Abstract
There is substantial evidence that oxidative stress participates in the pathophysiology of cardiovascular disease. Biochemical, molecular and pharmacological studies further implicate xanthine oxidoreductase (XOR) as a source of reactive oxygen species in the cardiovascular system. XOR is a member of the molybdoenzyme family and is best known for its catalytic role in purine degradation, metabolizing hypoxanthine and xanthine to uric acid with concomitant generation of superoxide. Gene expression of XOR is regulated by oxygen tension, cytokines and glucocorticoids. XOR requires molybdopterin, iron–sulphur centres, and FAD as cofactors and has two interconvertible forms, xanthine oxidase and xanthine dehydrogenase, which transfer electrons from xanthine to oxygen and NAD+, respectively, yielding superoxide, hydrogen peroxide and NADH. Additionally, XOR can generate superoxide via NADH oxidase activity and can produce nitric oxide via nitrate and nitrite reductase activities. While a role for XOR beyond purine metabolism was first suggested in ischaemia–reperfusion injury, there is growing awareness that it also participates in endothelial dysfunction, hypertension and heart failure. Importantly, the XOR inhibitors allopurinol and oxypurinol attenuate dysfunction caused by XOR in these disease states. Attention to the broader range of XOR bioactivity in the cardiovascular system has prompted initiation of several randomised clinical outcome trials, particularly for congestive heart failure. Here we review XOR gene structure and regulation, protein structure, enzymology, tissue distribution and pathophysiological role in cardiovascular disease with an emphasis on heart failure.
Xanthine oxidoreductase (XOR), first identified a century ago in milk (Schardinger, 1902), is a highly conserved member of the molybdoenzyme family, which also includes aldehyde oxidase (AO) and sulphite oxidase (SO) (Fig. 1A) (Kisker et al. 1997). XOR has two interconvertible forms, xanthine dehydrogenase (XDH) and xanthine oxidase (XO) (Stirpe & Della Corte, 1969). They differ in that XO only reduces oxygen, whereas XDH can reduce either oxygen or NAD+ but has greater affinity for the latter (Waud & Rajagopalan, 1976a). Both forms catalyse the conversion of hypoxanthine to xanthine and xanthine to uric acid (UA) (Hille & Nishino, 1995), the terminal two reactions of the purine degradation pathway (Fig. 2). XOR cofactors include (a) molybdopterin (Mo–Co) (b) two iron–sulphur centres (Fe2–S2), and (c) flavin adenine dinucleotide (FAD) (Hille & Nishino, 1995). Interest in XOR has grown over the past two decades because of its ability to generate reactive oxygen species (ROS), its suspected role in reperfusion injury, and most recently its pathophysiological role in congestive heart failure. Here we review the genetic and enzymatic properties of XOR, its physiological function, and its newly appreciated roles in cardiovascular disease.
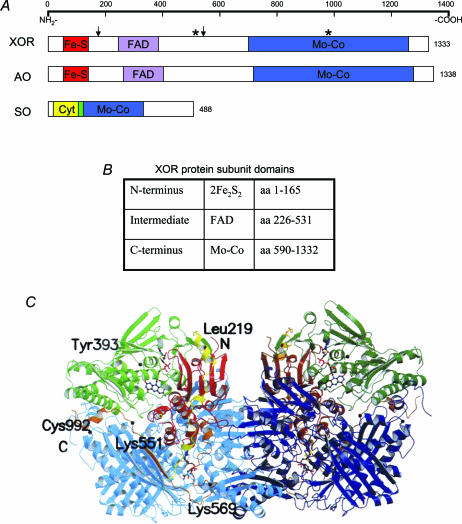
Figure 1. Secondary and tertiary structures of XOR.
A, secondary structure of molybdoenzymes XOR, AO, and SO. Arrows designate trypsin sites (Lys186, Lys552) (Amaya et al. 1990). Stars designate cysteine residues modified in reversible XOR conversion (Cys535, Cys992) (Nishino & Nishino, 1997). B, XOR subunit domains, their sizes, and their associated cofactors (Enroth et al. 2000). C, crystal structure of bovine XOR homodimer (Enroth et al. 2000). Copyright 2000 National Academy of Sciences, USA.
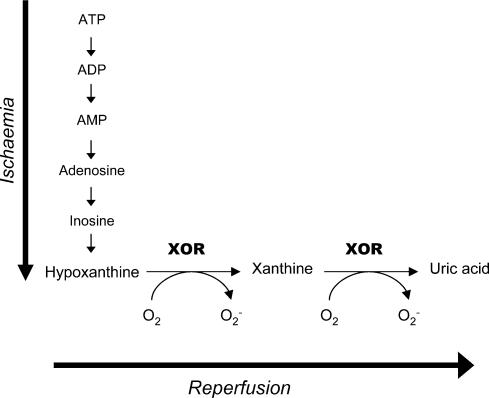
Figure 2. The purine degradation pathway.
Reprinted from Mathews & Van Holde (1996) with permission of Pearson Education Inc.
Gene structure
The gene encoding human XOR is >60 kb, comprises 36 exons (Xu et al. 1996), and is located on the short arm of chromosome 2 (Ichida et al. 1993; Xu et al. 1994b). The mRNA transcript contains an open reading frame of 3999 bp encoding 1333 amino acids (Ichida et al. 1993; Xu et al. 1994a, 1995). The amino acid sequence is 91% homologous with rat and mouse XOR (Xu et al. 1994a, 1995), with binding sites for XOR cofactors well conserved amongst human, chicken, rat and mouse XOR (Ichida et al. 1993). The Mo–Co binding site is the most conserved region of XOR with 94% homology between human, rat and mouse amino acid sequences (Xu et al. 1994a, 1995).
The gene encoding the related enzyme AO exhibits striking similarity to XOR. The intron–exon organization is almost identical between XOR and AO genes (Terao et al. 1998), and their amino acid sequences are 50% homologous as determined from bovine cDNA (Terao et al. 1997). Their similarities in primary structure extend to their tertiary structures, for they share the same cofactors and redox centre distribution (Fig. 1A), as well as many of the same substrates (Terao et al. 1998). The genes encoding AO and XOR are closely spaced on chromosome 2 (Terao et al. 1998), suggesting they arose from an ancestral gene via tandem duplication. The functional role of AO remains unknown but is likely to be different from XOR as it lacks dehydrogenase activity (Turner et al. 1995).
Several mutations have been identified in the XOR gene that cause classical xanthinuria type I (Ichida et al. 1997; Sakamoto et al. 2001), whereas mutations responsible for xanthinuria type II have been located in the gene for human molybdenum cofactor sulphurase, which is necessary for both XOR and AO activity (Ichida et al. 2001). More than 50% of patients with xanthinuria are asymptomatic, while the remainder present with xanthine calculi, renal failure and myopathy (Simmonds, 1994). In contrast, nearly all patients lacking SO activity due to congenital Mo–Co deficiency suffer from severe neurological disease that is often fatal (Simmonds, 1994). Interestingly, XOR knockout mice are runted and rarely survive up to 6 weeks, suggesting greater importance of this enzyme in lower mammals (Vorbach et al. 2002).
Gene expression and regulation
Basal expression of the XOR gene is mediated by several transcription factors. For example, various sequence motifs have been located upstream of the XOR coding sequence that bind C/EBP, ETS-1, AP-1, AP-2 and TF-IID (Xu et al. 1996, 2000). Additionally, nuclear factor Y has been shown to activate XOR transcription (Martelin et al. 2000). The basal activity of the human XOR promoter is nominal when compared with other mammals (Xu et al. 2000), probably due to putative repressor elements identified in non-coding regions of XOR. For example, the human promoter contains a unique TATA-like element not found in rodents, mutation of which enhances promoter activity (Xu et al. 2000). An E-box has also been located upstream of the human gene that may restrict transcriptional activity (Xu et al. 2000). Promoter suppression involving a constitutively expressed repressor protein or labile protein with RNase activity has been suspected, for inhibition of protein synthesis with cycloheximide results in elevated levels of XOR mRNA (Dupont et al. 1992; Pfeffer et al. 1994; Kurosaki et al. 1996).
Although the basal expression of human XOR is low, a variety of factors up-regulate transcription (Table 1). Many potential cytokine-responsive elements have been identified in the regulatory region upstream from the gene (Xu et al. 1996), along with putative glucocorticoid or hypoxia-responsive elements (Hoidal et al. 1997). In support of their postulated regulatory role in vivo, these entities activate XOR gene transcription in vitro. For example, Pfeffer et al. (1994) demonstrated that bovine renal epithelial cells up-regulate XOR transcription when treated with TNF-α, IFN-γ, IL-1, IL-6, or dexamethasone. In contrast, Dupont et al. (1992) found that only IFN-γ activated transcription in rat pulmonary vascular endothelial cells, suggesting regulation of XOR gene expression is cell-specific. In this regard, Hoidal et al. (1997) showed that endothelial and epithelial cells utilize different regions of the XOR promoter for basal transcription. Furthermore, cell location also influences promoter activity. For example, depending on their glandular location, murine mammary epithelial cells exhibit a differential response to glucocorticoids, which activate XOR expression in alveolar epithelial cells but not in epithelial cells of the lactiferous ducts (Kurosaki et al. 1996). Several studies have also demonstrated additive or synergistic effects using various cytokines and glucocorticoids (Pfeffer et al. 1994; Kurosaki et al. 1996; Hassoun et al. 1998; Page et al. 1998).
Table 1.
Regulation of XOR gene expression
| Positive regulators | Negative regulators |
|---|---|
| Hypoxia | Hyperoxia |
| Lipopolysaccharide | |
| Interferon γ | |
| Interleukin-1 | |
| Interleukin-6 | |
| Tumor necrosis factor α | |
| Dexamethasone | |
| Cortisol | |
| Prolactin |
In addition to these regulatory factors, oxygen tension also influences XOR gene expression (Lanzillo et al. 1996). Terada et al. (1997) demonstrated that hypoxia activates and hyperoxia inhibits transcription in endothelial cells. Similar results were obtained by Hassoun et al. (1998) when they exposed various rat and bovine cells to hypoxic conditions. These effects on XOR gene expression may be mediated by hypoxia-inducible factor-1. Interestingly, Terada et al. (1997) observed increasing XOR activity shortly after exposure to hypoxia, before there was a measurable change in XOR transcription. Likewise, in hyperoxic conditions, XOR activity declined at a faster rate than could be achieved with cycloheximide. Page et al. (1998) similarly found that increased XOR activity could not be accounted for by enhanced gene expression alone in cytokine-stimulated epithelial cells. These findings implicate oxygen tension and other transcriptional modifiers in both pretranslational and post-translational regulation of XOR expression.
