Abstract
The purpose of this study was to determine what contributions are made to the rat full-field electroretinogram (ERG) by ganglion cells (GCs). To that end, the ERG was assessed longitudinally following optic nerve transection (ONTx). Additional studies were conducted using intravitreal injections of pharmacologically active substances. The ERG was recorded simultaneously from both eyes of anaesthetized adult Brown-Norway rats (ketamine: xylazine: acepromazine, 55 : 5 : 1 mg kg−1) using custom silver chloride electrodes. Stimuli were brief, white xenon discharges delivered via a Ganzfeld under dark-adapted and light-adapted conditions (150 cd m−2). ERGs were obtained 1, 2, 3, 4 and 9 weeks after ONTx (n = 8) or sham (n = 8) operations. ONTx reduced both positive and negative components of the scotopic threshold response (pSTR and nSTR). Scotopic ERG responses to brighter flashes, including a-waves, b-waves and oscillatory potentials (OPs) were unaffected by ONTx. ONTx reduced the photopic b-wave and OPs. TTX (6 μm) reduced the pSTR and nSTR, but not the scotopic a-wave, b-wave or OPs. TTX had dramatic effects on the photopic ERG, surpassing the effects of ONTx. TTX application 9 weeks post-ONTx had little additional effect on the STR. Inhibition of inner retinal responses using GABA (10 mm) or NMDA (0.8 mm) reduced the nSTR substantially. Similar results were obtained with antagonists of AMPA/KA ionotropic glutamate receptors 6-cyano-7-nitroquinoxaline-2,3(1H,4H)-dione (CNQX, 0.2 mm) or cis-2,3-piperidinedicarboxylic acid (PDA, 5 mm); however, both also reduced the scotopic b-wave by ∼40 %. By contrast, the NMDA receptor antagonist D(-)-2-amino-7-phosphonoheptanoic acid (D-AP7, 0.2 mm) had no effect alone, but the combination of D-AP7 and CNQX completely abolished the STR. The results of this study indicate that: (1) both pSTR and nSTR components in the rat depend directly upon intact GC responses, and that amacrine cell contributions to these components are relatively small; (2) scotopic ERG response components to brighter flashes receive little influence from GCs; (3) the rat photopic ERG also reflects GC signals and may serve as an additional useful test of GC function; (4) TTX had dramatic effects on the rat photopic ERG that were not attributable to GC currents, but rather to voltage-gated sodium currents in amacrine or interplexiform cells; (5) a small residual negative STR persisted after ONTx that was likely to be generated by graded responses of third-order retinal cells, most likely amacrine cells.
The corneal ERG has long been known as a useful tool for objective assessment of retinal function. The ERG, which can be measured non-invasively at the corneal surface, is a compound field potential generated by various retinal cell types whose relative contributions depend on the stimulus conditions. For example, under dark-adapted conditions the ERG response to a bright stimulus flash manifests an a-wave and b-wave that primarily reflect the activity of photoreceptors (Penn & Hagins 1969; Baylor et al. 1984) and depolarizing bipolar cells (Stockton & Slaughter 1989; Robson & Frishman 1995), respectively. It is thought that these cell types dominate the ERG because they are orientated radially, and consequently so too are the currents generated by their light responses. By contrast, although third-order retinal neurons are known to influence the ERG (Stockton & Slaughter 1989; Robson & Frishman 1995; Robson et al. 2003), their contribution to the standard scotopic full-field ERG has proven more difficult to measure. Responses to very dim flashes under dark-adapted conditions or to moderately bright flashes under light-adapted conditions, however, may have greater utility in this regard.
For example, the dark-adapted ERG response to very weak flashes in cats, monkeys, humans, mice and rats is dominated by a negative potential thought to be generated post-receptorally (Wakabayashi et al. 1988; Bush et al. 1995); more specifically, within the innermost retinal layers proximal to the bipolar cells (Sieving et al. 1986; Frishman & Steinberg 1989b; Naarendorp & Sieving 1991; Robson & Frishman 1995; Naarendorp et al. 2001; Saszik et al. 2002b). This response has been termed the scotopic threshold response (STR) because it appears at the ERG threshold and is detectable only 0.5 log units above behavioural threshold in both humans (Sieving & Nino 1988; Frishman et al. 1996b) and rats (Naarendorp et al. 2001).
The precise generators of the STR, however, are still not completely understood, and they may be somewhat species dependent. For example, the STR is reduced by induction of experimental glaucoma in the non-human primate (Frishman et al. 1996a), whereas surprisingly, ONTx in cat and human had little effect on the STR (Sieving 1991). More recently, Saszik et al. (2002b) required a minimum of three subcomponents to model adequately the intensity response function of the mouse scotopic ERG near threshold, including two small, sensitive positive potentials generated in proximal retina.
A similarly slow negative potential generated by inner retinal activity has also been shown to contribute to the primate photopic ERG. Since studies by Viswanathan et al. (1999, 2000) demonstrated that the photopic negative response (PhNR) was sensitive to TTX and experimental glaucoma in monkeys, interest in the PhNR as another possible measure of GC function in human glaucoma has grown (Colotto et al. 2000; Cursiefen et al. 2001; Drasdo et al. 2001; Viswanathan et al. 2001). However, to date, little is known about the photopic ERG of the rat, nor of the specific contributions made by GCs to the rat full-field ERG. These gaps need to be addressed because use of the rat in experimental models of glaucoma, retinal ischaemia and neuroprotection of GCs continues to grow (Goldblum & Mittag 2002) and the ERG remains the measure of choice for assessment of retinal function in these models.
The purpose of the present study was to determine the contributions made by retinal GCs to the full-field flash ERG of the rat. This was approached by longitudinal comparison of the ERG from baseline to 9 weeks following intraorbital ONTx (Domenici et al. 1991). Additional studies were performed using intravitreal injections of pharmacological agents in both control eyes and in eyes 9 weeks after ONTx. A brief account of some of these findings has been published in abstract form (Bui et al. 2003).
Methods
Subjects
All experimental methods and animal care procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Legacy Institutional Animal Care and Use Committee. Subjects were adult Brown-Norway rats between 10 and 12 weeks of age (180–260 g, Charles River Laboratories Inc., Willmington, MA, USA). The rats were maintained in a 12 h light (<40 lux)/12 h dark environment with normal rat chow and water available ad libitum.
Electroretinography
Animals were dark adapted overnight (= 12 h) and prepared for recording under dim red light (λ > 600 nm). Anaesthesia was initially induced with an intramuscular thigh injection of ketamine (55 mg kg−1, Ketaset, Fort Dodge Animal Health, Fort Dodge, IA, USA), xylazine (5 mg kg−1, X-ject E, Phoenix Scientific Inc. St Joseph, MO, USA) and acepromazine maleate (1 mg kg−1, Aceproject, Phoenix Scientific Inc., St Joseph, MO, USA). Supplemental anaesthesia was provided at 45 min intervals (ketamine: xylazine: acepromazine 30 : 2 : 1 mg kg−1, i.m). Pupils were fully dilated (≥ 4 mm) with one drop each of tropicamide (0.5 %, Alcon Laboratories Inc., Fort Worth, TX, USA) and phenylephrine (2.5 %, Bausch and Lomb Pharmaceuticals Inc., Tampa, FL, USA). Corneal anaesthesia with achieved with a single drop of proparacaine hydrochloride (0.5 %, Alcon Laboratories Inc.). Animals were lightly secured to a stage with velcro strips across the upper and lower back to ensure a stable, reproducible position for recording the ERG. Body temperature was maintained between 37 and 38 °C with a water heat pad (TP500 T/Pump, Gaymar Industries, Orchard Park, NY, USA) that was fixed to the top of the stage. Sufficient depth of anaesthesia was sensitively monitored online by inspection of the ERG signal baseline; during pilot work it had been revealed that changes in the stability of eye position, as well as any increase in respiratory artefacts, whenever observed, were always more sensitive than tail-pinch responses or onset of whisker movements.
Following the recording session, which lasted 1.5–2.5 h, animals recovered on a warm water pad (Gaymar Industries Inc.). In all cases, animals recovered fully from anaesthesia, except when a terminal experiment was conducted (intravitreal injection). In total, animals in the longitudinal study of ONTx were anaesthetized six times (for surgery, and then 1, 2, 3, 4 and 9 weeks post-operatively). At the end of experimentation animals were killed using an i.p injection of pentobarbital sodium and phenytoin sodium (400 : 40 mg kg−1 Euthasol™, Delmarva Laboratories, Inc., Midlothian, VA, USA). Some animals were perfused for tissue harvest, which is described in greater detail below.
Full-field ERGs were recorded using a UTAS-E3000 system (LKC Technologies, Gaithersburg, MD, USA) simultaneously from both eyes with custom-made silver chloride electrodes. The tip of the active electrode was placed at the corneal apex and referenced to a ring-shaped electrode placed against the scleral conjunctiva around the equator of the eye. A platinum electrode (Grass, West Warwick, RI, USA) placed in the tail served as ground. Eyes were lubricated after electrode placement and periodically throughout the session with 1.0 % carboxymethylcellulose sodium (Celluvisc, Allergan, Irvine, CA, USA). Simultaneous recording effectively halved the recording time and allowed ERGs to be obtained from the control and treated eyes under identical states of anaesthesia and adaptation.
Following electrode placement, a minimum of 10 min additional dark adaptation was allowed before recording. Signals were recorded with band pass settings of 0.3–30 Hz for the STR response and 0.3–500 Hz for all other responses. Signals were digitized at 1 kHz and 2 kHz for STRs and all other responses, respectively.
