrast to Prox1-null mice, which fail to develop any lymphatic vasculature (Wigle and Oliver, 1999), T1α/podoplanin–/– mice develop a peripheral lymphatic vascular system; however, it exhibits pronounced defects in lymphatic vascular organization and function. Our findings indicate that T1α/podoplanin is important to regulate different aspects related to the later stages of lymphatic development and patterning. In contrast to other recently described gene targeting models, including mice deficient for angiopoietin-2 (Gale et al., 2002) or VEGF receptor-3 (Dumont et al., 1998), T1α/podoplanin deficiency selectively affects the lymphatic vascular system without any detectable effect on the development of the blood vascular system. Similarly to neuropilin-2 mutant mice which show only reduction of small lymphatic vessels but no alteration of the blood vascular system (Yuan et al., 2002), our findings in T1α/podoplanin-deficient mice are in agreement with the relatively late onset of T1α/podoplanin expression during vascular development, and with its predominant expression on lymphatic vascular endothelium.
Importantly, T1α/podoplanin–/– mice were characterized by congenital lymphedema, as manifested by the pronounced swelling of the limbs at birth. Intradermal dye injection into the foot pads of T1α/podoplanin–/– mice revealed several enlarged, plump lymphatic vessels, but failed to visualize the characteristic dermal capillary networks seen in wild-type and in T1α/podoplanin+/– mice. These networks were most probably filled from deeper lymphatic vessels through anastomozing vessels that still lack valves at the early stages of pre- and post-natal lymphatic development (Polonskaja, 1935). Because histological examination revealed the presence of dermal capillaries in T1α/podoplanin–/– mice, these findings indicate an insufficient formation of anastomozing lymphatic vessels between the superficial and subcutaneous lymphatic networks.
In the intestine of T1α/podoplanin–/– mice, lacteals were not detectable and many of the lymphatics of the submucosal plexus were enlarged. The physiological consequences of these developmental defects on the uptake of lipids from the intestine are not known, since these mice died immediately after birth (before the first feeding) and chyle transport could not be investigated. However, as previously described (Gale et al., 2002), angiopoietin-2-null mice develop chylous ascites shortly after birth, due to insufficient formation of lacteals. Because the observed morphological defect in T1α/podoplanin–/– mice is even more pronounced, we expect that lipid uptake in the intestine would also be defective. The observed enlargement of both dermal and submucosal intestinal lymphatics of T1α/podoplanin–/– mice is most likely to be related to the lack of connecting lymphatics between the superficial and deep networks, indicating that T1α/podoplanin is required for the formation of these specific lymphatic vessels.
We were unable to propagate lymphatic endothelial cells that were isolated from both wild-type and T1α/podoplanin–/– neonatal mice by using a modification of our recently established purification method for human dermal lymphatic endothelial cells (Hirakawa et al., 2003). At present, there are no published reports on the successful propagation of primary murine lymphatic endothelial cells, and suitable culture techniques still remain to be established. Therefore, we investigated the cellular effects of T1α/podoplanin overexpression in two types of immortalized human and murine vascular endothelial cells that express only moderate levels of T1α/podoplanin (Hong et al., 2002). The induction of elongated endothelial cell extensions and cytoskeletal reorganization, together with the enhanced adhesion and migration of stably T1α/podoplanin-transfected endothelial cells are in agreement with its effects on the motility of immortalized keratinocytes (Scholl et al., 1999) and indicate that this protein controls endothelial cell functions that are required for normal lymphatic patterning during development. These findings were confirmed further by the reduced endothelial cell adhesion observed after inhibition of endogenous T1α/podoplanin expression by siRNA transfection. Because efficient cell migration is dependent upon the polarity of the migrating cells, and since T1α/podoplanin is also expressed on the apical surface of polarized alveolar epithelial cells (Dobbs et al., 1988; Rishi et al., 1995), it may exert an important role for the polarization of cells and for the stabilization of cellular protrusions at the leading edge of migrating cells. This proposed function is supported further by our findings that overexpression of T1α/podoplanin in endothelial cells promoted the formation of tube-like structures on Matrigel which provide a link to the in vivo findings of incomplete lymphatic network formation in T1α/podoplanin–/– mice.