Protein structure
The XOR enzyme is a homodimer composed of catalytically independent subunits with an approximate molecular mass of 150 kDa each (Krenitsky et al. 1986). The interface between the subunits occurs such that the overall complex has a butterfly shape (Fig. 1C). Each subunit is organized into three domains associated with a specific cofactor (Fig. 1B). The N-terminal domain (amino acids 1–165) is composed of two subdomains, each with one Fe2–S2 centre coordinated to four cysteine residues. A linker peptide connects it to the intermediate domain (amino acids 226–531), which holds a deep binding pocket for FAD that positions the flavin ring in close proximity to an Fe2–S2 centre. Another linker peptide joins the FAD domain with the C-terminal domain (amino acids 590–1332), which is the largest domain and the location of Mo–Co binding (Enroth et al. 2000).
The crystal structure of aldehyde oxidoreductase, a molybdoenzyme from the organism Desulfovibrio gigas, demonstrates penta-coordination of the molybdenum ion with one oxo ligand, one water molecule, one sulphido group, and two dithiolene sulphur atoms from molybdopterin (Romão et al. 1995; Huber et al. 1996). In XOR, the sulphido group is necessary for maintaining enzyme activity, as the desulpho-form is unable to react with substrate (Kisker et al. 1997). Similarities between the crystal structures of aldehyde oxidoreductase and bovine XOR suggest the coordination of the molybdenum ion in aldehyde oxidoreductase may apply to XOR as well (Romão et al. 1995; Huber et al. 1996; Enroth et al. 2000). A more detailed discussion of XOR structure can be found in the review by Harrison (2002).
Conversion from XDH to XO
When the different forms of XOR were first discovered, the purification process converted variable amounts of enzyme to XO, implicating it as the predominant form. However, it has since been demonstrated that most XOR is in the XDH form in vivo (Della Corte et al. 1969; Stirpe & Della Corte, 1969; Waud & Rajagopalan, 1976a). Both forms catalyse reactions involving similar substrates, but they prefer different electron acceptors. Flavin active site probe studies suggested and crystal structure elucidation confirmed that changes at the FAD site dictate electron acceptor preferences (Massey et al. 1989; Saito et al. 1989; Enroth et al. 2000). Site-directed mutagenesis identified a group of amino acids (Phe549, Arg335, Trp336, Arg427) that is disrupted during conversion, resulting in loss of interaction between Phe549 and Trp336. This significantly alters the flavin active site structure, making it inaccessible to NAD+. However, the structural change also creates a channel that facilitates the approach of molecular oxygen (Kuwabara et al. 2003).
XOR conversion occurs via two different routes which determine its reversibility. When XDH is treated with proteases like trypsin, chymotrypsin, or pancreatin, it is irreversibly transformed into XO (Della Corte et al. 1969; Stirpe & Della Corte, 1969). Trypsinization of rat enzyme (Fig. 1A) yields three digestion products (20, 40 and 85 kDa) that associate strongly to maintain enzyme activity unless subjected to denaturing conditions (Amaya et al. 1990). Reversible conversion occurs due to conditions that oxidize thiol groups of Cys535 and Cys992 (Fig. 1A) (Nishino & Nishino, 1997), including storage at –20°C (Della Corte et al. 1969), incubation at 37°C (Della Corte & Stirpe, 1972), exposure to sulphydryl agents (Waud & Rajagopalan, 1976b), and exposure to anaerobic conditions (Della Corte et al. 1969). This mechanism of conversion is preventable and reversible by treatment with dithioerythritol or other thiol compounds, unlike proteolytic conversion (Waud & Rajagopalan, 1976a).
Enzymatic properties
Although XOR reacts with many substrates including purines, purine ribonucleosides, and 2′-deoxyribonucleosides (Krenitsky et al. 1974), it is best known as the rate-limiting enzyme of the purine degradation pathway (Fig. 2) (Parks & Granger, 1986). XOR converts hypoxanthine to xanthine and xanthine to UA, the end product of purine catabolism in humans. In lower mammals, urate oxidase further metabolizes UA to allantoin, but this enzyme is inactivated in most primates (Usuda et al. 1988).
Although aspects of the mechanism of XOR reaction with xanthine remain uncertain, proposed reaction schemes share some common features (Xia et al. 1999). The reductive half-reaction occurs at Mo–Co where XOR accepts two electrons from xanthine, reducing Mo(VI) to Mo(IV) (Fig. 3A) (Xia et al. 1999). From the xanthine-C8 position, a hydrogen is transferred to the sulphido ligand of molybdenum, such that Mo = S becomes Mo–SH (Bray et al. 1979). At the same time, nucleophilic attack by a hydroxyl group occurs at C8, thus forming UA. This hydroxyl group is ultimately derived from water (Murray et al. 1966), but it is unknown whether it reacts independently or as a Mo ligand. Several water molecules that may be involved have been identified in the active site of XOR by crystallography (Enroth et al. 2000). Crystal structure studies have also suggested that a basic amino acid from XOR assists in nucleophilic attack by playing a role in proton transfer. A glutamate residue (Glu869) potentially responsible for this was first designated in the crystal structure of D. gigas aldehyde oxidoreductase (Romão et al. 1995), and an analogous residue (Glu1261) was subsequently identified in XOR (Enroth et al. 2000). Of note, the hypoxanthine analogue allopurinol reacts with XOR at Mo–Co to yield alloxanthine (also known as oxypurinol), which binds to XOR via direct coordination to Mo, thereby inhibiting enzyme interaction with substrate (Massey et al. 1970; Truglio et al. 2002).
Figure 3. Mechanism of XOR reaction with xanthine.
A, reductive half-reaction. B, oxidative half-reaction.
In contrast to the reductive half-reaction, the oxidative half-reaction takes place at FAD (Fig. 3B) (Hille & Nishino, 1995). Intramolecular electron transfer between Mo–Co and FAD is mediated by the Fe2–S2 centres (Hille & Anderson, 1991), which serve as an electron reservoir or ‘sink’ to maintain Mo–Co as Mo(VI) and flavin as FADH2 for efficient reaction catalysis (Olson et al. 1974a). Electrons are subsequently transferred from FAD to NAD+ or O2 (Olson et al. 1974b; Hille & Massey, 1981). In the reoxidation of fully reduced XO, the first two steps each involve transfer of two electrons to O2, generating hydrogen peroxide (H2O2) (Olson et al. 1974b; Hille & Massey, 1981). XO then transfers its remaining electrons in separate steps, with each electron independently reducing O2 to produce superoxide (·O2−). Thus, the overall reoxidation of fully reduced XO yields two H2O2 and two ·O2− species (Hille & Massey, 1981). Interestingly, XDH actually generates more ·O2− per mole O2 (Saito & Nishino, 1989) due to greater thermodynamic stability of the FAD form that is reactive with oxygen (Hunt et al. 1993). However, despite greater efficiency in ·O2− production, XDH reacts slowly with oxygen, with a maximal rate of ·O2− generation that is 25% of XO Vmax (Saito & Nishino, 1989). In addition to this slow reaction rate, NAD+ exhibits tight, rapid binding to XDH (Harris & Massey, 1997a), so O2 is a very poor competitor. The mechanism of electron transfer from XDH to NAD+ is not as well elucidated as that for oxygen, but evidence suggests that XDH cycles between two and four electron-reduced states. NAD+ binds to XDH when it is reduced with four electrons, with subsequent transfer of two electrons to produce NADH (Harris & Massey, 1997a). Hille & Nishino (1995) have reviewed the kinetics and mechanism of reaction of XOR in greater detail.
Since NAD+ is the preferred electron acceptor for XDH, generation of ·O2− by XDH is limited when the supply of NAD+ is ample (Sanders et al. 1997). However, once NAD+ is converted to NADH, XDH can operate as an NADH oxidase (Harris & Massey, 1997b; Sanders et al. 1997; Zhang et al. 1998a). NADH directly transfers electrons to FAD, and subsequent reduction of oxygen generates ·O2−. This NADH oxidase activity is significant, reaching 40% of the XDH activity with xanthine (Harris & Massey, 1997b). In contrast to XDH, XO exhibits little reactivity with NADH (Waud & Rajagopalan, 1976a). Interestingly, NADH oxidase activity is insensitive to allopurinol binding (Sanders et al. 1997), since Mo–Co is not involved. In contrast, diphenyleneiodonium (DPI), a non-specific FAD site inhibitor, effectively blocks the NADH oxidase activity of XDH (Sanders et al. 1997; Zhang et al. 1998a).
Superoxide radical produced by either XO or XDH can combine with nitric oxide (NO) to form peroxynitrite (ONOO−), a potent non-radical oxidant species (Godber et al. 2000a). NO is usually generated by nitric oxide synthase (NOS). However, XOR may also be a contributory source of NO (Millar et al. 1998), especially in hypoxic conditions when NOS cannot generate NO. Li et al. (2003) recently demonstrated that NO generation occurs in hypoxic rat myocardium in the presence of NOS inhibitors but not allopurinol, implicating XOR in the production of NO. XOR has both inorganic nitrate reductase (Li et al. 2003) and nitrite reductase (Zhang et al. 1998b; Godber et al. 2000b; Li et al. 2001) activities at its Mo–Co site (Godber et al. 2000b; Li et al. 2001; Li et al. 2003). NO also may be generated by XOR via the reduction of organic nitrates like isosorbide dinitrate and glyceryl trinitrate (Doel et al. 2001) and organic nitrites like isoamyl nitrite (Doel et al. 2000). Doel et al. (2001) demonstrated that reduction of organic nitrates occurs at the FAD site, in contrast to the reduction of inorganic nitrate at Mo–Co. Harrison (2002) has reviewed the generation of NO by XOR-mediated metabolism of nitrovasodilators at great length.
In humans, there is a wide range of XOR activity, with greater than threefold variation in enzymatic activity amongst individuals (Guercionni et al. 1991). However, when compared with other mammals, the magnitude of enzyme activity in humans is relatively low (Parks & Granger, 1986), and suppressed gene expression cannot solely account for this. For example, although similar yields of XOR can be obtained from human and bovine milk, human XOR activity is variably 1–6% of bovine activity when xanthine is the substrate (Abadeh et al. 1992). When all substrates are considered, the difference between bovine and human XOR activity spans three orders of magnitude (Abadeh et al. 1992). Some of this disparity is due to the presence of demolybdo-XOR in human milk, which can account for 5% (Abadeh et al. 1992) to >95% (Godber et al. 1997) of total inactive XOR, depending on the sample. Other inactive forms of the enzyme, such as desulpho-XOR, may also be found in milk (Abadeh et al. 1992).