Stimuli were brief white flashes (xenon arc discharge, C.I.E. x = 0.32, y = 0.33) delivered via a Ganzfeld integrating sphere (UTAS-3000, LKC Technologies). Stimulus intensities were measured using a calibrated photometer (Spectra Pritchard PR-1980B, Photo Research, Chatsworth, CA, USA) with a (human) scotopic luminosity filter in place. STR responses were obtained for flash intensities ranging from −6.64 to −3.30 log (cd s) m−2 in 0.2 log unit increments, by averaging 20–60 responses per intensity (60 for the dimmest and 20 for the higher intensities), with an interstimulus interval of 2 s. Scotopic ERGs obtained for all intensities above −3.30 log (cd s) m−2 were recorded as single flash responses. For stimulus intensities between −3.04 and 2.72 log (cd s) m−2 the interval between flashes was progressively lengthened from 10 to 120 s to allow complete recovery of b-wave amplitude. After completion of the scotopic ERG intensity series, animals were light adapted for 15 min to a steady white background (150 cd m−2, x = 0.44, y = 0.41). Light-adapted flash responses were recorded for intensities between 0.97 and 2.72 log (cd s) m−2 in 0.25 log unit increments. Each record was an average of 20 responses obtained with a 2 s interstimulus interval.
Surgical procedure
Animals were randomly assigned to either the ONTx (n = 8) or the sham-operated (n = 8) group. In all cases, the operation was randomly assigned to one eye, and the contralateral eye served as an untreated control. Surgery was performed under general anaesthesia (ketamine, xylazine and acepromazine, 55 : 5 : 1 mg kg−1, i.m) with additional topical anaesthesia (0.5 % proparacaine HCl). For both groups, all surgical procedures were identical except for the final step. Following a lateral canthotomy, a 3 mm lateral incision was made along the superior conjunctiva about 1 mm posterior to the limbus. The superior rectus muscle was resected and the globe was rotated downward using forceps to grip the anterior portion of the rectus muscle and its tendon. The bulbar fascia was removed by blunt dissection along an anterior–posterior direction, using the superotemporal and superonasal vortex veins as guides, until the optic nerve sheath was reached at the posterior pole. Once the optic nerve was clearly visualized a longitudinal incision of 0.5–1 mm was made in the superior aspect of the meningeal sheath. The optic nerve was gently lifted up and away from the posterior ciliary artery and vein, which are attached to the optic nerve sheath inferiorly (Morrison et al. 1999; Bhutto & Amemiya 2001). The optic nerve was transected approximately 0.5–1 mm behind the globe using a pair of fine Vannas scissors, without damaging the ocular blood supply (Vidal-Sanz et al. 1987). Completeness of ONTx was confirmed by visualization of the posterior end of the stump in cross-section. Normal retinal perfusion was confirmed with direct ophthalmoscopy immediately following the operation and after each ERG recording session. In the sham-operated group, the surgical procedure was identical, up to and including the optic nerve sheath incision; however, in these animals the optic nerve was not transected. Post-operative cycloplegia was achieved using one drop of 1.0 % atropine sulphate (Bausch and Lomb Pharmaceuticals, Inc.). Post-operative analgesia was provided with i.m injections of buprenorphine hydrochloride (0.075 mg kg−1, Buprenex(tm), Reckitt and Colman Products, Richmond, VA, USA) every 8 h for 2–3 days. Lastly, eyes were dressed with a broad-spectrum antibiotic ointment (bacitracin and polymixin B sulphate, AK-POLY-BAC™, Akorn Inc., Buffalo Grove, IL, USA), which was re-applied twice daily for 4 days.
Intravitreal injections
Pharmacological agents or vehicle were delivered as a 2.5–5 μl aliquot into the vitreal chamber using a 30 gauge needle attached to a 10 μl Hamilton microsyringe (Hamilton Company, Reno, NV, USA) via a section of polyethylene tube. Another short section of polyethylene tube was used as a guard to restrict the effective needle length to 1.5 mm. In cases where a single pharmacological agent was delivered, a volume of 2.5 μl was injected. When two agents were injected in combination, the volume was increased to 5 μl to ensure adequate delivery. Dureau et al. (2001) have shown that injected volumes of up to 5 μl provide good reproducibility and minimize loss of solution. The needle was inserted through the sclera superiorly, ∼1 mm behind the limbus, at an angle of 45° to avoid contact with the lens. Data were excluded if opacification of the lens was detected. Pharmacological agents, TTX (6 μm), GABA (10 mm), NMDA (0.8 mm), CNQX (200 μm), D-AP7 (200 μm), L-(+)-2-amino-4-phosphonobyturic acid (L-AP4, 2 mm) and PDA (5 mm), were obtained from Sigma (Sigma Chemical Co., St Louis, MO, USA). All agents, with the exception of CNQX, were diluted in balanced salt solution (BSS), and equalized approximately to pH 7.4 using 1 m hydrochloric acid and 1 m sodium hydroxide (Sigma Chemical Co.). In the case of CNQX the vehicle used was dimethyl sulphoxide (Sigma Chemical Co.). The concentrations given above represent the estimated final vitreal concentrations, calculated by assuming an average vitreous chamber volume of 40 μl for the rat (Hughes 1979; Dureau et al. 2001).
Pharmacological experiments were performed on 2–5 naive control animals with each drug or drug combination as follows: TTX, n = 5; GABA, n = 4; NMDA, n = 4; CNQX, n = 4; D-AP7, n = 3; CNQX/D-AP7, n = 3; L-AP4, n = 2; PDA, n = 3; L-AP4/PDA, n = 2. Additionally, at 9 weeks following ONTx, two animals had intravitreal injections of TTX (6 μm). The effect of control injections was assessed using 5.5 μl of vehicle alone (BSS, n = 3).
Perfusion, tissue collection and preparation
Nine weeks following ONTx, animals were deeply anaesthetized and transcardially perfused with 4 % paraformaldehyde in 0.1 m phosphate buffer (∼200 ml) lasting ∼10 min. Following perfusion, both eyes were enucleated, a 4 mm incision was made in each cornea and fixation was continued overnight in chilled 4 % paraformaldehyde. Eyes were transferred to PBS following manual removal of the extraocular tissues. After a series of three washes in 0.1 m phosphate buffer the eyes were processed and placed in paraffin blocks. Vertical longitudinal sections were cut (6 μm) and those containing the optic nerve were stained (haematoxylin and eosin, H & E) for light microscopy (DMRXE, Leica Microsystems, Heidelberg, Germany). The total number of nuclei in the ganglion cell layer of each section was counted manually by a masked observer. Digital photographs of retinal sections were acquired adjacent to the optic nerve head both superiorly and inferiorly (Retiga 1300 digital camera and QCapture software, Q Imaging, Burnaby, BC, Canada).
Data analysis
ERG amplitudes were measured at fixed times after the stimulus flash. Criterion times were chosen to correspond with the peak (120 ms) and trough (220 ms) of control responses to dim flashes. For responses to brighter flashes, an 8 ms criterion time was used to measure a-wave amplitude. The amplitude of the photopic b-wave was measured using a 50 ms criterion time. The amplitudes of scotopic and photopic OPs were measured after raw data were band-pass filtered (−3 dB at 50 and 280 Hz) by summing the root mean square (RMS) over the entire OP complex.
Statistics
Analysis of variance (anova; Prism, v3.02, GraphPad Software Inc., San Diego, CA, USA) was applied to test the various null hypotheses, which in general, could be stated as: no effect of experimental treatment. Repeated measures ANOVA were used to test the significance of ERG differences between ONTx and sham-operated groups across the longitudinal follow-up period. Two-way ANOVA (ERG versus treatment and intensity) was used to evaluate individual experimental effects (e.g. ONTx at week 4, or TTX). In all cases, the alpha level was adjusted to 0.01 to correct for multiple comparisons (i.e. to limit type-two errors given that seven ERG parameters were evaluated after each experiment). For cases in which the anova result indicated a significant interaction between treatment and intensity, Bonferroni post hoc tests were used to evaluate amplitude differences among individual intensities.
Results
ERGs in control eyes
Figure 1A shows representative normal rat ERG responses (thin traces) to stimulus flashes of increasing intensity. The lowest intensity for which reliable responses could always be recorded (i.e. that were larger than noise-only records) was −6.64 log (cd s) m−2. The responses near scotopic ERG threshold always consisted of a positive potential followed by a slow negative component, respectively, known as the pSTR and nSTR. The positive and negative components had their peak amplitudes at ∼120 ms and ∼220 ms, respectively. Exclusively negative waveforms were never observed, not even at threshold [−6.64 log (cd s) m−2]. With increasing stimulus intensity, both the pSTR and nSTR grew in amplitude. The amplitude of the nSTR saturated at approximately −5.55 log (cd s) m−2, whereas the positive component continued to grow. The implicit time of both positive and negative components began to lengthen for flash intensities above −5.36 log (cd s) m−2. This intensity probably represents the point at which the bipolar cell component (PII) begins to influence the ERG waveform (Robson & Frishman 1995, 1998). At higher stimulus intensities (from ∼−4 to −2 log (cd s) m−2) the b-wave, which peaked at 135 ms, dominated the ERG.
Figure 1. The effect of optic nerve transection (ONTx) on the rat full-field ERG.
Dark- and light-adapted ERG waveforms 4 weeks following ONTx from the transected eye (bold traces) and the control eye (thin traces) for stimuli of various intensities. A, responses to the dimmest stimulus intensities [−6.64 to −3.51 log (cd s) m−2]. B, scotopic responses for stimulus intensities from −2.36 to 2.22 log (cd s) m−2. C, isolated scotopic OPs. D, photopic ERG responses. E, isolated photopic oscillatory potentials (OPs).
As shown in Fig. 1B, the emergence of a shallow a-wave was consistently observed at about −2 log (cd s) m−2, i.e. approximately 4.6 log units above ERG threshold. For these and higher intensities, the OPs became more prominent on the ascending limb of the b-wave and the b-wave implicit time shortened. Saturation of the trough-to-peak b-wave amplitude (1812 ± 75 μV) occurred at 1.72 log (cd s) m−2. Saturation of the maximal a-wave amplitude (736 ± 26 μV) occurred at approximately 2.72 log (cd s) m−2 (note, a-wave amplitudes at 3.22 were not larger than at 2.72 log (cd s) m−2 but data for the former are not shown as they were collected in only about half of the animals). Figure 1C shows that the average implicit time of the isolated scotopic OPs altered from 50 ms at −2.36 log (cd s) m−2 to 30 ms at 2.22 log (cd s) m−2. Over the same intensity range, scotopic OP amplitude grew approximately four-fold.