T1α/podoplanin is a mucin-type glycoprotein with extensive O-glycosylation and a high content of sialic acid (Gonzalez and Dobbs, 1998). This negatively charged structure probably is resistant to proteases and provides a physical barrier that protects cells from environmental agents (Zimmer et al., 1999). Immuno-nanogold electron microscopy showed that T1α/podoplanin is predominantly localized to the apical, luminal plasma membrane of intestinal lymphatic endothelial cells. This localization is similar to that reported for other cell types (Dobbs et al., 1988; Rishi et al., 1995; Williams et al., 1996; Breiteneder-Geleff et al., 1997; Zimmer et al., 1997) and is compatible with a protective function towards the proteinase-containing lymph (Bartos et al., 1979). It is of interest that T1α/podoplanin is also expressed by alveolar epithelial cells, cells of the choroid plexus, ependymal epithelia and mesothelia that are also exposed to an external or internal fluid compartment.
In conclusion, we propose that T1α/podoplanin is required to control different aspects of normal lymphatic vasculature formation. Lack of T1α/podoplanin leads to alterations in the final patterning of the lymphatic vasculature as well as in lymph transport. The future generation of a conditional inactivation of T1α/podoplanin will be a valuable tool to understand further its role in the normal function and in pathological alterations of the lymphatic vasculature.
Materials and methods
Generation of T1α/podoplanin knockout mice
T1α/podoplanin mutant mice were generated as described (Ramirez et al., 2003). Fragments were designed to replace ∼1.5 kb of the promoter sequence, 210 bp of the untranslated region (UTR), the first exon (67 bp) and 181 bp of the first intron. The efficiency of the disruption of the T1α/podoplanin gene was confirmed by northern and western blot analyses of newborn lung RNA and protein extracts that demonstrated the absence of T1α/podoplanin mRNA or protein in T1α/podoplanin–/– mice and reduced T1α/podoplanin mRNA levels in T1α/podoplanin+/– mice (Ramirez et al., 2003).
Tissue processing and immunostaining
Tissue samples were obtained from newborn T1α/podoplanin (+/+), (+/–) and (–/–) littermates that had been killed by intraperitoneal injection of barbital sodium within 10 min after birth and from wild-type embryos at days E10.5, E12.5 and E14.5, and were fixed in 4% paraformaldehyde (Fluka, Buchs, Switzerland). Immunofluorescence stains were performed on 6 µm cryostat sections of dorsal skin and of ileum, as well as on 10 µm sections of wild-type mouse embryos as described (Wigle et al., 2002), using polyclonal rabbit antibodies against Prox1 (Wigle et al., 2002) and LYVE-1 (Prevo et al., 2001; kindly provided by Dr D.Jackson, Oxford, UK), a monoclonal rat antibody against CD31 (BD Pharmingen, San Diego, CA), a hamster antibody against murine gp38 (Farr et al., 1992; clone 8.1.1, Developmental Studies Hybridoma Bank, University of Iowa), antibodies against murine K14, K10 and loricrin (Babco, Richmond, CA) and corresponding secondary antibodies labeled with AlexaFluor488 or AlexaFluor594 (Molecular Probes, Eugene, OR). Cell nuclei were counterstained with 20 µg/ml Hoechst bisbenzimide (Molecular Probes). Whole-mount samples of mouse ears and of ileum were stained as described elsewhere (Gale et al., 2002). The tissues were examined by using a Nikon E-600 microscope (Nikon, Melville, NY) and images were captured with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI). Computer-assisted morphometric analysis of lymphatics and blood vessels within the dermis of eight animals per group (three fields per sample at 20× magnification) were performed as described previously (Streit et al., 1999), using the IP-LAB software (Scanalytics, Fairfax, VA). The differences in vessel size were analyzed by the two-sided unpaired Student’s t-test.