Post-translational regulation
Oxygen tension, which affects XOR activity by regulating gene expression, also regulates XOR activity at a post-translational level (Terada et al. 1997). Poss et al. (1996) demonstrated that XOR activity in bovine aortic endothelial cells doubles after exposure to hypoxia without any increase in mRNA expression for 24 h. Terada et al. (1997) observed a similar response to hypoxia in fibroblasts. Interestingly, XOR is inactivated by high oxygen tension, also via post-translational modification (Terada et al. 1988; Panus et al. 1992). Terada et al. (1997) detected a relatively fast decline in XOR activity in association with hyperoxia, more rapid than would be expected if only de novo synthesis was affected. The mechanism by which oxygen tension regulates enzyme activity may involve phosphorylation of XOR, for Kayyali et al. (2001) demonstrated that XOR phosphorylation in hypoxia is associated with enhanced enzyme activity. Interestingly, XOR phosphorylation is partially blocked by p38 kinase inhibitors, and p38 is up-regulated in hypoxia, suggesting a possible role in regulation of XOR activity (Kayyali et al. 2001).
In addition to oxygen tension, NO also may regulate XOR activity. Several studies have established that XOR is progressively inactivated during reduction of nitrates and nitrites (Doel et al. 2000, 2001; Godber et al. 2000b), and inactivation of XOR by exogenous NO has also been demonstrated (Ichimori et al. 1999). Doel et al. (2001) hypothesized that progressive inactivation of XOR may be responsible for the clinical phenomenon of tolerance that occurs in patients with continuous exposure to organic nitrates. Sulphuration of inactivated XOR has been shown to restore activity, indicating the mechanism of inactivation involves conversion to desulpho-XOR (Ichimori et al. 1999; Doel et al. 2000, 2001; Godber et al. 2000b). In contrast to these results, other investigators have found that NO does not affect XOR activity but peroxynitrite does, suggesting that observations of XOR inhibition by NO are artifacts of peroxynitrite production and subsequent XOR inactivation (Houston et al. 1998; Lee et al. 2000).
Other products and byproducts of XOR reactions have also been implicated as negative regulators of XOR activity, including ·O2− (Terada et al. 1988), H2O2 (Terada et al. 1991a) and hydroxyl radical (·OH) (Terada et al. 1991a). Such product-mediated regulation may play a role in XOR inactivation in hyperoxia.
Tissue distribution and subcellular localization
The determination of XOR tissue distribution has been complicated by inconsistent results obtained using different methods of XOR detection. Antibody-dependent methods like immunohistochemistry recognize active and inactive forms of the enzyme in intact tissues, whereas biochemical techniques rely on detection of enzyme activity in tissue homogenates. This distinction becomes especially important in human tissue, since a large proportion of human XOR is inactive (Abadeh et al. 1992).
In mammals, the highest levels of XOR activity are found in the liver and small intestine (Parks & Granger, 1986). XOR has also been identified in other mammalian tissues, including bovine heart (Jarasch et al. 1986; de Jong et al. 1990) and rat heart (Muxfeldt & Schaper, 1987; de Jong et al. 1990). The abundance of XOR is relatively low in human tissue, but it has been consistently identified in liver, small intestine and mammary gland (Linder et al. 1999). Furthermore, several (Muxfeldt & Schaper, 1987; Abadeh et al. 1993; Vickers et al. 1998; Cappola et al. 2001) but not all (Eddy et al. 1987; Linder et al. 1999) studies have identified XOR protein and/or activity in the human heart. Importantly, Jarasch et al. (1981) consistently located XOR in bovine capillary endothelial cells using immunohistochemistry, and similar experiments established the presence of XOR in human endothelial cells (Jarasch et al. 1981; Bruder et al. 1984). This same group of investigators successfully detected bovine and human XOR activity in various tissue samples using an ultrasensitive radioimmunoassay (Bruder et al. 1983; Jarasch et al. 1986). However, the XOR activity in these tissue fractions was 10- to 1000-fold lower than activity in fractions from liver or lactating mammary gland, suggesting XOR activity may have been below the limit of detection of less sensitive methods used in other studies (Bruder et al. 1983).
Subcellular localization methods have demonstrated the presence of XOR both in the cytoplasm (Rouquette et al. 1998; Frederiks & Vreeling-Sindelarova, 2002) and on cell membranes (Rouquette et al. 1998), with cell surface binding likely mediated by glycosaminoglycans (Adachi et al. 1993; Radi et al. 1997; Rouquette et al. 1998). XOR affinity for glycosaminoglycans, especially heparin, has been implicated by in vitro and in vivo studies. For example, heparin is very effective at displacing XOR in affinity chromatography (Radi et al. 1997), and the addition of heparin or heparinase to endothelial cell cultures increases free XOR in the culture media (Adachi et al. 1993). Similarly, injection of human subjects with heparin rapidly results in a two- to threefold increase in plasma XOR (Adachi et al. 1993). In the past XOR has been reported to be solely cytosolic (Ichikawa et al. 1992), but recently it has been identified by electron microscopy in the peroxisomes of hepatocytes and in various organelles of Kupffer and sinusoidal cells, including rough endoplasmic reticulum, lysosomes, and endocytic vesicles (Frederiks & Vreeling-Sindelarova, 2002). Thus, the subcellular localization of XOR remains controversial and incompletely described.
Physiological functions of XOR
For many decades, the sole purpose of XOR was presumed to be purine catabolism (Fig. 2), but growing evidence suggests a much broader biological role for this enzyme. For example, XOR has antimicrobial properties, as it inhibits the growth of bacteria in vitro in an NO-dependent manner (Hancock et al. 2002). There is also evidence that XOR plays an antimicrobial role in vivo, for infants who receive breast milk rich in XOR are less likely to develop gastroenteritis than those who are fed formula (Stevens et al. 2000). Furthermore, in mice with chronic granulomatous disease, allopurinol decreases clearance of pathogens and reduces killing efficiency in vivo, suggesting XOR may contribute to host defence against oxidant-sensitive organisms (Segal et al. 2000). Although XOR clearly participates in antimicrobial defense, patients with xanthinuria are not immunocompromised, indicating the role of XOR in host defense is non-essential (see review by Harrison, 2002).
UA production by XOR may itself have broader biological consequences beyond purine degradation, for it possesses antioxidant properties (Becker, 1993). Importantly, humans have higher UA concentrations compared with other mammals, as urate oxidase is inactivated in primates (Usuda et al. 1988). Watanabe et al. (2002) hypothesized that this provided a survival advantage for humans because hyperuricaemia maintains blood pressure in the face of low dietary salt. Furthermore, Ames et al. (1981) have speculated that UA contributes to increased life span in humans by providing protection against oxidative stress-provoked ageing and cancer.
A role for XOR in other physiological processes including oxidant-mediated signal transduction has been suggested (see review by Meneshian & Bulkley, 2002).
Pathophysiological role of XOR
XOR-generated ROS are implicated in both tissue structural damage and cell signalling interference. Classically, ROS can cause lipid peroxidation, resulting in disruption of membrane architecture and lysosomal enzyme release (Weiss, 1986), and DNA and amino acid oxidation, causing genetic mutations and enzyme dysfunction or proteolysis (Kehrer, 2000). In regard to XOR, oxidative injury is often achieved via the byproducts of ·O2− and H2O2 generation. For example, in the Haber–Weiss reaction, iron(III) reacts with ·O2− to yield iron(II) and molecular oxygen; iron(II) then reacts with H2O2 to generate highly reactive ·OH (Kehrer, 2000). Hydroxyl radicals have a half-life of 1 ns and thus indiscriminately oxidize their closest target (Weiss, 1986; Kehrer, 2000). In another example of byproduct-mediated damage, peroxynitrite, which is generated from ·O2− and NO (Houston et al. 1998), is a powerful oxidant involved in nitration of tyrosine residues (Sawa et al. 2000). Dysfunction of proteins due to nitration has been implicated in the pathophysiology of several cardiovascular diseases, including autoimmune myocarditis, hypertension, and heart failure (Turko & Murad, 2002), although this remains controversial.
In 1981 Granger and colleagues hypothesized that XOR-generated ROS cause ischaemic bowel injury due to ATP catabolism during hypoxia and increased electron acceptor availability on reperfusion (Fig. 4). In their study, ischaemic damage only occurred on reperfusion and was attenuated by superoxide dismutase (SOD; Granger et al. 1981). Since this concept of ischaemia–reperfusion (I-R) injury was introduced (Granger et al. 1981; McCord, 1985), several lines of reasoning have supported XOR involvement. First, XOR products increase after I-R, as demonstrated in human aortic endothelial cells using electron paramagnetic resonance (EPR) spectroscopy (Zweier et al. 1994). EPR studies have also identified bursts of ·O2−, ·OH, and ONOO− from reperfused rabbit and rat hearts (Zweier, 1988; Wang & Zweier, 1996). Second, XOR products are associated with cardiac tissue damage, further supporting XOR's role in I-R injury. For example, reperfusion of ischaemic rat hearts results in elevated H2O2 levels and ventricular dysfunction, which can be partially attenuated with radical scavengers or allopurinol (Brown et al. 1988). Similarly, ischaemic rabbit hearts pretreated with allopurinol experience less ventricular dysfunction on reperfusion compared with untreated hearts (Terada et al. 1991b).
Figure 4. Ischaemia–reperfusion injury hypothesis.
Derived from Granger et al. (1981) and McCord (1985).
In regard to peroxynitrite-mediated damage, Ferdinandy and colleagues showed that myocardial contractile function declines with increasing XOR activity and ONOO− generation (Ferdinandy et al. 1999). Furthermore, myocardial reperfusion damage can be attenuated with SOD or NOS inhibitors, which prevent ONOO− formation (Zweier et al. 2001). Peroxynitrite also causes myocardial injury via activation of matrix metalloproteinases (MMP) (Wang et al. 2002a) like MMP-2, which degrades troponin I (Wang et al. 2002b). Activated MMPs are associated with depressed functional recovery of ischaemic rat hearts after reperfusion (Cheung et al. 2000). Other MMPs, including type I collagenase, are also activated by oxidative stress, suggesting a potential role in myocardial remodelling (Siwik et al. 2001).
In addition to tissue damage, XOR-generated ROS can also exert cardiotoxicity via interference with cell signalling (Chiamvimonvat et al. 1995; Thomas et al. 1995). For instance, ROS modify thiol-containing proteins involved in calcium cycling, such as the ryanodine receptor (Campbell et al. 1996) and Ca2+-ATPase (Xu et al. 1998), which may result in diminished myocardial performance. Pérez et al. (1998) demonstrated XOR may be responsible for such oxidant-induced effects, showing that allopurinol and oxypurinol improve force generated by stunned cardiac myofilaments while at the same time reducing systolic Ca2+ transients, thereby enhancing myofilament Ca2+ sensitivity. Moreover, subsequent studies using rat models of heart failure have suggested that changes in calcium cycling due to XOR inhibition may be an adapative response to the depression of myofilament force-generating capacity (Pérez et al. 1999).