Figure 1D shows representative light-adapted, full-field flash responses for control rats (thin traces). A positive component (b-wave) dominated the photopic response, which grew in amplitude (to a maximum of ∼300 μV) and became broader as intensity increased. Photopic b-wave implicit time increased from 55 ms at 1.22 log (cd s) m−2 to 70 ms at the highest intensity [2.72 log (cd s) m−2]. Even with the most intense stimuli only a small photopic a-wave (<15 μV) could be observed for flashes on this background. As shown in Fig. 1E, the photopic OPs grew in amplitude with increasing stimulus intensity; however, unlike scotopic OPs, the implicit times of photopic OPs changed minimally with intensity.
ERGs after ONTx
The bold traces in Fig. 1 show ERG responses 4 weeks post-ONTx in one representative animal (thin traces show control responses from the fellow eye described above). Figure 1A shows that ONTx reduced both pSTR and nSTR components, leaving only a small residual negative potential, which peaked at 160 ms. This remnant negativity grew with intensity and saturated at approximately −6.04 log (cd s) m−2. Between −5.55 and −5.36 log (cd s) m−2 a positive component began to appear in the waveform, and by −5.12 log (cd s) m−2 a clear positive peak at 135 ms was discernible. This positive potential emerged at about the same intensity that the positive potential with the slower peak time, PII, began to influence control responses. The amplitude of this positive component grew rapidly and became similar to the control eye amplitude (thin traces) over the top 4 log units of the stimulus intensity range (Fig. 1B, above −1.22 log (cd s) m−2). Figure 1B shows that ONTx had no appreciable effect on scotopic ERG a-waves or b-waves over this intensity range. Figure 1C suggests that ONTx enhanced scotopic OP amplitudes between −2.36 and −1.22 log (cd s) m−2; however, above −1.22 log (cd s) m−2, OP amplitudes were similar between the two eyes. Figure 1D shows that ONTx reduced the amplitude of the photopic b-wave for all intensities measured, whereas Fig. 1E suggests that ONTx reduced photopic OP amplitudes only at 2.22 and 1.72 log (cd s) m−2.
Intensity response functions: scotopic ERG
Figure 2 demonstrates the relationship between stimulus intensity and ERG amplitude at fixed times following the stimulus. The data in Fig. 2 are displayed as the group mean (± s.e.m.) for control eyes (open circles, n = 8) and ONTx eyes at 4 weeks post-transection (filled circles, n = 8). For dim flashes, response amplitude of the pSTR and nSTR was measured at 120 and 220 ms, respectively. The 120 ms criterion was extended throughout the entire intensity range to characterize growth of the b-wave as well. The results shown in Fig. 2A and D confirm the findings for the STR described in Fig. 1A: the amplitudes of the pSTR (Fig. 2A) and nSTR (Fig. 2D) were markedly reduced 4 weeks after ONTx (pSTR, F = 83.6, P < 0.0001; nSTR, F = 75.1, P < 0.0001). To visualize the effects of ONTx on the pSTR more clearly, these data were plotted on log-linear coordinates in Fig. 2B, which shows that ONTx completely abolished the pSTR for intensities below −5.12 log (cd s) m−2.
Figure 2. Intensity-response functions in control and transected eyes.
ERG amplitude (group mean ± s.e.m.) versus stimulus intensity for ONTx eyes (filled symbols, n = 8) and fellow control eyes (open symbols, n = 8) 4 weeks after surgery. A, scotopic (circles) and photopic (squares) ERG amplitudes measured at fixed criterion times of 120 ms and 50 ms after the stimulus flash, respectively. B, pSTR amplitude shown on linear-log coordinates. C, isolated OP amplitude. D, nSTR amplitude (220 ms criterion time). E, A-wave amplitude (8 ms criterion time).
The group data shown in Fig. 2E confirm that ONTx did not significantly affect scotopic a-wave amplitudes at 4 weeks (F = 1.0, P = 0.31). The effect of ONTx on the scotopic b-wave was relatively small, but significant (Fig. 2A, F = 62.8, P < 0.0001); above −3.0 log (cd s) m−2 the b-wave was smaller by 10–15 %. By contrast, scotopic OPs (Fig. 2C, F = 7.3, P = 0.008) were actually enhanced after ONTx (by 20–40 %, below ∼0.72 log (cd s) m−2).
Intensity response functions: photopic ERG
Figure 2A also shows that ONTx caused a significant reduction in the photopic b-wave amplitude across the full range of measured intensities (filled squares, F = 156.7, P < 0.0001). Photopic OPs are plotted in Fig. 2C (squares) alongside those for scotopic OPs (circles, described above). Here it is shown more clearly by the group data, than by the individual example in Fig. 1C, that ONTx (filled squares) led to a significant reduction in photopic OP amplitude (F = 77.6, P < 0.0001). The effect of ONTx on photopic OPs was largest for intermediate stimulus intensities (treatment * intensity, F = 4.0, P = 0.0006).
Control data: ERGs after sham surgery
ERGs for one representative sham-operated animal, 4 weeks after surgery, are shown in Fig. 3A and the group data (n = 8) are reported in Fig. 3B–E as intensity-response functions. Figure 3A shows that ERGs recorded from the sham-operated eye (bold traces) and control eye (thin traces) were similar, if not identical, across the entire spectrum of stimulus intensity. Figure 3B–E show that no significant differences were observed between the sham-operated group (filled symbols) and their fellow control eyes (open symbols) for any of the measured ERG parameters: pSTR (Fig. 3B, circles, < −3.30 log (cd s) m−2, F = 3.1, P = 0.08), nSTR (Fig. 3C, F = 0.9, P = 0.35), scotopic a-wave (Fig. 3E, F = 1.3, P = 0.25), scotopic b-wave (Fig. 3B, circles, > −3.30 log (cd s) m−2, F = 3.3, P = 0.07), scotopic OPs (Fig. 3D, circles, F = 0.1, P = 0.73), photopic b-wave (Fig. 3B, squares, F = 5.8, P = 0.02) and photopic OPs (Fig. 3D, squares, F = 3.1, P = 0.08).
Figure 3. The effect of sham operation on the rat full-field ERG.
ERG responses for sham-operated and fellow control eyes. A, representative ERG waveforms from the sham-operated (bold traces) and control eye (thin traces) for a single animal 4 weeks after surgery. B, scotopic (circles) and photopic (squares) ERG amplitudes (group mean ± s.e.m.) for sham-operated (n = 8, filled symbols) and fellow control eyes (n = 8, open symbols) 4 weeks after surgery. C, nSTR amplitude. D, scotopic (circles) and photopic (squares) OP amplitudes. E, a-wave amplitude. Details as in Fig. 2.
Time course of ERG changes after ONTx
In rodents, nearly all GCs die and degenerate 1–4 weeks after ONTx (Thanos et al. 1993; Villegas-Perez et al. 1993; Berkelaar et al. 1994; Mansour-Robaey et al. 1994), although the precise time course can vary depending on the target projection, or cell type (Villegas-Perez et al. 1993; Russelakis-Carneiro et al. 1996), and the distance between the injury and the soma (Domenici et al. 1991). Therefore, longitudinal data on retinal function were collected at 1, 2, 3, 4 and 9 weeks after ONTx and shown in Fig. 4. Results for the full ERG protocol were obtained at each time point, but for brevity, responses to the chosen intensities are sufficient to represent results for each of the major ERG subcomponents previously delineated. The scotopic a-wave, b-wave and OP amplitudes are reported for 2.22 log (cd s) m−2 flashes; the photopic b-wave and OPs for 2.72 log (cd s) m−2; the pSTR for −5.12 log (cd s) m−2; and the nSTR for −6.04 log (cd s) m−2. ERG amplitude data for ONTx (n = 8, filled symbols) and sham-operated eyes (n = 8, open symbols) are expressed relative to fellow eyes (mean percentage ± s.e.m.) as a function of time (weeks) after surgery.
Figure 4. Longitudinal effects of optic nerve transection on full-field ERG parameters.
ERGs were assessed at 1, 2, 3, 4 and 9 weeks after either ONTx (filled symbols, n = 8) or sham-operation (open symbols, n = 8). ERG amplitudes for the treated eye (either ONTx or sham operation) are expressed relative to the fellow control eye (group mean percentage ± s.e.m.). A, a-wave amplitude [2.22 log (cd s) m−2]. B, pSTR [upward triangles −5.12 log (cd s) m−2] and nSTR [downward triangles −6.04 log (cd s) m−2] amplitudes. C, scotopic (diamonds) and photopic (squares) b-wave amplitudes [2.22 log (cd s) m−2]. D, scotopic (diamonds) and photopic (squares) OP amplitudes [2.22 log (cd s) m−2].
The results in Fig. 4A suggest that surgical trauma, but not ONTx per se, caused a mild transient reduction of outer retinal function. That is, at 1 week, a-wave amplitude was equally reduced to about 84 ± 6 % of control by both ONTx and sham surgical procedures (no significant effect of treatment, Bonferroni post test week 1, t = 0.45, P > 0.05). During the second, third, fourth and ninth post-operative weeks the a-wave amplitude recovered to normal in both groups (>95 %). Overall, there were no significant differences between ONTx and sham-operated groups for a-wave amplitudes (F = 0.59, P = 0.45), nor for their pattern of change over time (treatment * time; F = 3.1, P = 0.75).