Immuno-nanogold electron microscopy
Small intestine samples were collected from genetically modified mice and their wild-type littermates immediately after birth. Tissue samples were fixed in 4% paraformaldehyde and transferred to 30% sucrose/phosphate-buffered saline (PBS). After embedding the tissues in OCT compound (Miles, Elkhart, IN), cryostat sections (5 µm) were immersed in 50 mM glycine. After washing, sections were immersed in normal goat serum (Vector Laboratories, Burlingame, CA) and incubated with anti-LYVE-1 or 8.1.1 antibody, followed by incubation with goat anti-rabbit Fab′, conjugated with 1.4 nm nanogold particles (Nanoprobes, Stony Brook, NY), or with goat anti-hamster IgG, conjugated with 0.8 nm nanogold particles (Aurion, Wageningen, The Netherlands). Sections were post-fixed in 1% PBS-buffered glutaraldehyde, developed with HQ silver enhancement solution (Nanoprobes), fixed in 5% sodium thiosulfate, post-fixed in 1% osmium tetroxide in sym-collidine buffer, washed in 0.05 M sodium maleate buffer, and stained with 2% uranyl acetate in 0.05 M sodium maleate buffer. Tissues were dehydrated in graded ethanols, infiltrated with propylene oxide–eponate (Ted Pella, Redding, CA) and embedded by inverting eponate-filled plastic capsules over the slide-attached tissue sections. After polymerization, eponate blocks were separated from the glass slides and thin sections were cut on an ultratome (Reichert Ultracuts, Austria), collected on uncoated 200 mesh copper grids (Ted Pella) and examined with a CM 10 transmission electron microscope (Philips, Eindhoven, The Netherlands). Specificity controls included replacement of the primary antibody with irrelevant rabbit IgG and omission of the specific primary antibody.
Cell culture and transfection
Immortalized HMEC-1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) that contained 10% fetal bovine serum (FBS), 2 mM l-glutamine and antibiotics (Life Science, Grand Island, NY). The murine hemangioendothelioma-derived cell line EOMA (Obeso et al., 1990) was maintained in DMEM containing 20% FBS and 4 mM l-glutamine. Primary human dermal lymphatic endothelial cells were isolated and propagated as recently described (Hirakawa et al., 2003). The rat T1α/podoplanin coding sequence (Rishi et al., 1995; GenBank accession No. U07797) was cloned into a pIRES2-EGFP vector (Clontech, Palo Alto, CA) which contains a cytomegalovirus (CMV) enhancer promoter and a neomycin selection cassette. The nucleotide sequence was verified using the Applied Biosystems Big Dye Terminator kits. After linerarization with BsaI, HMEC-1 and EOMA cells were transfected either with the full-length rat T1α/podoplanin cDNA or with pIRES2-EGFP vector alone using the SuperFect transfection reagent (Qiagen, Chatsworth, CA). Stably transfected cells were selected in growth medium containing 200 µg/ml neomycin (Gibco, Carlsbad, CA). For all experiments, pooled transfected HMEC-1 cells and three clones of stably transfected EOMA cells were used for T1α/podoplanin overexpression and for vector controls. To determine T1α/podoplanin expression levels, we performed SYBR Green-based real-time RT–PCRs as described (Hong et al., 2002), using the ABI Prism 7000 Sequence Detection System. The following forward and reverse primers were used: 5′-GACATGGTGAACCCAGGTCT-3 and 5′-AATGGGAGGCTGTGTTGGTA-3. Total RNAs were isolated using Tri-reagent (Sigma, St Louis, MO) and were treated with RNase-free RQ-DNase (Promega, Madison, WI). A 100 ng aliquot of RNA was used for each reaction. SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) was used for reactions with the addition of MultiScribe reverse transcriptase (Applied Biosystems). Protein expression was confirmed by immunofluorescence staining with a specific monoclonal anti-rat T1α/podoplanin antibody (Wetterwald et al., 1996). For F-actin staining, phalloidin–tetramethylrhodamine B isothiocyanate conjugate (Sigma) was used (Scholl et al., 1999).