In corroboration with this evidence supporting XOR involvement in I-R injury, XOR activity is increased after I-R (Brown et al. 1988; Ferdinandy et al. 1999). Weinbroum et al. (1995) found that XOR activity in postischaemic hepatic effluent and lung lavage fluid increases over 1000-fold and 100-fold, respectively. Elevated XOR activity also occurs on reperfusion of human aortic endothelial cells, with an eightfold elevation in UA production (Zweier et al. 1994). Elevations in circulating XOR after I–R (Weinbroum et al. 1995) can lead to tissue damage at distant sites that are low in endogenous XOR activity (Nielsen et al. 1994). One reason that XOR activity increases after I-R is that substrate availability increases significantly during ischaemia because of ATP degradation. High performance liquid chromatography (HPLC) of rat heart extracts demonstrated that ATP is most abundant under normoxic conditions. However, after 30 min of ischaemia, AMP predominates and hypoxanthine and xanthine also appear. After 5 min of reperfusion, hypoxanthine/xanthine levels are almost undetectable, whereas ATP levels have nearly recovered (Xia & Zweier, 1995). This increase in substrate availability also occurs in vivo, for urinary hypoxanthine/xanthine levels double in acute coronary syndrome patients (Turgan et al. 1999) and serum xanthine increases in patients with acute myocardial infarction (Kock et al. 1994).
Despite the supporting evidence, the role of XOR in I-R injury remains controversial. First, as discussed above, some studies of tissue distribution have found that XOR is absent from major tissues known to be adversely affected by I-R, including cardiac and brain tissue. However, many of these studies utilized functional assays that were not sensitive enough to detect the small proportion of XOR that is active in human tissues. Moreover, as described earlier, XOR has been localized to the endothelial cells of all tissues using immunohistochemistry. In addition to this, circulating XOR has been demonstrated in human, rat and rabbit serum (Nielsen et al. 1994; Weinbroum et al. 1995; Mathru et al. 1996). Elevation of circulating XOR occurs after I-R, so tissues with low endogenous XOR activity may be damaged by circulating and/or endothelium-bound XOR in the vasculature (Nielsen et al. 1994). Another argument refuting the role of XOR in I-R injury calls attention to the predominance of the XDH form in vivo, assuming that conversion from XDH to XO is essential for XOR to produce sufficient ROS to cause I-R damage. However, the time required for XOR conversion has been reported with inconsistent results varying from seconds (McCord, 1985) to hours (Engerson et al. 1987). In support of conversion of XOR in I-R injury, several studies have demonstrated an increase in the percentage of XO relative to total XOR activity during ischaemia (Chambers et al. 1985; Parks et al. 1988), but this may be due to inhibition of XDH by accumulating NADH rather than conversion from XDH to XO. Even the mechanism of conversion is controversial, with studies implicating both reversible (McKelvey et al. 1988; Brass et al. 1991) and proteolytic conversion (McCord, 1985; Engerson et al. 1987).
The significance of XOR involvement in I-R injury has also been debated because of many studies suggesting neutrophils may be the primary source of ROS. Both the depletion of neutrophils (Romson et al. 1983; Hernandez et al. 1987) and prevention of neutrophil adherence to endothelial cells with neutralizing antibody to CD18 (Hernandez et al. 1987; Duilio et al. 2001) attenuate the injury associated with tissue I-R. However, tissue damage after reperfusion has also been demonstrated in experimental systems devoid of neutrophils (Ratych et al. 1987; Brown et al. 1988). A complimentary role is likely, with ROS generated from both XOR and neutrophils causing I-R injury. This is supported by evidence that the pretreatment of feline intestine with allopurinol attenuates neutrophil infiltration after reperfusion (Grisham et al. 1986), and allopurinol and tungsten treatment inhibit neutrophil adhesion to endothelial cells (Terada et al. 1992). Interestingly, neutrophil chemotaxis may be mediated by XOR products, for tissue injection of superoxide prompts infiltration of neutrophils without additional signs of inflammation, and the infiltration is inhibited by SOD (McCord & Roy, 1982).
In response to some of these concerns, Harrison (1997) expanded the I-R hypothesis to include the NADH oxidase activity of XOR, suggesting it too may contribute to I-R oxidative stress. XDH actually exhibits preference for oxidation of NADH rather than xanthine when physiological pH is decreased to 6.5, as may occur locally in tissue ischaemia (Harrison, 1997). Furthermore, NADH levels increase two- to sixfold in guinea-pig and rat hearts during ischaemia (Williamson, 1966; Varadarajan et al. 2001), thereby increasing substrate availability for NADH oxidase activity. Interestingly, the NADH oxidase activity of XDH has a maximal rate of ·O2− production fourfold greater than XO, and it can still maintain rates of ·O2− production comparable to XO in the presence of 20 μm NAD+ (Sanders et al. 1997), suggesting conversion from XDH to XO is not necessary for superoxide-mediated I-R injury. Harrison's hypothesis is especially interesting because NADH oxidase activity is maintained in demolybdo- and desulpho-XOR, so traditional XOR assays may not detect this activity. Moreover, it provides a rationale consistent with the apparently low activity of XOR in human tissues. Importantly, NADH oxidase activity is not inhibited by allopurinol, since it is independent of the Mo–Co site (Sanders et al. 1997; Zhang et al. 1998a). Therefore, in many studies where allopurinol was unable to completely attenuate injury due to ·O2− (Brown et al. 1988; Thompson-Gorman & Zweier, 1990; Terada et al. 1992), NADH oxidase activity may have been responsible. The NADH oxidase activity of XOR is effectively inhibited by DPI (Sanders et al. 1997), which also inhibits neutrophil NADPH oxidase, a fact not considered in some investigations of the relative contributions of XOR and neutrophils to I-R injury. Recently, Zima et al. (2003) demonstrated that NADH inhibits cardiac ryanodine receptor function, and it is plausible to speculate that the NADH oxidase activity of XOR may be involved. As with Granger's original hypothesis, Harrison's proposal does not exclude other means of ROS generation and I-R injury, and it may be that superoxide production by both XO and XDH-NADH oxidase contribute to I-R injury in vivo.
XOR in cardiovascular disease
The success of allopurinol in attenuation of myocardial I-R in animal studies prompted clinical trials to determine if it might clinically benefit patients. In aortocoronary bypass surgery, allopurinol administration to patients attenuated histological I-R damage to the myocardium and resulted in a threefold reduction in the need for inotropic support postoperatively (Gimpel et al. 1995). Similar benefits were demonstrated in patients with acute myocardial infarction undergoing primary coronary angioplasty; those who received allopurinol before reperfusion had significantly better left ventricular function both immediately after the procedure and 6 months later (Guan et al. 2003).
Oxidative stress contributes significantly to endothelial dysfunction in cardiovascular disease, as superoxide radicals readily inactivate endothelial NO, thereby impairing vasorelaxation. In fact, NO and .O2− react at a rate threefold greater than the rate at which SOD can eliminate .O2− (Cai & Harrison, 2000), and thus oxidative stress may overpower antioxidant protection in many disease states, resulting in endothelial dysfunction. Houston et al. (1999) established that XOR contributes to impairment of vasorelaxation by showing that endothelium-bound XOR inhibits NO-dependent cGMP production in smooth muscle cells. Furthermore, many clinical studies have established that XOR inhibition attenuates endothelial dysfunction. For example, allopurinol increases NO-stimulated blood flow in smokers (Guthikonda et al. 2003) and diabetics (Butler et al. 2000), reversing endothelial dysfunction. XOR may also contribute to endothelial dysfunction in hypercholesterolaemia. In cholesterol-fed rabbits ·O2− production is threefold greater in diseased vessels than normal vessels (Ohara et al. 1993), and oxypurinol improves endothelial function in the diseased vessels (Ohara et al. 1993; White et al. 1996). Further studies in rabbits have indicated ·O2− production can be attenuated by injection of heparin or heparin-binding SOD, since heparin can out-compete XOR for binding to endothelial cell surface glycosaminoglycans. Interestingly, heparin administration is also associated with improved endothelial function (White et al. 1996). The role of XOR in endothelial dysfunction in diseased coronary arteries has been directly demonstrated by measurement of endothelium-bound XOR activity, which is elevated >200% in diseased vessels and exhibits a negative relationship with endothelium-mediated vasodilatation (Landmesser et al. 2002). Furthermore, cholesterol and XOR have been colocalized in atherosclerotic plaques from carotid endarterectomy specimens. Local concentrations of UA are elevated five- to sixfold in such plaques, indicating up-regulation of XOR activity (Patetsios et al. 2001).
XOR has also been implicated in the pathogenesis of hypertension. For example, tungsten-rich diets, which inactivate XOR, successfully lower blood pressure in Dahl salt-sensitive hypertensive rats but have no effect on Dahl salt-resistant rats, indicating a potential role for XOR-generated ROS in salt-induced hypertension (Swei et al. 1999). Furthermore, treatment with tungsten (Suzuki et al. 1998), oxypurinol (Nakazono et al. 2003), or heparin-binding SOD (Nakazono et al. 2003) lowers blood pressure in spontaneously hypertensive rats (SHR). Laakso et al. (1998) measured renal XOR activity in both of these rat models and demonstrated that increased sodium intake elevates activity in a dose-dependent manner in Dahl salt-sensitive rats. XOR activity in SHR kidneys also increases in response to increased salt intake, but in contrast, renal XOR activity is already elevated at baseline in SHR when compared with normotensive rats (Laakso et al. 1998). In contrast to other studies (Nakazono et al. 2003), Laakso et al. (1998) found that XOR inhibition did not affect systolic blood pressure in SHR, suggesting induction of XOR renal activity may occur secondary to increased salt intake or salt-induced hypertension.
There is evidence that UA levels correlate with blood pressure (Mazzali et al. 2001), independent of XOR activity. When mild hyperuricaemia is induced in rats with oxonic acid (urate oxidase inhibitor), hypertension develops that is preventable by treatment with allopurinol or a uricosuric agent (Mazzali et al. 2001). Once elevated, blood pressure can be decreased by allopurinol, withdrawal of oxonic acid, or treatment with enalapril or l-arginine. Oxonic acid-treated rats also develop renal injury attenuated by enalapril or l-arginine (Mazzali et al. 2001). In rats fed low-salt diets, oxonic acid results in systemic and glomerular hypertension preventable with allopurinol (Sánchez-Lozada et al. 2002). Renal biopsies from oxonic acid-treated rats show afferent arteriolar thickening that correlates with blood pressure and uric acid levels and is preventable with allopurinol (Sánchez-Lozada et al. 2002). The importance of UA is further supported by the finding that elevated serum UA is associated with increased cardiovascular risk in hypertensive patients, regardless of whether their hypertension is treated (Alderman et al. 1999). Recently, UA has also been shown to have prognostic value in patients with heart failure (Anker et al. 2003).
Heart failure (HF) is often the ultimate result of pathogenetic mechanisms that cause endothelial dysfunction and hypertension. Thus, studies of XOR in these disease states have prompted investigation of its role in HF. Patients with HF have significantly reduced endothelium-associated SOD activity when compared with controls, whereas their endothelium-bound XOR activity is enhanced >200% (Landmesser et al. 2002). Randomized, placebo-controlled trials of allopurinol have demonstrated attenuation of endothelial dysfunction in HF patients (Doehner et al. 2002; Farquharson et al. 2002), with improved endothelial-dependent vasodilatation and enhanced peak blood flow (Doehner et al. 2002).