Figure 4B shows that 1 week after ONTx both pSTR and nSTR amplitudes were reduced to 24 ± 8 % and 41 ± 8 %, respectively, of fellow (control) eye values. By contrast, pSTR (91 ± 11 %) and nSTR (91 ± 9 %) amplitudes in sham-operated eyes were similar to controls, despite the mild photoreceptor deficit described above. The amplitude of the pSTR continued to decline after ONTx, reaching maximal loss at 2 weeks after surgery (−3 ± 9 % of control, t = 9.0, P < 0.001); however, the effect of time after surgery was not significant (treatment * time, F = 1.3, P = 0.30). The nSTR amplitude was significantly lower in the ONTx group (ONTx versus sham, F = 123.4, P < 0.0001) and declined to reach maximal attenuation 9 weeks post-transection (26 ± 5 %); however, again time post-surgery was not a significant factor (treatment * time, F = 0.23, P = 0.67). In the sham-operated group both pSTR and nSTR amplitudes recovered nearly completely (average for weeks 2–9, 94 ± 4 % and 97 ± 6 %, respectively).
Figure 4C summarizes the time course for the scotopic (diamonds) and photopic (squares) b-wave. Although there was no significant effect of ONTx on the scotopic b-wave compared with the sham group over time (F = 1.9, P = 0.18), ONTx did significantly reduce photopic b-wave amplitude (F = 28.8, P < 0.0001). Similarly, the photopic OPs (Fig. 4D) were significantly reduced after ONTx as compared with sham-operated controls (F = 13.1, P = 0.001); by contrast, the scotopic OPs showed a more transient change (F = 15.3, P = 0.0005) – having decreased through the second week (t = 3.1, P < 0.05) they recovered to normal by week 9.
Histological findings after ONTx
Representative histological sections are shown in Fig. 5. Nine weeks after ONTx (Fig. 5A), the density of cell bodies (arrowheads) in the GC layer was reduced to nearly half that observed in fellow control eyes (Fig. 5B) at equivalent eccentricities. Expressed as a percentage of the fellow eye, the total number of nuclei remaining in the GC layer 9 weeks after ONTx was 61.2 ± 9.8 % (F = 6.0, P = 0.03). The thickness of the nerve fibre layer (NFL) was also reduced as compared with control eyes, whereas all other retinal layers appeared to be intact and similar to control eyes. In sham-operated eyes (Fig. 5C), the number of cell bodies in the GC layer and the thickness of the NFL were both similar to fellow control eyes (Fig. 5D). The total number of nuclei remaining in the GC layer 9 weeks after sham surgery was 101.1 ± 10.2 %. Similarly, at 9 weeks after sham surgery the histological appearance of all other retinal layers was also indistinguishable from fellow control eyes.
Figure 5. Histological findings after ONTx and sham-operations.
Histological sections sampled approximately 1 mm from the inferior edge of the optic nerve 9 weeks after: A, ONTx; B, fellow control eye; C, sham-operated eye; D, sham fellow control eye. NFL, nerve fibre layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; and Ph, photoreceptor layer. Arrowheads point to cell bodies in the GCL. Scale bar = 100 μm.
ERGs after TTX
TTX did not completely abolish the STR in naive animals, but it did alter the waveform considerably (Fig. 6). After TTX application (bold traces), a small (∼2.5 μV) negative component with an implicit time of 110 ms became apparent as the first feature to evolve after the stimulus. A positive component also persisted after TTX, but this was smaller and slower than the control pSTR, with an implicit time of ∼160 ms. At an intensity of −5.12 log (cd s) m−2 a positive component began to grow in amplitude and eventually merged with the scotopic b-wave, although its peak time (165 ms) was initially much slower than that of control waveforms. Generally, the amplitude of this positive component exceeded that of fellow control eyes at intermediate intensities (−3.57 to −1.22 log (cd s) m−2, also see Fig. 7A). At the brightest intensities (above −1.22 log (cd s) m−2) TTX had negligible effects on either scotopic a-waves or scotopic b-waves (Fig. 6B). However, the scotopic OPs were generally smaller and slower after TTX, at least for all intensities below 2.22 log (cd s) m−2 (Fig. 6C). The effects of TTX on the photopic ERG were greater than the effects of ONTx. In particular, the b-wave was reduced to less than half the control amplitude (Fig. 6D) and there was a near total loss of photopic OPs (Fig. 6E).
Figure 6. The effect of TTX on the full-field ERG of control animals.
Dark- and light-adapted ERG waveforms 60 min after intravitreal injection of TTX (6 μm); injected eye (bold traces), control eye (thin traces). A, responses to the dimmest stimulus intensities [−6.64 to −3.51 log (cd s) m−2]. B, scotopic responses for stimulus intensities from −2.36 to 2.22 log (cd s) m−2. C, isolated scotopic OPs. D, photopic ERG responses. E, isolated photopic oscillatory potentials (OPs).
Figure 7. The effect of TTX on intensity-response functions.
ERG amplitude (group mean ± s.e.m.) versus stimulus intensity after intravitreal injection of TTX (filled symbols, n = 5) and fellow control eyes (open symbols, n = 5). A, scotopic (circles) and photopic (squares) ERG amplitudes. B, pSTR amplitude shown on linear-log coordinates. C, isolated OP amplitude. D, nSTR amplitude. E, a-wave amplitude. Other details as in Fig. 2.
Intensity-response functions after TTX
Figure 7A and B confirm that TTX caused a significant reduction of pSTR amplitude (F = 8.3, P = 0.005). The pSTR data from Fig. 7A were plotted in Fig. 7B on log-linear coordinates to show that after TTX, pSTR amplitudes (measured at 120 ms) actually had negative values until flash intensity reached −4.60 log (cd s) m−2. The nSTR was also reduced after TTX (Fig. 7D, F = 142.3, P < 0.0001), whereas a small but significant enhancement of scotopic b-wave amplitudes was observed (Fig. 7A, circles, F = 12.4, P = 0.0006). No significant change was detected post-TTX for either scotopic a-wave (Fig. 7E, F = 0.8, P = 0.37) or OP amplitudes (Fig. 7C, circles, F = 4.2, P = 0.04). By contrast with the relatively subtle effects of ONTx, application of TTX led to a large reduction of both the photopic b-wave (Fig. 7A, squares, F = 1270, P < 0.0001) and OP amplitudes (Fig. 7C, squares, F = 506, P < 0.0001).
Control data: ERGs after vehicle injection
To ensure that the injection technique, volume or vehicle used did not contribute to the functional changes described above, the ERG was assessed following intravitreal injection of 5.5 μl vehicle (n = 3). In all three cases the effect of the vehicle injection was negligible after 10–20 min, during which the recovery from a minor (presumed) volume effect could be tracked. Figure 8 shows representative waveforms from a vehicle-injected eye (bold traces) and its fellow control eye (thin traces) 30 min after injection (actually 30–90 min, given the 1 h duration of the ERG protocol). Figure 8 shows that ERGs recorded under both scotopic and photopic conditions were unaffected by the intravitreal injection procedure itself.
Figure 8. Intravitreal injection control experiment.
ERG responses from the vehicle-injected eye (bold traces) and control eye (thin traces) for one representative animal.
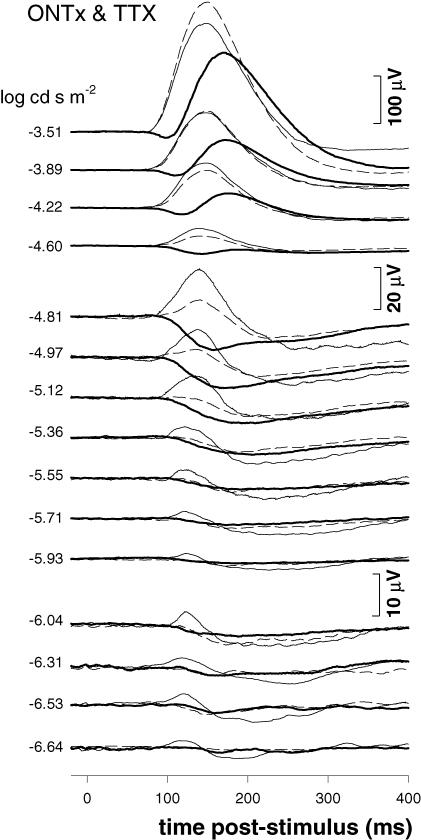
ERGs after ONTx and TTX
The STR was not completely abolished even 9 weeks after ONTx, which suggests that retinal neurons other than GCs may contribute a negative potential to the rat ERG near threshold. Previous studies in other species have suggested that amacrine cells may be one of the major sources of the STR (Sieving 1991; Korth et al. 1994). The dominant population of amacrine cells in the rat, the narrow-field AII amacrine cells, are known to be a critical link in the rod pathway throughput and to be one of the few other cell types that produce TTX-sensitive action potentials (Boos et al. 1993). TTX-sensitive Na+ channel activation in rat retina may also contribute to, but is not required for, transmitter (GABA) release by wide-field A17 amacrine cells (Hartveit 1999). The following experiment was designed to evaluate what contributions, if any, are made to the STR by TTX-sensitive retinal signals (spikes) in cells other than GCs. The effects of intravitreal TTX injection were evaluated in two animals each 9 weeks after ONTx.
Figure 9 presents the results for one of these animals, which was a good replica of the other. Dim scotopic ERGs from the control eye (thin traces) are superimposed on the records from the ONTx eye 1 h after TTX injection (bold traces). For reference, the ERGs from the same experimental eye collected 4 weeks post-ONTx (i.e. 5 weeks prior to TTX injection) are also shown (dashed traces). The data in Fig. 9 show that there was no additional effect of TTX after ONTx for the lowest flash intensities (−5.36 log (cd s) m−2 and below). For intensities above −5.12 log (cd s) m−2 the difference between ONTx alone and ONTx/TTX became more apparent. Importantly though, for these brighter intensities and above, scotopic and photopic ERG responses in ONTx/TTX were indistinguishable from those observed after TTX alone (Fig. 6), suggesting that graded responses in GCs contributed little to the rat ERG far above scotopic threshold.
Figure 9. The combined effect of ONTx and TTX on the rat full-field ERG.
TTX was injected 9 weeks after ONTx (n = 2). Scotopic ERG responses from the control eye (thin traces) are superimposed on the records from the TTX-treated ONTx eye (ONTx/TTX, bold traces). The ERGs from the same experimental eye collected 4 weeks post-ONTx are also shown (dashed traces).