Cell transfection with human T1α/podoplanin siRNAs
The following siRNA oligonucleotides were synthesized by Dharmacon (Lafayette, CO): (R1) 5′-GCGAAGACCGCUAUAAGUCdTdT-3′, (R2) 5′-AAGAUGGUUUGUCAACAGUdTdT-3′ and (R3) 5′-AGAUGA CACUGAGACUACAdTdT-3′. Primary human lymphatic endothelial cells (Hirakawa et al., 2003) were transfected or not with siRNA oligonucleotides (500 nmol) or with equimolar concentrations of control plasmid vector by using the Nucleofector kit (Amaxa, Cologne, Germany) according to the manufacturer’s instructions. Cells were harvested at 2 and 4 days after transfection. For western analyses, cell lysates were obtained as described (Hong et al., 2002) and 30 ng of protein per sample were immunoblotted with an antibody against human T1α/podoplanin (Zimmer et al., 1997; kindly provided by Dr G.Herrler, Hanover, Germany) and with an antibody against human LYVE-1 (kindly provided by Dr D.Jackson, Oxford, UK).
Cell migration, adhesion and tube formation assays
For cell migration assays, 24-well FluoroBlok inserts (Falcon, Franklin Lakes, NJ; 8 µm pore size) were coated on the underside with 10 µg/ml fibronectin (BD Bioscience, Bedford, MA) or with 50 µg/ml type I collagen (Vitrogen, Palo Alto, CA) for 1 h, followed by addition of 100 µg/ml bovine serum albumin (BSA; Sigma) to block the remaining protein-binding sites. Cells (2 × 105 cells/ml) were seeded in serum-free EBM-2 medium (Clonetics, Watersville, MD) containing 0.2% de-lipidized BSA into each well and cells were incubated for 3 h at 37°C. Cells on the underside of inserts were stained with Calcein AM (Molecular Probes), and the fluorescence intensity was measured using the Victor2 Fluorometer (PerkinElmer, Boston, MA). Cell adhesion assays were performed as described (Streit et al., 1999). Twelve-well plates were coated with 10 µg/ml fibronectin or with 50 µg/ml type I collagen for 1 h at 4°C, followed by blocking with 100 µg/ml BSA. Cells (1 × 105 cells in 200 µl of serum-free DMEM) were added to each well and were incubated at 37°C for 20 min. Unattached cells were removed by three gentle washes with PBS; attached cells were fixed with 4% paraformaldehyde, stained with Hoechst bisbenzimide and the number of attached cells/mm2 was determined. Tube formation assays were performed on Matrigel-coated 24-well plates (BD Bioscience) as described previously (Obeso et al., 1990). EOMA cells were seeded at a density of 2 × 105 cells/ml in growth medium and were incubated for 24 h at 37°C. After fixation with 4% paraformaldehyde, images were captured and the total length of tube-like structures per area was measured using the IP-LAB software. All studies were performed in triplicate. Statistical analyses were performed using the unpaired Student’s t-test.
Acknowledgments
Acknowledgements
The authors thank J.Bertoncini, L.Janes, M.Constant and G.Millien for technical assistance, Dr D.Jackson for the gift of the LYVE-1 antibody, Sam W.Lee for valuable advice regarding siRNA experiments, and Kathryn Pyne for photographic assistance with the electron micrographs. This work was supported by NIH grants CA69184, CA86410, CA92644 (M.D.), HL47049 (M.C.W.), AI33372 and AI44066 (A.M.D.), by American Cancer Society Program Project Grant 99-23901 (M.D.), by the Deutsche Forschungsgemeinschaft (V.S.), by the Francis Family Foundation (M.I.R.) and by the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd Agreement (M.D.).