In HF patients, XOR also adversely affects myocardial function, specifically myocardial energetics. In both experimental animals and humans, allopurinol is effective in attenuating the mechanoenergetic uncoupling that occurs in HF. For example, in dogs with pacing-induced HF, allopurinol decreases myocardial oxygen consumption and increases myocardial contractility (Ekelund et al. 1999). In addition to improving left ventricular function at rest in HF dogs, allopurinol also enhances contractile performance of the myocardium in response to exercise- and dobutamine-induced β-adrenergic stimulation (Ukai et al. 2001). We have demonstrated that high dose ascorbate mimicks these effects of allopurinol. Interestingly, NOS inhibition prevents the benefits of allopurinol and ascorbate in HF dogs, suggesting XOR-generated ·O2− may interfere with NO regulation of myocardial energetics. NOS inhibition also decreases myocardial efficiency in healthy dogs, further substantiating the role of XOR and NO cross-talk in HF (Saavedra et al. 2002). Normal interaction between XOR and NO may be disrupted in HF because XOR abundance in failing myocardium is elevated (Fig. 5) (Ekelund et al. 1999; de Jong et al. 2000; Cappola et al. 2001). Recently Kögler et al. (2003) found that rat myofilament twitch tension is enhanced by oxypurinol. However, the effect is much more pronounced in failing myocardium, where total XOR activity is elevated relative to normal myocardium, with a fractional increase in twitch tension that is threefold higher in failing myocardium (Kögler et al. 2003). Thus, the inotropic consequences of oxypurinol are in effect relatively selective for heart failure, where XOR activity is elevated. In regard to the potential therapeutic use of oxypurinol in HF patients, oxypurinol does not affect resting tension or intracellular Ca2+ transients; thus diastolic myocardial function is not impaired (Kögler et al. 2003). Most importantly, in corroboration with these animal studies, we have demonstrated that allopurinol infusion into the coronary circulation of HF patients improves myocardial efficiency by diminishing oxygen consumption (Cappola et al. 2001), suggesting XOR inhibition is a potential strategy for the treatment of human heart failure.
Figure 5. XOR is up-regulated in heart failure.
A, XOR immunoblot of myocardial extracts from patients with idiopathic dilated cardiomyopathy (CM) or normal cardiac function (NL) probed with monoclonal anti-XDH antibody. Bands correspond to XDH (145 kDa) and XO (125, 85 kDa). B, densitometry depicting average XOR signal from all patients. Signal is increased 60% in patients with idiopathic dilated cardiomyopathy (*P < 0.05, Student's unpaired t test). From Cappola et al. 2001). Reprinted with permission from Lippincott Williams & Wilkins.
Conclusion
Over a century after its discovery, our knowledge of the biological activity of XOR continues to grow. In the past 20 years, greater understanding of its genetic and biochemical properties has enhanced appreciation of XOR participation in human physiology and pathophysiology. Both laboratory and clinical investigations have implicated XOR in oxidative stress-related diseases, most recently in cardiovascular disease. The precise mechanisms by which XOR inflicts tissue damage and disrupts cell signalling in the cardiovascular system remain to be elucidated, but many studies suggest XOR-oriented therapy holds promise for patients with heart failure and other cardiovascular diseases. The new appreciation of the role of XOR in cardiovascular pathophysiology has led to a wave of clinical trials. The largest of these studies, entitled ‘A Phase II–III Prospective, Randomized, Double-Blind, Placebo-Controlled Efficacy and Safety Study of Oxypurinol Added to Standard Therapy in Patients with NYHA Class III–IV Congestive Heart Failure’ (OPT-CHF), is a 400 patient study currently enrolling participants in the United States and Canada. If positive, the results of this trial have the potential to substantially influence heart failure management in the future.
Acknowledgments
This work was supported by a Paul Beeson Physician Faculty Scholars in Ageing Research Award and NIH RO1 HL-065455. The authors are indebted to Ryan M. Kretzer for his careful review of this manuscript.
Disclosure statement
J.M.H. is a paid consultant at Cardiome Pharma Corporation. The terms of this arrangement are being managed by The John Hopkins University in accordance with its conflict of interest policies.
References
- Abadeh S, Case PC, Harrison R. Purification of xanthine oxidase from human heart. Biochem Soc Trans. 1993;21:99S. doi: 10.1042/bst021099s. [DOI] [PubMed] [Google Scholar]
- Abadeh S, Killacky J, Benboubetra M, Harrison R. Purification and partial characterization of xanthine oxidase from human milk. Biochim Biophys Acta. 1992;1117:25–32. doi: 10.1016/0304-4165(92)90157-p. [DOI] [PubMed] [Google Scholar]
- Adachi T, Fukushima T, Usami Y, Hirano K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial cell surface. Biochem J. 1993;289:523–527. doi: 10.1042/bj2890523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderman MH, Cohen H, Madhavan S, Kivlighn S. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension. 1999;34:144–150. doi: 10.1161/01.hyp.34.1.144. [DOI] [PubMed] [Google Scholar]
- Amaya Y, Yamazaki K, Sato M, Noda K, Nishino T, Nishino T. Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. Amino acid sequence of rat liver xanthine dehydrogenase and identification of the cleavage sites of the enzyme protein during irreversible conversion by trypsin. J Biol Chem. 1990;265:14170–14175. [PubMed] [Google Scholar]
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJS. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional and hemodynamic staging. Circulation. 2003;107:1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med. 1993;14:615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- Brass CA, Narciso J, Gollan JL. Enhanced activity of the free radical producing enzyme xanthine oxidase in hypoxic rat liver. J Clin Invest. 1991;87:424–431. doi: 10.1172/JCI115013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray RC, Gutteridge S, Stotter DA, Tanner SJ. The mechanism of action of xanthine oxidase: the relationship between the rapid and very rapid molybdenum electron-paramagnetic-resonance signals. Biochem J. 1979;177:357–360. doi: 10.1042/bj1770357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Terada LS, Grosso MA, Whitmann GJ, Velasco SE, Patt A, Harken AH, Repine JE. Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J Clin Invest. 1988;81:1297–1301. doi: 10.1172/JCI113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder G, Heid HW, Jarasch ED, Mather IH. Immunological identification and determination of xanthine oxidase in cells and tissues. Differentiation. 1983;23:218–225. doi: 10.1111/j.1432-0436.1982.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Bruder G, Jarasch ED, Heid HW. High concentrations of antibodies to xanthine oxidase in human and animal sera: molecular characterization. J Clin Invest. 1984;74:783–794. doi: 10.1172/JCI111494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R, Morris AD, Belch JJF, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular disease: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes: dual mechanism regulation by nitric oxide and S-nitrosothiols. J General Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marbán E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- Chambers DE, Parks DA, Patterson G, Roy R, McCord JM, Yoshida S, Parmley LF, Downey JM. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol. 1985;17:145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- Cheung P, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation. 2000;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- Chiamvimonvat N, O'Rourke B, Kamp TJ, Kallen RG, Hofmann F, Flockerzi V, Marban E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ Res. 1995;76:325–334. doi: 10.1161/01.res.76.3.325. [DOI] [PubMed] [Google Scholar]
- Della Corte E, Gozzetti G, Novello F, Stirpe F. Properties of the xanthine oxidase from human liver. Biochim Biophys Acta. 1969;191:164–166. doi: 10.1016/0005-2744(69)90327-1. [DOI] [PubMed] [Google Scholar]
- Della Corte E, Stirpe F. The regulation of rat liver xanthine oxidase: involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972;126:739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehner W, Schoene N, Rachhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJS, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- Doel JJ, Godber BLJ, Eisenthal R, Harrison R. Reduction of organic nitrates catalysed by xanthine oxidoreductase under anaerobic conditions. Biochim Biophys Acta. 2001;1527:81–87. doi: 10.1016/s0304-4165(01)00148-9. [DOI] [PubMed] [Google Scholar]
- Doel JJ, Godber BLJ, Goult TA, Eisenthal R, Harrison R. Reduction of organic nitrites to nitric oxide catalyzed by xanthine oxidase: possible role in metabolism of nitrovasodilators. Biochem Biophys Res Commun. 2000;270:880–885. doi: 10.1006/bbrc.2000.2534. [DOI] [PubMed] [Google Scholar]
- Duilio C, Ambrosio G, Kuppusamy P, Dipaula A, Becker LC, Zweier JL. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am J Physiol. 2001;280:H2649–H2657. doi: 10.1152/ajpheart.2001.280.6.H2649. [DOI] [PubMed] [Google Scholar]
- Dupont GP, Huecksteadt T, Marshall BC, Ryan US, Michael JR, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity and gene expression in cultured rat pulmonary endothelial cells. J Clin Invest. 1992;89:197–202. doi: 10.1172/JCI115563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy LJ, Stewart JR, Jones HP, Engerson TD, McCord JM, Downey JM. Free radical-producing enzyme, xanthine oxidase, is undetectable in human hearts. Am J Physiol. 1987;253:H709–H711. doi: 10.1152/ajpheart.1987.253.3.H709. [DOI] [PubMed] [Google Scholar]