ERG response with other inhibitors of inner retinal activity
Given that some small component of the rat STR persisted after ONTx plus TTX, we hypothesized that its source may arise from graded responses of inner retinal neurons other than GCs, perhaps amacrine cells for example. Previous studies in cats, rats and mice have shown that the STR was selectively attenuated by pharmacological agents that block inner retinal light responses, including GABA, glycine and NMDA (Sieving et al. 1986; Naarendorp & Sieving 1991; Robson & Frishman 1995; Naarendorp et al. 2001; Saszik et al. 2002b). The effects of these and other agents are presented in Figs 10–14.
Figure 10. The effect of GABA on the rat full-field ERG.
ERG responses for GABA-injected (10 mm) and fellow control eyes. A, representative ERG waveforms from the GABA-injected (bold traces) and control eye (thin traces) for a single animal. B, scotopic (circles) and photopic (squares) ERG amplitudes (group mean ± s.e.m.) for GABA-injected (n = 4, filled symbols) and fellow control eyes (n = 4, open symbols). C, pSTR amplitude. D, scotopic (circles) and photopic (squares) OP amplitudes. E, nSTR amplitude. F, a-wave amplitude. Details as in Fig. 2.
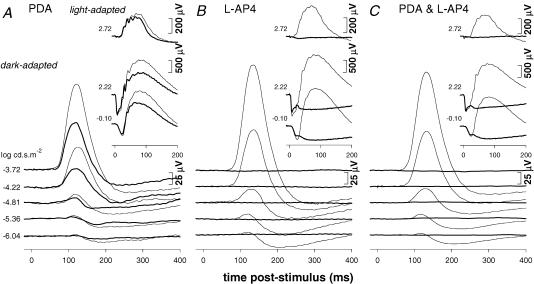
Figure 14. The effect of PDA and L-AP4 on the rat full-field ERG.
A, ERG responses 60 min after injection of PDA (5 mm, bold traces); ERGs for fellow control eye (thin traces). B, ERG responses 60 min after injection of L-AP4 (2 mm, bold traces); ERGs for fellow control eye (thin traces). C, ERG responses 60 min after injection (bold traces) of the combination of PDA (5 mm) and L-AP4 (2 mm); fellow control eye ERGs (thin traces).
Figure 10 shows that the primary effects of GABA (10 mm, n = 4) were attenuation of the nSTR (Fig. 10A and E) and reduction of OP amplitudes (Fig. 10A and D). Interestingly, the amplitude of the pSTR was unaffected by GABA (Fig. 10A and C). Scotopic a-wave (Fig. 10A and F) and b-wave amplitudes (Fig. 10A and B) were also unchanged after GABA, as was the photopic b-wave (Fig. 10A and B, squares).
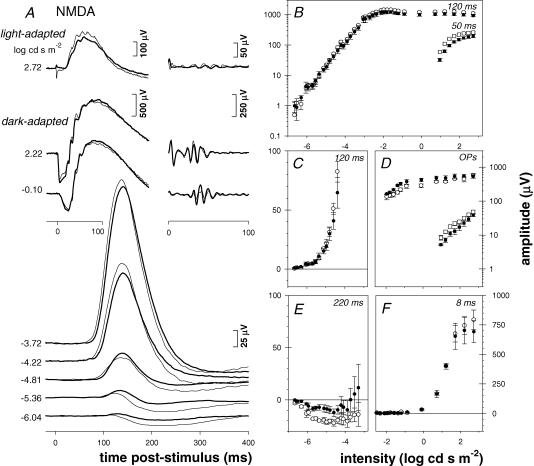
The effects of NMDA (0.8 mm, n = 4, Fig. 11) were similar to GABA except that in addition to suppression of the nSTR and OPs, the photopic b-wave was also reduced by ∼30 % (Fig. 11A and B, squares). Suppression of the nSTR after NMDA (Fig. 10A and E), although not complete, was slightly greater than that after GABA. Thus the effect also manifested as an increase in the pSTR implicit time from 115 ms to 130 ms, a larger shift than was observed after GABA. But like GABA, NMDA did not alter the amplitude of the pSTR, scotopic a-wave or b-wave (Fig. 11C, F and B, circles, respectively).
Figure 11. The effect of NMDA on the rat full-field ERG.
ERG responses for NMDA-injected (0.8 mm) and fellow control eyes. A, representative ERG waveforms from the NMDA-injected (bold traces) and control eye (thin traces) for a single animal. B, scotopic (circles) and photopic (squares) ERG amplitudes (group mean ± s.e.m.) for NMDA-injected (n = 4, filled symbols) and fellow control eyes (n = 4, open symbols). C, pSTR amplitude. D, scotopic (circles) and photopic (squares) OP amplitudes. E, nSTR amplitude. F, a-wave amplitude. Details as in Fig. 2.
The effects of CNQX (200 μm), an antagonist of the AMPA/KA class of ionotropic glutamate receptor, were more complicated than those of GABA or NMDA. Although suppression of the nSTR was even stronger than that produced by NMDA or GABA, indeed nearly complete below −5.36 log (cd s) m−2 (Fig. 12A and E), there were also substantial effects on the scotopic b-wave (Fig. 12A and B, circles). For example, after CNQX the scotopic b-wave maximum amplitude was reduced by 39 ± 14 %. The effects on the photopic b-wave (Fig. 12A and B, squares), however, were small (14 ± 4 % reduction) in comparison with the scotopic b-wave. Similar to its effect on b-waves, CNQX reduced the scotopic OPs, particularly at higher flash intensities (Fig. 12A and D, circles), but had little effect on photopic OPs (Fig. 12A and D, squares). As anticipated, CNQX did not affect the scotopic a-wave (Fig. 12A and F).
Figure 12. The effect of CNQX on the rat full-field ERG.
ERG responses for CNQX-injected (200 μm) and fellow control eyes. A, representative ERG waveforms from the CNQX-injected (bold traces) and control eye (thin traces) for a single animal. B, scotopic (circles) and photopic (squares) ERG amplitudes (group mean ± s.e.m.) for CNQX-injected (n = 4, filled symbols) and fellow control eyes (n = 4, open symbols). C, pSTR amplitude. D, scotopic (circles) and photopic (squares) OP amplitudes. E, nSTR amplitude. F, a-wave amplitude. Details as in Fig. 2.
Whereas NMDA is thought to suppress light responses of third-order retinal neurons by maximally depolarizing their membranes, alterations in the ERG after NMDA application cannot be considered as specifically representative of signals mediated by NMDA-type ionotropic glutamate receptors. Therefore, we inhibited NMDA receptors using the selective NMDA receptor antagonist D-AP7 (Diamond & Copenhagen 1995). Figure 13A shows that D-AP7 (200 μm) alone had little effect on either scotopic or photopic ERG responses. This null effect was replicated in two other animals (data not shown). However, the combination of D-AP7 (200 μm) and CNQX (200 μm) resulted in greater attenuation of the scotopic b-wave (>50 %) as compared with CNQX alone (Fig. 12). Moreover, this combination also completely abolished the STR below −5.12 log (cd s) m−2 (Fig. 13B). The combination of the D-AP7/CNQX did not alter the scotopic a-wave (Fig. 13B).
Figure 13. The effect of D-AP7 and the combination of D-AP7/CNQX on the rat full-field ERG.
A, ERG responses 60 min after injection of D-AP7 (200 μm, bold traces); ERGs for fellow control eye (thin traces). B, ERG responses 60 min after injection (bold traces) of the combination of CNQX (200 μm) and D-AP7 (200 μm); fellow control eye ERGs (thin traces).
The glutamate receptor antagonists L-AP4 and PDA were used to block neurotransmission from photoreceptors to depolarizing (‘ON’) and hyperpolarizing (‘OFF’) bipolar cells, respectively (Stockton & Slaughter 1989; Sieving et al. 1994). The latter also blocks glutamatergic transmission to horizontal cells, and together L-AP4 and PDA isolate the photoreceptor contribution to the rat ERG. Figure 14A shows that PDA (5 mm, n = 3) alone reduced both the pSTR and the nSTR, but the greater effect was on the nSTR. At higher stimulus intensities, PDA also reduced the scotopic b-wave, as well as the photopic b-wave, but had minimal effects on the scotopic a-wave. As expected from their similar pharmacology, the effects of PDA alone were quite similar to those of CNQX (Fig. 12).
Inhibition of neurotransmission between photoreceptors and depolarizing bipolar cells with L-AP4 completely abolished the STR, as well as both the scotopic and the photopic b-waves (Fig. 14B). After L-AP4, the remaining waveform was dominated by a negative component (bold traces), whose leading edge closely matched the a-wave of the control eye waveforms (thin traces). This waveform is probably composed of both fast and slow PIII components (Green & Kapousta-Bruneau 1999; Karwoski & Xu 1999). A small cone-driven a-wave (∼15 μV) also remained after L-AP4. Figure 14C shows that the effect of the combination of PDA together with L-AP4 was qualitatively similar to that of L-AP4 alone.
Discussion
The purpose of the current study was to determine what contributions GCs make to the full-field ERG of the rat. The primary experimental approach was to examine changes in the ERG following complete ONTx. Interruption of axons along the optic nerve or optic tract leads to GC loss in all mammals studied (Quigley et al. 1977; Maffei & Fiorentini 1981; Allcutt et al. 1984; Misantone et al. 1984; Barron et al. 1986; Watanabe et al. 2001). A similar approach was previously used to reveal the dependence of the pattern-ERG, for example, on intact GC function in rat (Berardi et al. 1990) and other species (Maffei & Fiorentini 1981; Hollander et al. 1984; Maffei et al. 1985). The results of this study indicate that GC responses dominate the rat STR, both positive and negative components. The findings also show that GCs contribute to the generation of the rat photopic b-wave and OPs, yet appear to have little influence on the scotopic ERG a- and b-wave responses to brighter flashes, like those most commonly measured in other studies. Each of the major findings is discussed in further detail below.