References
- Bartos V., Kolc,J., Vanecek,R. and Malek,P. (1979) Lymph and blood enzymes and pathologic alterations in canine experimental pancreatitis after administration of benzo-pyrones. Scand. J. Gastroenterol., 14, 343–347. [DOI] [PubMed] [Google Scholar]
- Breiteneder-Geleff S., Matsui,K., Soleiman,A., Meraner,P., Poczewski,H., Kalt,R., Schaffner,G. and Kerjaschki,D. (1997) Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol., 151, 1141–1152. [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. (2000) Mechanisms of angiogenesis and arteriogenesis. Nat. Med., 6, 389–395. [DOI] [PubMed] [Google Scholar]
- Detmar M. and Hirakawa,S. (2002) The formation of lymphatic vessels and its importance in the setting of malignancy. J. Exp. Med., 196, 713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L.G., Williams,M.C. and Gonzalez,R. (1988) Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim. Biophys Acta, 970, 146–156. [DOI] [PubMed] [Google Scholar]
- Dumont D.J., Jussila,L., Taipale,J., Lymboussaki,A., Mustonen,T., Pajusola,K., Breitman,M. and Alitalo,K. (1998) Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science, 282, 946–949. [DOI] [PubMed] [Google Scholar]
- Farr A.G., Berry,M.L., Kim,A., Nelson,A.J., Welch,M.P. and Aruffo,A. (1992) Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J. Exp. Med., 176, 1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. and Byrne,C. (1994) The epidermis: rising to the surface. Curr. Opin. Genet. Dev., 4, 725–736. [DOI] [PubMed] [Google Scholar]
- Gale N. et al. (2002) Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning and only the latter role is rescued by angiopoietin-1. Dev. Cell, 3, 411–423. [DOI] [PubMed] [Google Scholar]
- Gandarillas A., Scholl,F.G., Benito,N., Gamallo,C. and Quintanilla,M. (1997) Induction of PA2.26, a cell-surface antigen expressed by active fibroblasts, in mouse epidermal keratinocytes during carcinogenesis. Mol. Carcinog., 20, 10–18. [DOI] [PubMed] [Google Scholar]
- Gonzalez R.F. and Dobbs,L.G. (1998) Purification and analysis of RTI40, a type I alveolar epithelial cell apical membrane protein. Biochim. Biophys Acta, 1429, 208–216. [DOI] [PubMed] [Google Scholar]
- Hirakawa S., Hong,Y.K., Harvey,N., Schacht,V., Matsuda,K., Libermann,T. and Detmar,M. (2003) Transcriptional profiling of isolated human lymphatic versus blood vascular endothelial cells. Am. J. Pathol., 162, 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y.K., Harvey,N., Noh,Y.H., Schacht,V., Hirakawa,S., Detmar,M. and Oliver,G. (2002) Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn., 225, 351–357. [DOI] [PubMed] [Google Scholar]
- Kriehuber E., Breiteneder,G.S., Groeger,M., Soleiman,A., Schoppmann,S.F., Stingl,G., Kerjaschki,D. and Maurer,D. (2001) Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med., 194, 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekinen T. et al. (2001) Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J., 20, 4762–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon E.C. and Ryan,T.J. (1994) Lymphedema and wound healing. Clin. Dermatol., 12, 89–93. [DOI] [PubMed] [Google Scholar]
- Nose K., Saito,H. and Kuroki,T. (1990) Isolation of a gene sequence induced later by tumor-promoting 12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells (MC3T3-E1) and expressed constitutively in ras-transformed cells. Cell Growth Differ., 1, 511–518. [PubMed] [Google Scholar]
- Obeso J., Weber,J. and Auerbach,R. (1990) A hemangioendothelioma-derived cell line: its use as a model for the study of endothelial cell biology. Lab. Invest., 63, 259–269. [PubMed] [Google Scholar]