- Ekelund UEG, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, Kass DA, Marbán E, Hare JM. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ Res. 1999;85:437–445. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- Engerson TD, McKelvey TG, Rhyne DB, Boggio EB, Snyder SJ, Jones HP. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest. 1987;79:1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth C, Eger BT, Okamoto K, Nishino T, Nishino T, Pai EF. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion. Proc Natl Acad Sci U S A. 2000;97:10723–10728. doi: 10.1073/pnas.97.20.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquharson CAJ, Butler R, Hill A, Belch JJF, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Panas D, Schulz R. Peroxynitrite contributes to spontaneous loss of cardiac efficiency in isolated working rat hearts. Am J Physiol. 1999;276:H1861–H1867. doi: 10.1152/ajpheart.1999.276.6.H1861. [DOI] [PubMed] [Google Scholar]
- Frederiks WM, Vreeling-Sindelarova H. Ultrastructural localization of xanthine oxidoreductase activity in isolated rat liver cells. Acta Histochem. 2002;104:29–37. doi: 10.1078/0065-1281-00629. [DOI] [PubMed] [Google Scholar]
- Gimpel JA, Lahpor JR, van der Molen A, Damen J, Hitchcock JF. Reduction of reperfusion injury of human myocardium by allopurinol: a clinical study. Free Radic Biol Med. 1995;19:251–255. doi: 10.1016/0891-5849(94)00242-c. [DOI] [PubMed] [Google Scholar]
- Godber BLJ, Doel JJ, Durgan J, Eisenthal R, Harrison R. A new route to peroxynitrite: a role for xanthine oxidoreductase. FEBS Lett. 2000a;475:93–96. doi: 10.1016/s0014-5793(00)01639-2. [DOI] [PubMed] [Google Scholar]
- Godber BLJ, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem. 2000b;275:7757–7763. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- Godber BLJ, Sanders S, Harrison R, Eisenthal R, Bray RC. >95% of xanthine oxidase in human milk is present as the demolybdo form, lacking molybdopterin. Biochem Soc Trans. 1997;25:519S. doi: 10.1042/bst025519s. [DOI] [PubMed] [Google Scholar]
- Granger DN, Rutili G, McCord J. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Guan W, Osanai T, Kamada T, Hanada H, Ishizaka H, Onodera H, Iwasa A, Fujita N, Kudo S, Ohkubo T, Okumura K. Effect of allopurinol pretreatment on free radical generation after primary coronary angioplasty for acute myocardial infarction. J Cardiovasc Pharmacol. 2003;41:699–705. doi: 10.1097/00005344-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Guercionni R, Szumlanski C, Weinshilboum RM. Human liver xanthine oxidase: nature and extent of individual variation. Clin Pharmacol Ther. 1991;50:663–672. doi: 10.1038/clpt.1991.205. [DOI] [PubMed] [Google Scholar]
- Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–421. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- Hancock JT, Salisbury V, Ovejero-Boglione MC, Cherry R, Hoare C, Eisenthal R, Harrison R. Antimicrobial properties of milk: dependence on presence of xanthine oxidase and nitrite. Antimicrob Agents Chemother. 2002;46:3308–3310. doi: 10.1128/AAC.46.10.3308-3310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CM, Massey V. The oxidative half-reaction of xanthine dehydrogenase with NAD; reaction kinetics and steady-state mechanism. J Biol Chem. 1997a;272:28335–28341. doi: 10.1074/jbc.272.45.28335. [DOI] [PubMed] [Google Scholar]
- Harris CM, Massey V. The reaction of reduced xanthine dehydrogenase with molecular oxygen – reaction kinetics and measurement of superoxide radical. J Biol Chem. 1997b;272:8370–8379. doi: 10.1074/jbc.272.13.8370. [DOI] [PubMed] [Google Scholar]
- Harrison R. Human xanthine oxidoreductase: in search of a function. Biochem Soc Trans. 1997;25:786–791. doi: 10.1042/bst0250786. [DOI] [PubMed] [Google Scholar]
- Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med. 2002;33:774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- Hassoun PM, Yu F, Cote CG, Zulueta JJ, Sawhney R, Skinner KA, Skinner HB, Parks DA, Lanzillo JJ. Upregulation of xanthine oxidase by lipopolysaccharide, interleukin-1, and hypoxia: role in acute lung injury. Am J Resp Crit Care Med. 1998;158:299–305. doi: 10.1164/ajrccm.158.1.9709116. [DOI] [PubMed] [Google Scholar]
- Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- Hille R, Anderson RF. Electron transfer in milk xanthine oxidase as studied by pulse radiolysis. J Biol Chem. 1991;266:5608–5615. [PubMed] [Google Scholar]
- Hille R, Massey V. Studies on the oxidative half-reaction of xanthine oxidase. J Biol Chem. 1981;256:9090–9095. [PubMed] [Google Scholar]
- Hille R, Nishino T. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 1995;9:995–1003. [PubMed] [Google Scholar]
- Hoidal JR, Xu P, Huecksteadt T, Sanders KA, Pfeffer K. Transcriptional regulation of human xanthine dehydrogenase/xanthine oxidase. Biochem Soc Trans. 1997;25:796–799. doi: 10.1042/bst0250796. [DOI] [PubMed] [Google Scholar]
- Houston M, Chumley P, Radi R, Rubbo H, Freeman BA. Xanthine oxidase reaction with nitric oxide and peroxynitrite. Arch Biochem Biophys. 1998;355:1–8. doi: 10.1006/abbi.1998.0675. [DOI] [PubMed] [Google Scholar]
- Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, Freeman BA. Binding of xanthine oxidase to vascular endothelium: kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem. 1999;274:4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- Huber R, Hof P, Duarte RO, Moura JJG, Moura I, Liu M, LeGall J, Hille R, Archer M, Romao M. A structure-based catalytic mechanism for the xanthine oxidase family of molybdenum enzymes. Proc Natl Acad Sci U S A. 1996;93:8846–8851. doi: 10.1073/pnas.93.17.8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J, Massey V, Dunham WR, Sands RW. Redox potentials of milk xanthine dehydrogenase – room temperature measurement of the FAD and 2Fe/2S center potentials. J Biol Chem. 1993;268:18685–18691. [PubMed] [Google Scholar]
- Ichida K, Amaya Y, Kamatani N, Nishino T, Hosoya T, Sakai O. Identification of two mutations in human xanthine dehydrogenase gene responsible for classical type I xanthinuria. J Clin Invest. 1997;99:2391–2397. doi: 10.1172/JCI119421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida K, Amaya Y, Noda K, Minoshima S, Hosoya T, Sakai O, Shimizu N, Nishino T. Cloning of the cDNA encoding human xanthine dehydrogenase (oxidase): structural analysis of the protein and chromosomal location of the gene. Gene. 1993;133:279–284. doi: 10.1016/0378-1119(93)90652-j. [DOI] [PubMed] [Google Scholar]
- Ichida K, Matsumara T, Sakuma R, Hosoya T, Nishino T. Mutation of human molybdenum cofactor sulfurase gene is responsible for classical xanthinuria type II. Biochem Biophys Res Commun. 2001;282:1194–1200. doi: 10.1006/bbrc.2001.4719. [DOI] [PubMed] [Google Scholar]
- Ichikawa M, Nishino T, Nishino T, Ichikawa A. Subcellular localization of xanthine oxidase in rat hepatocytes: high-resolution immunoelectron microscopic study combined with biochemical analysis. J Histochem Cytochem. 1992;40:1097–1103. doi: 10.1177/40.8.1619276. [DOI] [PubMed] [Google Scholar]
- Ichimori K, Fukahori M, Nakazawa H, Okamoto K, Nishino T. Inhibition of xanthine oxidase and xanthine dehydrogenase by nitric oxide: nitric oxide converts reduced xanthine-oxidizing enzymes into the desulfo-type inactive form. J Biol Chem. 1999;274:7763–7768. doi: 10.1074/jbc.274.12.7763. [DOI] [PubMed] [Google Scholar]
- Jarasch ED, Bruder G, Heid HW. Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol Scand. 1986;548:39–46. [PubMed] [Google Scholar]
- Jarasch ED, Grund C, Bruder G, Heid HW, Keenan TW, Franke WW. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981;25:67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- de Jong JW, Schoemaker RG, de Jonge R, Bernocchi P, Keijzer E, Harrison R, Sharma HS, Ceconi C. Enhanced expression and activity of xanthine oxidoreductase in the failing heart. J Mol Cell Cardiol. 2000;32:2083–2089. doi: 10.1006/jmcc.2000.1240. [DOI] [PubMed] [Google Scholar]
- de Jong JW, van der Meer P, Nieukoop S, Huizer T, Stroeve RJ, Bos E. Xanthine oxidoreductase activity in perfused hearts of various species, including humans. Circ Res. 1990;67:770–773. doi: 10.1161/01.res.67.3.770. [DOI] [PubMed] [Google Scholar]
- Kayyali US, Donaldson C, Huang H, Abdelnour R, Hassoun PM. Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. J Biol Chem. 2001;276:14359–14365. doi: 10.1074/jbc.M010100200. [DOI] [PubMed] [Google Scholar]
- Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Kisker C, Schindelin H, Rees DC. Molybdenum cofactor-containing enzymes: structure and mechanism. Annu Rev Biochem. 1997;66:233–267. doi: 10.1146/annurev.biochem.66.1.233. [DOI] [PubMed] [Google Scholar]
- Kock R, Delvoux B, Sigmund M, Greiling H. A comparative study of the concentrations of hypoxanthine, xanthine, uric acid, and allantoin in the peripheral blood of normals and patients with acute myocardial infarction and other ischaemic diseases. Eur J Clin Chem Clin Biochem. 1994;32:837–842. doi: 10.1515/cclm.1994.32.11.837. [DOI] [PubMed] [Google Scholar]
- Kögler H, Fraser H, McCune S, Altschuld R, Marbán E. Disproportionate enhancement of myocardial contractility by the xanthine oxidase inhibitor oxypurinol in failing rat myocardium. Cardiovasc Res. 2003;59:582–592. doi: 10.1016/s0008-6363(03)00512-1. [DOI] [PubMed] [Google Scholar]
- Krenitsky TA, Spector T, Hall WW. Xanthine oxidase from human liver: purification and characterization. Arch Biochem Biophys. 1986;247:108–119. doi: 10.1016/0003-9861(86)90539-4. [DOI] [PubMed] [Google Scholar]
- Krenitsky TA, Tuttle JV, Cattau EL, Wang P. A comparison of the distribution and electron acceptor specificities of xanthine oxidase and aldehyde oxidase. Comp Biochem Physiol B. 1974;49:687–703. doi: 10.1016/0305-0491(74)90256-9. [DOI] [PubMed] [Google Scholar]
- Kurosaki M, Zanotta S, Li Calzi M, Garattini E, Terao M. Expression of xanthine oxidoreductase in mouse mammary epithelium during pregnancy and lactation: regulation of gene expression by glucocorticoids and prolactin. Biochem J. 1996;319:801–810. doi: 10.1042/bj3190801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara Y, Nishino T, Okamoto K, Matsumara T, Eger BT, Pai EF, Nishino T. Unique amino acids cluster for switching from the dehydrogenase to oxidase form of xanthine oxidoreductase. Proc Natl Acad Sci U S A. 2003;100:8170–8175. doi: 10.1073/pnas.1431485100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso J, Mervaala E, Himberg J, Teräväinen T, Karppanen H, Vapaatalo H, Lapatto R. Increased kidney xanthine oxidoreductase activity in salt-induced experimental hypertension. Hypertension. 1998;32:902–906. doi: 10.1161/01.hyp.32.5.902. [DOI] [PubMed] [Google Scholar]