Ganglion cells provide major contribution to the rat STR
In this study the rat STR was profoundly altered but not completely eliminated by ONTx. This result generally supports previous studies that found that the STR is generated by cells within the innermost retinal layers (Sieving et al. 1986; Frishman et al. 1988; Frishman & Steinberg 1989a). The STR was removed by the combination of L-AP4 and PDA (Fig. 14C), which is consistent with previous studies that used aspartate and/or L-AP4 (Wakabayashi et al. 1988; Frishman & Steinberg 1989a; Sieving & Wakabayashi 1991; Bush et al. 1995) to establish that there was no direct photoreceptoral contribution to the STR. That the STR was eliminated by L-AP4 alone (Fig. 14B) also confirms that it is generated by an ON-pathway circuit (Frishman & Steinberg 1989b), whose input is probably the rod bipolar cell (Yamashita & Wassle 1991; Euler et al. 1996).
However, with regard to whether GC responses per se contribute to the STR, previous studies have returned mixed results; taken together, they indicate substantial variation between species. For example, in cat and human, it is thought that most of the STR is generated by amacrine cell responses (Sieving 1991). By contrast, in monkey it has been argued that the STR depends more directly on intact GC function (Frishman et al. 1996a; Ahmed et al. 1999). This study suggests that the nSTR and, to even greater extent, the pSTR both depend on intact GC responses in rat. Indeed, the pSTR was reduced dramatically, perhaps even eliminated completely, by ONTx. Similar findings have been demonstrated by Saszik et al. (2002a) in mouse.
Additional evidence to support this conclusion derives from the close match between the time course of changes in the STR observed in this study (Fig. 4) and the pattern of GC loss found in anatomical studies of ONTx in rat (Thanos et al. 1993; Berkelaar et al. 1994; Mansour-Robaey et al. 1994). Although the precise time course of GC loss depends on the type (cut versus crush) and location of injury (distance from the GC soma) (Radius & Anderson 1978; Domenici et al. 1991; Villegas-Perez et al. 1993), in general, when ONTx is performed close to the eye in rats, greater than 90 % of GCs are lost within 4 weeks (Thanos et al. 1993; Berkelaar et al. 1994; Mansour-Robaey et al. 1994).
Another important consideration is the possibility of retrograde transynaptic degeneration. Amacrine cells in the inner nuclear layer and amacrine cells displaced into the GC layer were reported to be unaffected by ONTx near the eye in rats (Carter et al. 1987). However, Hollander et al. (1984) reported a small reduction of inner nuclear layer thickness 4 months after ONTx (near the chiasm) in cats. In the current study, the normal appearance of the inner nuclear layer and the presence of numerous cell bodies in the GC layer, which were probably displaced amacrine cells (Perry 1981; Perry et al. 1983), provide some evidence against substantial transynaptic degeneration 9 weeks after ONTx. Similarly, that the scotopic OPs were grossly intact, if not actually enhanced, also argues against major loss of amacrine cell function (Wachtmeister 1998).
STR remnant after ONTx
The remnant nSTR observed to persist even 9 weeks after ONTx probably arises from amacrine cell responses. Specifically, this study presents evidence that the residual nSTR is produced by graded amacrine cell light responses, rather than by action potentials that are known to occur in two of the major classes of rat amacrine cells (Boos et al. 1993; Hartveit 1999). For example, intravitreal injection of TTX 9 weeks after ONTx produced no additional change in the remnant nSTR (Fig. 9). Yet the STR was completely eliminated by the combination of D-AP7 and CNQX, antagonists of NMDA and AMPA/KA ionotropic glutamate receptors, respectively (Fig. 13). This combination would be expected to block nearly all glutamatergic neurotransmission beyond the rod bipolar cell (i.e. to all third-order retinal neurons) (Boos et al. 1993; Hartveit 1999; Menger & Wassle 2000). This finding also suggests that signal transmission solely via metabotropic glutamate receptors (Hartveit et al. 1995) is insufficient to generate an STR in rat, consistent with their proposed modulatory role within inner retinal circuits (Euler & Wassle 1998; Thoreson & Witkovsky 1999).
By contrast, when applied alone GABA (10 mm), NMDA (0.8 mm) or CNQX (200 μm) each reduced the amplitude of the nSTR, but did not significantly affect the amplitude of the pSTR. The findings with GABA were somewhat unexpected because GABA has been used to suppress completely the STR in cats (Naarendorp & Sieving 1991) and mice (Saszik et al. 2002b), although the concentration required to do so in mice [35 mm (Saszik et al. 2002a)] may be higher than that applied here. Perhaps differences between species and/or the concentration of GABA could explain the discrepant findings.
Incomplete suppression of the STR by NMDA was also somewhat surprising. Naarendorp et al. (2001) showed that the amplitude of a small positive potential that persisted after NMDA (0.1 mm) grew linearly with intensity at low stimulus intensities in the rat. They concluded that NMDA reduced contributions from third-order retinal neurons (including the pSTR) and unmasked linear growth of the b-wave just above its threshold. By contrast, Saszik et al. (2002b) reported that NMDA did not completely suppress the STR in mice, but rather left a small residual negative STR component. Again, species differences and/or concentration differences may account for some of these discrepancies.
The effects of NMDA, the prototypical agonist for the NMDA-type ionotropic glutamate receptor, probably manifest through depolarization of third-order neurons and possibly by interactions with other receptor types (Diamond & Copenhagen 1993; Yu & Miller 1996; Thoreson & Witkovsky 1999). NMDA receptor subunits have been localized on virtually all GCs and displaced amacrine cells, as well as a subset of amacrine cells in the inner nuclear layer (Hartveit et al. 1994; Fletcher et al. 2000). However, in the rat retina, the NMDA-type receptor is thought to play a relatively minor role in direct signal transmission to either amacrine cells (Boos et al. 1993; Hartveit 1999; Menger & Wassle 2000) or GCs (Massey & Miller 1990; Cohen & Miller 1994; Yu & Miller 1995), but perhaps rather more of a modulatory role, similar to that of metabotropic glutamate receptors (Euler & Wassle 1998; Thoreson & Witkovsky 1999). In the current study, the NMDA receptor antagonist D-AP7 had little effect on the rat ERG (Fig. 13A), and similar results were obtained with MK-801 (data not shown).
By contrast, the AMPA/KA receptor antagonists CNQX (Fig. 12) and PDA (Fig. 14A) blocked the majority of the nSTR and also a substantial proportion of the scotopic b-wave. It is well known that both AII and A17 amacrine cells receive the majority of their feed-forward excitatory input from rod bipolar cells via CNQX-sensitive AMPA/KA receptors (Boos et al. 1993; Hartveit 1999; Menger & Wassle 2000). Excitatory inputs to GCs are also thought to be dominated by AMPA/KA receptors as opposed to NMDA receptors (Boos et al. 1990; Massey & Miller 1990; Diamond & Copenhagen 1993; Cohen & Miller 1994; Velte et al. 1997). Thus, CNQX might have been expected to block the STR more completely, that is to block also the pSTR. One possible explanation is that AMPA/KA and NMDA receptor-mediated signals interact during generation of the pSTR (Diamond & Copenhagen 1993; Yu & Miller 1996), as combined antagonism of both receptor types led to complete block of the STR (including the pSTR, see above). The latter finding is consistent with results in cat (Robson & Frishman 1995).
The reduction of scotopic b-wave amplitude produced by AMPA/KA receptor antagonists CNQX and PDA was a reliable effect, but it is difficult to explain. One study reported decreased scotopic b-wave amplitudes in the connexin36 knock-out mouse (Cx36−/−), suggesting that loss of functional coupling between AII amacrine cells and ON cone bipolar could produce this effect (Guldenagel et al. 2001). However, others have found that scotopic b-wave amplitudes in Cx36−/− mice are similar to those of wild-type mice (Seeliger et al. 2003).
Classical (brighter flash) scotopic ERG in rat is unaffected by ONTx
The results of this study also indicate that scotopic ERG responses to brighter stimulus flashes [analogous to recommended standard scotopic ERG stimuli (Marmor & Zrenner 1998)] appeared to receive little influence from GCs. This finding is consistent with previous studies that showed ONTx had no effect on the scotopic full-field ERG in rats (Berardi et al. 1990), cats (Maffei & Fiorentini 1981; Hollander et al. 1984), non-human primates (Maffei et al. 1985) or humans (Dawson et al. 1982; Harrison et al. 1987; Sieving 1991). More recently, however, pharmacological studies in rabbit and salamander retina provided evidence that third-order neurons influence the scotopic b-wave (Dong & Hare 2000; Awatramani et al. 2001). In this study, ONTx had little effect on the scotopic b-wave, but TTX delayed the onset of the b-wave and increased its amplitude at intermediate light levels (Fig. 6, −5.12 to −3.57 log (cd s) m−2), even after ONTx (Fig. 9). The former is consistent with previously reported results in rabbit (Dong & Hare 2000) and salamander retina (Awatramani et al. 2001) and the latter suggests that these effects of TTX might be attributable to blockade of voltage-gated sodium currents in cells other than GCs, such as amacrine cells (Boos et al. 1993; Bloomfield 1996), interplexiform cells (Gustincich et al. 1997) or possibly retinal astrocytes (Barres et al. 1989).
ERG threshold of the rat
This study found that the threshold for detection of a reliable ERG response (−5 μV) in rat was approximately −6.53 log (cd s) m−2, which is similar to that reported previously for rats (−6.1 log (cd s) m−2), mice (−6.3 log (cd s) m−2), as well as for cats and monkeys (Naarendorp & Sieving 1991; Sieving & Wakabayashi 1991; Naarendorp et al. 2001; Saszik et al. 2002b). The ERG threshold for humans is reportedly higher than that in other species (Sieving & Nino 1988; Frishman et al. 1996b). In the rat, it has been suggested that the pSTR has a higher threshold than the nSTR (Bush et al. 1995; Naarendorp et al. 2001). However, in the present study, purely negative STR responses were never observed. This is consistent with studies in other species, including mouse (Saszik et al. 2002b), monkey (Frishman et al. 1996a) and human (Frishman et al. 1996b), which have all found more similar thresholds for the pSTR and nSTR.