- Oliver G. and Detmar,M. (2002) The rediscovery of the lymphatic system. Old and new insights into the development and biological function of lymphatic vascular system. Genes Dev., 16, 773–783. [DOI] [PubMed] [Google Scholar]
- Petrova T.V. et al. (2002) Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J., 21, 4593–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonskaja R. (1935) The connections between the superficial and deep lymphatic vessels of the lower limb. Anat. Anz., 81, 247–256. [Google Scholar]
- Prevo R., Banerji,S., Ferguson,D.J., Clasper,S. and Jackson,D.G. (2001) Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem., 276, 19420–19430. [DOI] [PubMed] [Google Scholar]
- Ramirez M.I., Millien,G., Hinds,A., Cao,Y., Seldin,D.C. and Williams,M.C. (2003) T1α, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev. Biol., 256, 61–72. [DOI] [PubMed] [Google Scholar]
- Rishi A.K., Joyce-Brady,M., Fisher,J., Dobbs,L.G., Floros,J., VanderSpek,J., Brody,J.S. and Williams,M.C. (1995) Cloning, characterization and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev. Biol., 167, 294–306. [DOI] [PubMed] [Google Scholar]
- Sabin F.R. (1902) On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat., 1, 367–389. [Google Scholar]
- Scholl F.G., Gamallo,C., Vilaro,S. and Quintanilla,M. (1999) Identification of PA2.26 antigen as a novel cell-surface mucin-type glycoprotein that induces plasma membrane extensions and increased motility in keratinocytes. J. Cell Sci., 112, 4601–4613. [DOI] [PubMed] [Google Scholar]
- Skobe M. and Detmar,M. (2000) Structure, function and molecular control of the skin lymphatic system. J. Investig. Dermatol. Symp. Proc., 5, 14–19. [DOI] [PubMed] [Google Scholar]
- Streit M., Velasco,P., Brown,L.F., Skobe,M., Richard,L., Riccardi,L., Lawler,J. and Detmar,M. (1999) Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am. J. Pathol., 155, 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterwald A., Hoffstetter,W., Cecchini,M.G., Lanske,B., Wagner,C., Fleisch,H. and Atkinson,M. (1996) Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone, 18, 125–132. [DOI] [PubMed] [Google Scholar]
- Wigle J.T. and Oliver,G. (1999) Prox1 function is required for the development of the murine lymphatic system. Cell, 98, 769–778. [DOI] [PubMed] [Google Scholar]
- Wigle J.T., Harvey,N., Detmar,M., Lagutina,I., Grosveld,G., Gunn,M.D., Jackson,D.G. and Oliver,G. (2002) An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J., 21, 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.C. (2003) Alveolar type I cells: molecular phenotype and development. Annu. Rev. Physiol., 65, 669–695. [DOI] [PubMed] [Google Scholar]
- Williams M.C., Cao,Y., Hinds,A., Rishi,A.K. and Wetterwald,A. (1996) T1 α protein is developmentally regulated and expressed by alveolar type I cells, choroid plexus and ciliary epithelia of adult rats. Am. J. Respir. Cell Mol. Biol., 14, 577–585. [DOI] [PubMed] [Google Scholar]
- Yuan L., Moyon,D., Pardanaud,L., Breant,C., Karkkainen,M.J., Alitalo,K. and Eichmann,A. (2002) Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development, 129, 4797–4806. [DOI] [PubMed] [Google Scholar]
- Zimmer G., Lottspeich,F., Maisner,A., Klenk,H.D. and Herrler,G. (1997) Molecular characterization of gp40, a mucin-type glycoprotein from the apical plasma membrane of Madin–Darby canine kidney cells (type I). Biochem. J., 326, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer G., Oeffner,F., Von Messling,V., Tschernig,T., Groness,H.J., Klenk,H.D. and Herrler,G. (1999) Cloning and characterization of gp36, a human mucin-type glycoprotein preferentially expressed in vascular endothelium. Biochem. J., 341, 277–284. [PMC free article] [PubMed] [Google Scholar]