- Laakso J, Vaskonen T, Mervaala E, Vapaatalo H, Lapatto R. Inhibition of nitric oxide synthase induces renal xanthine oxidoreductase activity in spontaneously hypertensive rats. Life Sci. 1999;65:2679–2685. doi: 10.1016/s0024-3205(99)00536-6. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- Lanzillo JJ, Yu F, Stevens J, Hassoun PM. Determination of xanthine dehydrogenase mRNA by a reverse transcription-coupled competitive quantitative polymerase chain reaction assay: regulation in rat endothelial cells by hypoxia and hyperoxia. Arch Biochem Biophys. 1996;335:377–380. doi: 10.1006/abbi.1996.0519. [DOI] [PubMed] [Google Scholar]
- Lee C, Liu X, Zweier JL. Regulation of xanthine oxidase by nitric oxide and peroxynitrite. J Biol Chem. 2000;275:9369–9376. doi: 10.1074/jbc.275.13.9369. [DOI] [PubMed] [Google Scholar]
- Li H, Samouilov A, Liu XI, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction: evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry. 2003;42:1150–1159. doi: 10.1021/bi026385a. [DOI] [PubMed] [Google Scholar]
- Linder N, Rapola J, Raivio KO. Cellular expression of xanthine oxidoreductase protein in normal human tissues. Laboratory Invest. 1999;79:967–974. [PubMed] [Google Scholar]
- Martelin E, Palvimo JJ, Lapatto R, Raivio KO. Nuclear factor Y activates the human xanthine oxidoreductase gene promoter. FEBS Lett. 2000;480:84–88. doi: 10.1016/s0014-5793(00)01909-8. [DOI] [PubMed] [Google Scholar]
- Massey V, Komai H, Palmer G, Elion GB. On the mechanism of inactivation of xanthine oxidase by allopurinol and other pyrazolo[3,4-d]pyrimidines. J Biol Chem. 1970;245:2837–2844. [PubMed] [Google Scholar]
- Massey V, Schopfer LM, Nishino T, Nishino T. Differences in protein structure of xanthine dehydrogenase and xanthine oxidase revealed by reconstitution with flavin active site probes. J Biol Chem. 1989;264:10567–10573. [PubMed] [Google Scholar]
- Mathews CK, Van Holde KE. Biochemistry. 2nd. edn. Menlo Park, CA, USA: Benjamin/Cummings Publishing Co., Inc.; 1996. Nucleotide metablism; pp. 793–793. [Google Scholar]
- Mathru M, Dries DJ, Barnes L, Tonino P, Sukhani R, Rooney MW. Tourniquet-induced exsanguination in patients requiring lower limb surgery. An ischemia-reperfusion model of oxidant and antioxidant metabolism. Anesthesiology. 1996;84:14–22. doi: 10.1097/00000542-199601000-00003. [DOI] [PubMed] [Google Scholar]
- Mazzali M, Hughes J, Kim Y, Jefferson A, Kang D, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McCord JM, Roy RS. The pathophysiology of superoxide: roles in inflammation and ischemia. Can J Physiol Pharmacol. 1982;60:1346–1352. doi: 10.1139/y82-201. [DOI] [PubMed] [Google Scholar]
- McKelvey TG, Höllwarth ME, Granger DN, Engerson TD, Landler U, Jones HP. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am J Physiol. 1988;254:G753–G760. doi: 10.1152/ajpgi.1988.254.5.G753. [DOI] [PubMed] [Google Scholar]
- Meneshian A, Bulkley GB. The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirc. 2002;9:161–175. doi: 10.1038/sj.mn.7800136. [DOI] [PubMed] [Google Scholar]
- Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- Murray KN, Watson JG, Chaykin S. Catalysis of the direct transfer of oxygen from nicotinamide N-oxide to xanthine by xanthine oxidase. J Biol Chem. 1966;241:4798–4801. [PubMed] [Google Scholar]
- Muxfeldt M, Schaper W. The activity of xanthine oxidase in heart of pigs, guinea pigs, rabbits, rats, and humans. Basic Res Cardiol. 1987;82:486–492. doi: 10.1007/BF01907096. [DOI] [PubMed] [Google Scholar]
- Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Iwasaki T. Does superoxide underlie the pathogenesis of hypertension. Proc Natl Acad Sci U S A. 2003;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen VG, Weinbroum A, Tan S, Samuelson PN, Gelman S, Parks DA. Xanthine oxidoreductase release after descending thoracic aorta occlusion and reperfusion in rabbits. J Thorac Cardiovasc Surg. 1994;107:1222–1227. [PubMed] [Google Scholar]
- Nishino T, Nishino T. The conversion from the dehydrogenase type to the oxidase type of rat liver xanthine dehydrogenase by modification of cysteine residues with fluorodinitrobenzene. J Biol Chem. 1997;272:29859–29864. doi: 10.1074/jbc.272.47.29859. [DOI] [PubMed] [Google Scholar]
- Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JS, Ballou DP, Palmer G, Massey V. The mechanism of action of xanthine oxidase. J Biol Chem. 1974a;249:4363–4382. [PubMed] [Google Scholar]
- Olson JS, Ballou DP, Palmer G, Massey V. The reaction of xanthine oxidase with molecular oxygen. J Biol Chem. 1974b;249:4350–4362. [PubMed] [Google Scholar]
- Page S, Powell D, Benboubetra M, Stevens CR, Blake DR, Selase F, Wolstenholme AJ, Harrison R. Xanthine oxidoreductase in human mammary epithelial cells: activation in response to inflammatory cytokines. Biochim Biophys Acta. 1998;1381:191–202. doi: 10.1016/s0304-4165(98)00028-2. [DOI] [PubMed] [Google Scholar]
- Panus PC, Wright SA, Chumley P, Radi R, Freeman BA. The contribution of vascular endothelial xanthine dehydrogenase/oxidase to oxygen-mediated cell injury. Arch Biochem Biophys. 1992;294:695–702. doi: 10.1016/0003-9861(92)90743-g. [DOI] [PubMed] [Google Scholar]
- Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution, and physiology. Acta Physiol Scand. 1986;548:87–99. [PubMed] [Google Scholar]
- Parks DA, Williams TK, Beckman JS. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol. 1988;254:G768–G744. doi: 10.1152/ajpgi.1988.254.5.G768. [DOI] [PubMed] [Google Scholar]
- Patetsios P, Song M, Shutze WP, Pappas C, Rodino W, Ramirez JA, Panetta TF. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am J Cardiol. 2001;88:188–191. doi: 10.1016/s0002-9149(01)01621-6. [DOI] [PubMed] [Google Scholar]
- Pérez NG, Gao WD, Marbán E. Novel myofilament Ca2+-sensitizing property of xanthine oxidase inhibitors. Circ Res. 1998;83:423–430. doi: 10.1161/01.res.83.4.423. [DOI] [PubMed] [Google Scholar]
- Pfeffer K, Huecksteadt T, Hoidal JR. Xanthine dehydrogenase and xanthine oxidase activity and gene expression in renal epithelial cells: cytokine and steroid regulation. J Immunol. 1994;153:1789–1797. [PubMed] [Google Scholar]
- Poss WB, Huecksteadt T, Panus PC, Freeman BA, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia. Am J Physiol. 1996;270:L941–L946. doi: 10.1152/ajplung.1996.270.6.L941. [DOI] [PubMed] [Google Scholar]
- Radi R, Rubbo H, Bush K, Freeman BA. Xanthine oxidase binding to glycosaminoglycans: kinetics and superoxide dismutase interactions of immobilized xanthine oxidase-heparin complexes. Arch Biochem Biophys. 1997;339:125–135. doi: 10.1006/abbi.1996.9844. [DOI] [PubMed] [Google Scholar]
- Ratych RE, Chuknyiska RS, Bulkley GB. The primary localization of free radical generation after anoxia/reoxygenation in isolated endothelial cells. Surgery. 1987;102:122–131. [PubMed] [Google Scholar]
- Romão MJ, Archer M, Moura I, Moura JJG, LeGall J, Engh R, Schneider M, Hof P, Huber R. Crystal structure of the xanthine oxidase-related aldehyde oxidoreductase from D. gigas. Science. 1995;270:1170–1176. doi: 10.1126/science.270.5239.1170. [DOI] [PubMed] [Google Scholar]
- Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Rouquette M, Page S, Bryant R, Benboubetra M, Stevens CR, Blake DR, Whish WD, Harrison R, Tosh D. Xanthine oxidoreductase is asymmetrically localised on the outer surface of human endothelial and epithelial cells in culture. FEBS Lett. 1998;426:397–401. doi: 10.1016/s0014-5793(98)00385-8. [DOI] [PubMed] [Google Scholar]
- Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie J, Harrison RW, Zeichner J, Mudrick D, Marbán E, Kass DA, Hare JM. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- Saito T, Nishino T. Differences in redox and kinetic properties between NAD-dependent and O2-dependent types of rat liver xanthine dehydrogenase. J Biol Chem. 1989;264:10015–10022. [PubMed] [Google Scholar]
- Saito T, Nishino T, Massey V. Differences in environment of FAD between NAD-dependent and O2-dependent types of rat liver xanthine dehydrogenase shown by active site probe study. J Biol Chem. 1989;264:15930–15935. [PubMed] [Google Scholar]
- Sakamoto N, Yamamoto T, Moriwaki Y, Teranishi T, Toyoda M, Onishi Y, Kuroda S, Sakaguchi K, Fujisawa T, Maeda M, Hada T. Identification of a new point mutation in the human xanthine dehydrogenase gene responsible for a case of classical type I xanthinuria. Hum Genet. 2001;108:279–283. doi: 10.1007/s004390100477. [DOI] [PubMed] [Google Scholar]
- Sánchez-Lozada LG, Tapia E, Avila-Casado C, Soto V, Franco M, Santamaría J, Nakagawa T, Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Mild hyperuricemia induces glomerular hypertension in normal rats. Am J Physiol. 2002;283:F1105–F1110. doi: 10.1152/ajprenal.00170.2002. [DOI] [PubMed] [Google Scholar]
- Sanders S, Eisenthal RS, Harrison R. NADH oxidase activity of human xanthine oxidoreductase – generation of superoxide anion. Eur J Biochem. 1997;245:541–548. doi: 10.1111/j.1432-1033.1997.00541.x. [DOI] [PubMed] [Google Scholar]
- Sawa T, Akaike T, Maeda H. Tyrosine nitration by peroxynitrite formed from nitric oxide and superoxide generated by xanthine oxidase. J Biol Chem. 2000;275:32467–32474. doi: 10.1074/jbc.M910169199. [DOI] [PubMed] [Google Scholar]
- Schardinger F. Über das Verhalten der Kuhmilch gegen Methylenblau und seine Verwendung zur Unterscheidung von ungekochter und gekochter Milch. Untersuch Nahrungs Genussmittel. 1902;5:1113–1121. [Google Scholar]
- Segal BH, Sakamoto N, Patel M, Maemura K, Klein AS, Holland SM, Bulkley GB. Xanthine oxidase contributes to host defense against Burkholderia cepacia in the p47phox−/− mouse model of chronic granulomatous disease. Infect Immun. 2000;68:2374–2378. doi: 10.1128/iai.68.4.2374-2378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds HA. Purine and pyrimidine disorders. In: Holton JB, editor. The Inherited Metabolic Diseases. 2nd. edn. New York: Churchill Livingstone; 1994. pp. 297–307. [Google Scholar]
- Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- Stevens CR, Millar TM, Clinch JG, Kanczler J, Bodamyali T, Blake DR. Antibacterial properties of xanthine oxidase in human milk. Lancet. 2000;356:829–830. doi: 10.1016/s0140-6736(00)02660-x. [DOI] [PubMed] [Google Scholar]
- Stirpe F, Della Corte E. The regulation of rat liver xanthine oxidase – conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O) J Biol Chem. 1969;244:3855–3863. [PubMed] [Google Scholar]
- Suzuki H, DeLano FA, Parks DA, Jamshidi N, Granger DN, Ishii H, Suematsu M, Zweifach BW, Schmid-Schönbein G. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc Natl Acad Sci U S A. 1998;95:4754–4759. doi: 10.1073/pnas.95.8.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swei A, Lacy F, DeLano FA, Parks DA, Schmid-Schönbein G. A mechanism of oxygen free radical production in the Dahl hypertensive rat. Microcirc. 1999;6:179–187. [PubMed] [Google Scholar]
- Terada LS, Beehler CJ, Banerjee A, Brown JM, Grosso MA, Harken AH, McCord JM, Repine JE. Hyperoxia and self- or neutrophil-generated O2 metabolites inactivate xanthine oxidase. J Appl Physiol. 1988;65:2349–2353. doi: 10.1152/jappl.1988.65.5.2349. [DOI] [PubMed] [Google Scholar]
- Terada LS, Guidot DM, Leff JA, Willingham IR, Hanley ME, Piermattei D, Repine JE. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc Natl Acad Sci U S A. 1992;89:3362–3366. doi: 10.1073/pnas.89.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada LS, Leff JA, Guidot DM, Willingham IR, Repine JE. Inactivation of xanthine oxidase by hydrogen peroxide involves site-directed hydroxyl radical formation. Free Radic Biol Med. 1991a;10:61–68. doi: 10.1016/0891-5849(91)90022-u. [DOI] [PubMed] [Google Scholar]
- Terada LS, Piermattei D, Shibao GN, McManaman JL, Wright RM. Hypoxia regulates xanthine dehydrogenase activity at pre- and post-translational levels. Arch Biochem Biophys. 1997;348:163–168. doi: 10.1006/abbi.1997.0367. [DOI] [PubMed] [Google Scholar]
- Terada LS, Rubinstein JD, Lesnefsky EJ, Horwitz LD, Leff JA, Repine JE. Existence and participation of xanthine oxidase in reperfusion injury of ischemic rabbit myocardium. Am J Physiol. 1991b;29:H805–H810. doi: 10.1152/ajpheart.1991.260.3.H805. [DOI] [PubMed] [Google Scholar]
- Terao M, Kurosaki M, Demontis S, Zanotta S, Garattini E. Isolation and characterization of the human aldehyde oxidase gene: conservation of intron/exon boundaries with the xanthine oxidoreductase gene indicates a common origin. Biochem J. 1998;332:383–393. doi: 10.1042/bj3320383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao M, Kurosaki M, Zanotta S, Garattini E. The xanthine oxidoreductase gene: structure and regulation. Biochem Soc Trans. 1997;25:791–796. doi: 10.1042/bst0250791. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Poland B, Honzatko R. Protein sulfhydryls and their role in the antioxidant function of protein S-thiolation. Arch Biochem Biophys. 1995;319:1–9. doi: 10.1006/abbi.1995.1261. [DOI] [PubMed] [Google Scholar]
- Thompson-Gorman SL, Zweier JL. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J Biol Chem. 1990;266:6656–6663. [PubMed] [Google Scholar]
- Truglio JJ, Theis K, Leimkühler S, Rappa R, Rajagopalan KV, Kisker C. Crystal structures of the active and alloxanthine-inhibited forms of xanthine dehydrogenase from Rhodobacter capsulatus. Structure. 2002;10:115–125. doi: 10.1016/s0969-2126(01)00697-9. [DOI] [PubMed] [Google Scholar]
- Turgan N, Boydak B, Habif S, Gülter C, Senol B, Mutaf I, Özmen D, Bayindir O. Urinary hypoxanthine and xanthine levels in acute coronary syndromes. Int J Clin Laboratory Res. 1999;29:162–165. doi: 10.1007/s005990050084. [DOI] [PubMed] [Google Scholar]
- Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54:619–634. doi: 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- Turner NA, Doyle WA, Ventom AM, Bray RC. Properties of rabbit liver aldehyde oxidase and the relationship of the enzyme to xanthine oxidase and dehydrogenase. Eur J Biochem. 1995;232:646–657. [PubMed] [Google Scholar]
- Ukai T, Cheng C, Tachibana H, Igawa A, Zhang Z, Cheng H, Little WC. Allopurinol enhances the contractile response to dobutamine and exercise in dogs with pacing-induced heart failure. Circulation. 2001;103:750–755. doi: 10.1161/01.cir.103.5.750. [DOI] [PubMed] [Google Scholar]
- Usuda N, Reddy MK, Hashimoto T, Rao MS, Reddy JK. Tissue specificity and species differences in the distribution of urate oxidase in peroxisomes. Laboratory Invest. 1988;58:100–111. [PubMed] [Google Scholar]
- Varadarajan SG, An J, Novalija E, Smart SC, Stowe DF. Changes in [Na+]i, compartmental [Ca2+], and NADH with dysfunction after global ischemia in intact hearts. Am J Physiol. 2001;280:H280–H293. doi: 10.1152/ajpheart.2001.280.1.H280. [DOI] [PubMed] [Google Scholar]
- Vickers S, Schiller HJ, Hildreth JEK, Bulkley GB. Immunoaffinity localization of the enzyme xanthine oxidase on the outside surface of endothelial cell plasma membrane. Surgery. 1998;124:551–560. [PubMed] [Google Scholar]
- Vorbach C, Scriven A, Capecchi MR. The housekeeper gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes Dev. 2002;16:3223–3235. doi: 10.1101/gad.1032702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Sawicki G, Schulz R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc Res. 2002a;53:165–174. doi: 10.1016/s0008-6363(01)00445-x. [DOI] [PubMed] [Google Scholar]
- Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JRB, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002b;106:1543–1549. doi: 10.1161/01.cir.0000028818.33488.7b. [DOI] [PubMed] [Google Scholar]
- Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart: evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- Waud WR, Rajagopalan KV. Purification and properties of the NAD+-dependent (Type D) and O2-dependent (Type O) forms of rat liver xanthine dehydrogenase. Arch Biochem Biophys. 1976a;172:354–364. doi: 10.1016/0003-9861(76)90087-4. [DOI] [PubMed] [Google Scholar]
- Waud WR, Rajagopalan KV. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (Type D) to an O2-dependent form (Type O) Arch Biochem Biophys. 1976b;172:365–379. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kang D, Feng L, Nakagawa T, Kanellis J, Lan H, Mazzali M, Johnson RJ. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40:355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- Weinbroum A, Nielsen VG, Tan S, Gelman S, Matalon S, Skinner KA, Bradley E, Jr, Parks DA. Liver ischemia-reperfusion increases pulmonary permeability in rat: role of circulating xanthine oxidase. Am J Physiol. 1995;268:G988–G996. doi: 10.1152/ajpgi.1995.268.6.G988. [DOI] [PubMed] [Google Scholar]
- Weiss SJ. Oxygen, ischemia, and inflammation. Acta Physiol Scand. 1986;548:9–37. [PubMed] [Google Scholar]
- White CR, Darley-Usmar V, Berrington WR, McAdams M, Gore JZ, Thompson JA, Parks DA, Tarpey M, Freeman BA. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci U S A. 1996;93:8745–8749. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JR. Glycolytic control mechanisms II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J Biol Chem. 1966;241:5026–5036. [PubMed] [Google Scholar]
- Xia M, Dempski R, Hille R. The reductive half-reaction of xanthine oxidase – reaction with aldehyde substrates and identification of the catalytically labile oxygen. J Biol Chem. 1999;274:3323–3330. doi: 10.1074/jbc.274.6.3323. [DOI] [PubMed] [Google Scholar]
- Xia Y, Zweier JL. Substrate control of free radical generation from xanthine oxidase in the postischemic heart. J Biol Chem. 1995;270:18797–18803. doi: 10.1074/jbc.270.32.18797. [DOI] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- Xu P, Huecksteadt T, Harrison R, Hoidal JR. Molecular cloning, tissue expression of human xanthine dehydrogenase. Biochem Biophys Res Commun. 1994a;199:998–1004. doi: 10.1006/bbrc.1994.1328. [DOI] [PubMed] [Google Scholar]
- Xu P, Huecksteadt T, Harrison R, Hoidal JR. Molecular cloning, tissue expression of human xanthine dehydrogenase. Biochem Biophys Res Commun. 1995;215:429. doi: 10.1006/bbrc.1995.2482. [DOI] [PubMed] [Google Scholar]
- Xu P, Huecksteadt T, Hoidal JR. Molecular cloning and characterization of the human xanthine dehydrogenase gene (XDH) Genomics. 1996;34:173–180. doi: 10.1006/geno.1996.0262. [DOI] [PubMed] [Google Scholar]
- Xu P, LaVallee P, Hoidal JR. Repressed expression of the human xanthine oxidoreductase gene: E-box and TATA-like elements restrict ground state transcriptional activity. J Biol Chem. 2000;275:5918–5926. doi: 10.1074/jbc.275.8.5918. [DOI] [PubMed] [Google Scholar]
- Xu P, Zhu XL, Huecksteadt T, Brothman AR, Hoidal JR. Assignment of human xanthine dehydrogenase gene to chromosome 2p22. Genomics. 1994b;23:289–291. doi: 10.1006/geno.1994.1498. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Blake DR, Stevens CR, Winyard PG, Symons MC, Benboubetra M, Harrison R. A reappraisal of xanthine dehydrogenase and oxidase in hypoxic reperfusion injury: the role of NADH as an electron donor. Free Radic Res. 1998a;28:151–164. doi: 10.3109/10715769809065801. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Naughton D, Winyard PG, Benjamin N, Blake DR, Symons MCR. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: a potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem Biophys Res Commun. 1998b;249:767–772. doi: 10.1006/bbrc.1998.9226. [DOI] [PubMed] [Google Scholar]
- Zima AV, Copello AJ & Blatter LA. Differential modulation of cardiac and skeletal muscle ryanodine receptors by NADH. FEBS Lett. 2003;547:32–36. doi: 10.1016/s0014-5793(03)00664-1. [DOI] [PubMed] [Google Scholar]
- Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart: evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–1357. [PubMed] [Google Scholar]
- Zweier JL, Broderick R, Kuppusamy P, Thompson-Gorman SL, Lutty GA. Determination of the mechanism of free radical generation in human aortic endothelial cells exposed to anoxia and reoxygenation. J Biol Chem. 1994;269:24156–24162. [PubMed] [Google Scholar]
- Zweier JL, Fertman J, Wei G. Nitric oxide and peroxynitrite in postischemic myocardium. Antioxid Redox Signal. 2001;3:11–22. doi: 10.1089/152308601750100443. [DOI] [PubMed] [Google Scholar]