The photopic ERG of the rat
An unexpected finding of this study was the extreme effects that TTX had on the photopic ERG of the rat (Figs 6 and 7). The photopic b-wave and OPs were both reduced by ONTx (by 25–30 %), suggesting that both are at least partially dependent on intact GC function – albeit to a lesser extent than the STR. However, TTX reduced the photopic b-wave by more than half its control amplitude and the photopic OPs nearly 10-fold. These effects of TTX were larger than those after GABA, NMDA, CNQX, PDA or the combination of CNQX and D-AP7, and thereby defy simple explanation. The current results also show that TTX exerts a much greater effect on cone-driven, as compared with rod-driven, OPs in the rat retina.
Acknowledgments
We would like to thank the following for their help with these experiments: Drs John Morrison and Jack Cioffi for surgical technique; Dr Jin Dong for histology; Dr Beth Edmunds for masked cell counts; and all of the above for their critical commentary. This research was supported by Legacy Research Services, NH & MRC Australia CJ Martin Fellowship (B.V.B.), MJ Murdock Charitable Trust (B.F.) and NIH grant EY05231 (G. A Cioffi).
References
- Ahmed J, Frishman L, Robson JG. Pharmacological removal of positive and negative STRs is required to isolate bipolar-cell responses in the macaque scotopic electroretinogram. Invest Ophthalmol Vis Sci. 1999;40(suppl.):S15. [Google Scholar]
- Allcutt D, Berry M, Sievers J. A quantitative comparison of the reactions of retinal ganglion cells to optic nerve crush in neonatal and adult mice. Brain Res. 1984;318:219–230. doi: 10.1016/0165-3806(84)90027-0. [DOI] [PubMed] [Google Scholar]
- Awatramani G, Wang J, Slaughter MM. Amacrine and ganglion cell contributions to the electroretinogram in amphibian retina. Vis Neurosci. 2001;18:147–156. doi: 10.1017/s0952523801181149. [DOI] [PubMed] [Google Scholar]
- Barres BA, Chun LL, Corey DP. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron. 1989;2:1375–1388. doi: 10.1016/0896-6273(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Barron KD, Dentinger MP, Krohel G, Easton SK, Mankes R. Qualitative and quantitative ultrastructural observations on retinal ganglion cell layer of rat after intraorbital optic nerve crush. J Neurocytol. 1986;15:345–362. doi: 10.1007/BF01611437. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Domenici L, Gravina A, Maffei L. Pattern ERG in rats following section of the optic nerve. Exp Brain Res. 1990;79:539–546. doi: 10.1007/BF00229323. [DOI] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutto IA, Amemiya T. Microvascular architecture of the rat choroid: corrosion cast study. Anat Rec. 2001;264:63–71. doi: 10.1002/ar.1102. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA. Effect of spike blockade on the receptive-field size of amacrine and ganglion cells in the rabbit retina. J Neurophysiol. 1996;75:1878–1893. doi: 10.1152/jn.1996.75.5.1878. [DOI] [PubMed] [Google Scholar]
- Boos R, Muller F, Wassle H. Actions of excitatory amino acids on brisk ganglion cells in the cat retina. J Neurophysiol. 1990;64:1368–1379. doi: 10.1152/jn.1990.64.5.1368. [DOI] [PubMed] [Google Scholar]
- Boos R, Schneider H, Wassle H. Voltage- and transmitter-gated currents of all-amacrine cells in a slice preparation of the rat retina. J Neurosci. 1993;13:2874–2888. doi: 10.1523/JNEUROSCI.13-07-02874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui BV, Fortune B, Morrison JC, Gioffi AC. Ganglion cell contribution to the rat full-field electroretinogram. Invest Ophthalmol Vis Sci. 2003;44:36. ARVO Meeting Extracts. [Google Scholar]
- Bush RA, Hawks KW, Sieving PA. Preservation of inner retinal responses in the aged Royal College of Surgeons rat. Evidence against glutamate excitotoxicity in photoreceptor degeneration. Invest Ophthalmol Vis Sci. 1995;36:2054–2062. [PubMed] [Google Scholar]
- Carter DA, Vidal-Sanz M, Aguayo AJ. Long-term preservation of intrinsic retinal neurons after axotomy-induced death of retinal ganglion cells. Soc Neurosci. 1987;13:1390. abstract. [Google Scholar]
- Cohen ED, Miller RF. The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Vis Neurosci. 1994;11:317–332. doi: 10.1017/s0952523800001668. [DOI] [PubMed] [Google Scholar]
- Colotto A, Falsini B, Salgarello T, Iarossi G, Galan ME, Scullica L. Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci. 2000;41:2205–2211. [PubMed] [Google Scholar]
- Cursiefen C, Korth M, Horn FK. The negative response of the flash electroretinogram in glaucoma. Doc Ophthalmol. 2001;103:1–12. doi: 10.1023/a:1017539018387. [DOI] [PubMed] [Google Scholar]
- Dawson WW, Maida TM, Rubin ML. Human pattern-evoked retinal responses are altered by optic atrophy. Invest Ophthalmol Vis Sci. 1982;22:796–803. [PubMed] [Google Scholar]
- Diamond JS, Copenhagen DR. The contribution of NMDA and non-NMDA receptors to the light-evoked input–output characteristics of retinal ganglion cells. Neuron. 1993;11:725–738. doi: 10.1016/0896-6273(93)90082-3. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Copenhagen DR. The relationship between light-evoked synaptic excitation and spiking behaviour of salamander retinal ganglion cells. J Physiol. 1995;487:711–725. doi: 10.1113/jphysiol.1995.sp020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici L, Gravina A, Berardi N, Maffei L. Different effects of intracranial and intraorbital section of the optic nerve on the functional responses of rat retinal ganglion cells. Exp Brain Res. 1991;86:579–584. doi: 10.1007/BF00230531. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Hare WA. Contribution to the kinetics and amplitude of the electroretinogram b-wave by third-order retinal neurons in the rabbit retina. Vision Res. 2000;40:579–589. doi: 10.1016/s0042-6989(99)00203-5. [DOI] [PubMed] [Google Scholar]
- Drasdo N, Aldebasi YH, Chiti Z, Mortlock KE, Morgan JE, North RV. The s-cone PHNR and pattern ERG in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001;42:1266–1272. [PubMed] [Google Scholar]
- Dureau P, Bonnel S, Menasche M, Dufier JL, Abitbol M. Quantitative analysis of intravitreal injections in the rat. Curr Eye Res. 2001;22:74–77. doi: 10.1076/ceyr.22.1.74.6974. [DOI] [PubMed] [Google Scholar]
- Euler T, Schneider H, Wassle H. Glutamate responses of bipolar cells in a slice preparation of the rat retina. J Neurosci. 1996;16:2934–2944. doi: 10.1523/JNEUROSCI.16-09-02934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Wassle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol. 1998;79:1384–1395. doi: 10.1152/jn.1998.79.3.1384. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Hack I, Brandstatter JH, Wassle H. Synaptic localization of NMDA receptor subunits in the rat retina. J Comp Neurol. 2000;420:98–112. [PubMed] [Google Scholar]
- Frishman LJ, Du Shen FFL, Robson JG, Harwerth RS, Smith EL, 3rd, Carter-Dawson L, Crawford ML. The scotopic electroretinogram of macaque after retinal ganglion cell loss from experimental glaucoma. Invest Ophthalmol Vis Sci. 1996a;37:125–141. [PubMed] [Google Scholar]
- Frishman LJ, Reddy MG, Robson JG. Effects of background light on the human dark-adapted electroretinogram and psychophysical threshold. J Opt Soc Am A. 1996b;13:601–612. doi: 10.1364/josaa.13.000601. [DOI] [PubMed] [Google Scholar]
- Frishman LJ, Sieving PA, Steinberg RH. Contributions to the electroretinogram of currents originating in proximal retina. Vis Neurosci. 1988;1:307–315. doi: 10.1017/s0952523800001966. [DOI] [PubMed] [Google Scholar]
- Frishman LJ, Steinberg RH. Intraretinal analysis of the threshold dark-adapted ERG of cat retina. J Neurophysiol. 1989a;61:1221–1232. doi: 10.1152/jn.1989.61.6.1221. [DOI] [PubMed] [Google Scholar]
- Frishman LJ, Steinberg RH. Light-evoked increases in [K+]o in proximal portion of the dark-adapted cat retina. J Neurophysiol. 1989b;61:1233–1243. doi: 10.1152/jn.1989.61.6.1233. [DOI] [PubMed] [Google Scholar]
- Goldblum D, Mittag T. Prospects for relevant glaucoma models with retinal ganglion cell damage in the rodent eye. Vision Res. 2002;42:471–478. doi: 10.1016/s0042-6989(01)00194-8. [DOI] [PubMed] [Google Scholar]
- Green DG, Kapousta-Bruneau NV. A dissection of the electroretinogram from the isolated rat retina with microelectrodes and drugs. Vis Neurosci. 1999;16:727–741. doi: 10.1017/s0952523899164125. [DOI] [PubMed] [Google Scholar]
- Guldenagel M, Ammermuller J, Feigenspan A, Teubner B, Degen J, Sohl G, Willecke K, Weiler R. Visual transmission deficits in mice with targeted disruption of the gap junction gene connexin36. J Neurosci. 2001;21:6036–6044. doi: 10.1523/JNEUROSCI.21-16-06036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustincich S, Feigenspan A, Wu DK, Koopman LJ, Raviola E. Control of dopamine release in the retina: a transgenic approach to neural networks. Neuron. 1997;18:723–736. doi: 10.1016/s0896-6273(00)80313-x. [DOI] [PubMed] [Google Scholar]
- Harrison JM, O'Connor PS, Young RS, Kincaid M, Bentley R. The pattern ERG in man following surgical resection of the optic nerve. Invest Ophthalmol Vis Sci. 1987;28:492–499. [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Hartveit E, Brandstatter JH, Enz R, Wassle H. Expression of the mRNA of seven metabotropic glutamate receptors (mGluR1–7) in the rat retina. An in situ hybridization study on tissue sections and isolated cells. Eur J Neurosci. 1995;7:1472–1483. doi: 10.1111/j.1460-9568.1995.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Hartveit E, Brandstatter JH, Sassoe-Pognetto M, Laurie DJ, Seeburg PH, Wassle H. Localization and developmental expression of the NMDA receptor subunit NR2A in the mammalian retina. J Comp Neurol. 1994;348:570–582. doi: 10.1002/cne.903480407. [DOI] [PubMed] [Google Scholar]
- Hollander H, Bisti S, Maffei L, Hebel R. Electroretinographic responses and retrograde changes of retinal morphology after intracranial optic nerve section. A quantitative analysis in the cat. Exp Brain Res. 1984;55:483–493. doi: 10.1007/BF00235279. [DOI] [PubMed] [Google Scholar]
- Hughes A. A schematic eye for the rat. Vision Res. 1979;19:569–588. doi: 10.1016/0042-6989(79)90143-3. [DOI] [PubMed] [Google Scholar]
- Karwoski CJ, Xu X. Current source-density analysis of light-evoked field potentials in rabbit retina. Vis Neurosci. 1999;16:369–377. doi: 10.1017/s0952523899162163. [DOI] [PubMed] [Google Scholar]
- Korth M, Nguyen NX, Horn F, Martus P. Scotopic threshold response and scotopic PII in glaucoma. Invest Ophthalmol Vis Sci. 1994;35:619–625. [PubMed] [Google Scholar]
- Maffei L, Fiorentini A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science. 1981;211:953–955. doi: 10.1126/science.7466369. [DOI] [PubMed] [Google Scholar]
- Maffei L, Fiorentini A, Bisti S, Hollander H. Pattern ERG in the monkey after section of the optic nerve. Exp Brain Res. 1985;59:423–425. doi: 10.1007/BF00230925. [DOI] [PubMed] [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor MF, Zrenner E. Standard for clinical electroretinography (1999 update). International Society for Clinical Electrophysiology of Vision. Doc Ophthalmol. 1998;97:143–156. doi: 10.1023/a:1002016531591. [DOI] [PubMed] [Google Scholar]
- Massey SC, Miller RF. N-methyl-D-aspartate receptors of ganglion cells in rabbit retina. J Neurophysiol. 1990;63:16–30. doi: 10.1152/jn.1990.63.1.16. [DOI] [PubMed] [Google Scholar]
- Menger N, Wassle H. Morphological and physiological properties of the A17 amacrine cell of the rat retina. Vis Neurosci. 2000;17:769–780. doi: 10.1017/s0952523800175108. [DOI] [PubMed] [Google Scholar]
- Misantone LJ, Gershenbaum M, Murray M. Viability of retinal ganglion cells after optic nerve crush in adult rats. J Neurocytol. 1984;13:449–465. doi: 10.1007/BF01148334. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Johnson EC, Cepurna WO, Funk RH. Microvasculature of the rat optic nerve head. Invest Ophthalmol Vis Sci. 1999;40:1702–1709. [PubMed] [Google Scholar]
- Naarendorp F, Sato Y, Cajdric A, Hubbard NP. Absolute and relative sensitivity of the scotopic system of rat: electroretinography and behavior. Vis Neurosci. 2001;18:641–656. doi: 10.1017/s0952523801184142. [DOI] [PubMed] [Google Scholar]
- Naarendorp F, Sieving PA. The scotopic threshold response of the cat ERG is suppressed selectively by GABA and glycine. Vision Res. 1991;31:1–15. doi: 10.1016/0042-6989(91)90068-g. [DOI] [PubMed] [Google Scholar]
- Penn RD, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969;223:201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Perry VH. Evidence for an amacrine cell system in the ganglion cell layer of the rat retina. Neuroscience. 1981;6:931–944. doi: 10.1016/0306-4522(81)90174-3. [DOI] [PubMed] [Google Scholar]
- Perry VH, Henderson Z, Linden R. Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. J Comp Neurol. 1983;219:356–368. doi: 10.1002/cne.902190309. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Davis EB, Anderson DR. Descending optic nerve degeneration in primates. Invest Ophthalmol Vis Sci. 1977;16:841–849. [PubMed] [Google Scholar]
- Radius RL, Anderson DR. Retinal ganglion cell degeneration in experimental optic atrophy. Am J Ophthalmol. 1978;86:673–679. doi: 10.1016/0002-9394(78)90189-7. [DOI] [PubMed] [Google Scholar]
- Robson JG, Frishman LJ. Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Vis Neurosci. 1995;12:837–850. doi: 10.1017/s0952523800009408. [DOI] [PubMed] [Google Scholar]
- Robson JG, Frishman LJ. Dissecting the dark-adapted electroretinogram. Doc Ophthalmol. 1998;95:187–215. doi: 10.1023/a:1001891904176. [DOI] [PubMed] [Google Scholar]
- Robson JG, Saszik SM, Ahmed J, Frishman LJ. Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol. 2003;547:509–530. doi: 10.1113/jphysiol.2002.030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russelakis-Carneiro M, Silveira LC, Perry VH. Factors affecting the survival of cat retinal ganglion cells after optic nerve injury. J Neurocytol. 1996;25:393–402. doi: 10.1007/BF02284810. [DOI] [PubMed] [Google Scholar]
- Saszik S, Robson JG, Frishman LJ. Contributions of spiking and non-spiking inner retinal neurons to the scotopic ERG of the mouse. Invest Ophthalmol Vis Sci. 2002a;43 E-Abstract # 1817. [Google Scholar]
- Saszik SM, Robson JG, Frishman LJ. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J Physiol. 2002b;543:899–916. doi: 10.1113/jphysiol.2002.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger MW, Saszik S, Mayser H, Frishman LJ, Hormuzdi S, Biel M, Humphries P, Willicke K, Monyer H, Weiler R. Connexin 36-dependent retinal function in mice with specific rod or cone photoreceptor input. Invest Ophthalmol Vis Sci. 2003;44 E-Abstract #1872. [Google Scholar]
- Sieving PA. Retinal ganglion cell loss does not abolish the scotopic threshold response (STR) of the cat and human ERG. Clin Vis Sci. 1991;2:149–158. [Google Scholar]
- Sieving PA, Frishman LJ, Steinberg RH. Scotopic threshold response of proximal retina in cat. J Neurophysiol. 1986;56:1049–1061. doi: 10.1152/jn.1986.56.4.1049. [DOI] [PubMed] [Google Scholar]
- Sieving PA, Murayama K, Naarendorp F. Push–pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci. 1994;11:519–532. doi: 10.1017/s0952523800002431. [DOI] [PubMed] [Google Scholar]
- Sieving PA, Nino C. Scotopic threshold response (STR) of the human electroretinogram. Invest Ophthalmol Vis Sci. 1988;29:1608–1614. [PubMed] [Google Scholar]
- Sieving PA, Wakabayashi K. Comparison of rod threshold ERG from monkey, cat and human. Clin Vision Sci. 1991;6:171–179. [Google Scholar]
- Stockton RA, Slaughter MM. B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J Gen Physiol. 1989;93:101–122. doi: 10.1085/jgp.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci. 1993;13:455–466. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Witkovsky P. Glutamate receptors and circuits in the vertebrate retina. Prog Retin Eye Res. 1999;18:765–810. doi: 10.1016/s1350-9462(98)00031-7. [DOI] [PubMed] [Google Scholar]
- Velte TJ, Yu W, Miller RF. Estimating the contributions of NMDA and non-NMDA currents to EPSPs in retinal ganglion cells. Vis Neurosci. 1997;14:999–1014. doi: 10.1017/s0952523800011731. [DOI] [PubMed] [Google Scholar]
- Vidal-Sanz M, Bray GM, Villegas-Perez MP, Thanos S, Aguayo AJ. Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci. 1987;7:2894–2909. doi: 10.1523/JNEUROSCI.07-09-02894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993;24:23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- Viswanathan S, Frishman LJ, Robson JG. The uniform field and pattern ERG in macaques with experimental glaucoma: removal of spiking activity. Invest Ophthalmol Vis Sci. 2000;41:2797–2810. [PubMed] [Google Scholar]
- Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL., 3rd The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1124–1136. [PubMed] [Google Scholar]
- Viswanathan S, Frishman LJ, Robson JG, Walters JW. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001;42:514–522. [PubMed] [Google Scholar]
- Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998;17:485–521. doi: 10.1016/s1350-9462(98)00006-8. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Gieser J, Sieving PA. Aspartate separation of the scotopic threshold response (STR) from the photoreceptor a-wave of the cat and monkey ERG. Invest Ophthalmol Vis Sci. 1988;29:1615–1622. [PubMed] [Google Scholar]
- Watanabe M, Inukai N, Fukuda Y. Survival of retinal ganglion cells after transection of the optic nerve in adult cats: a quantitative study within two weeks. Vis Neurosci. 2001;18:137–145. doi: 10.1017/s0952523801181137. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Wassle H. Responses of rod bipolar cells isolated from the rat retina to the glutamate agonist 2-amino-4-phosphonobutyric acid (APB) J Neurosci. 1991;11:2372–2382. doi: 10.1523/JNEUROSCI.11-08-02372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Miller RF. NBQX, an improved non-NMDA antagonist studied in retinal ganglion cells. Brain Res. 1995;692:190–194. doi: 10.1016/0006-8993(95)00665-d. [DOI] [PubMed] [Google Scholar]
- Yu W, Miller RF. The mechanism by which NBQX enhances NMDA currents in retinal ganglion cells. Brain Res. 1996;709:184–196. doi: 10.1016/0006-8993(95)01285-0. [DOI] [PubMed] [Google Scholar]