Abstract
Oxytocin is released from supraoptic magnocellular neurones and is thought to act at presynaptic receptors to inhibit transmitter release. We now show that this effect is mediated by endocannabinoids, but that oxytocin nonetheless plays an important role in endocannabinoid signalling. WIN55,212-2, a cannabinoid receptor agonist, mimicked the action of oxytocin and occluded oxytocin-induced presynaptic inhibition. The cannabinoid action is at the presynaptic terminal as shown by alteration in paired pulse ratio, a reduction in miniature EPSC frequency and immunohistochemical localization of CB1 receptors on presynaptic terminals. AM251, a CB1 receptor antagonist, blocked both the WIN55,212-2 and the oxytocin-induced presynaptic inhibition of EPSCs. Depolarization of postsynaptic magnocellular neurones (which contain fatty acid amide hydrolase, a cannabinoid catabolic enzyme) caused a transient inhibition of EPSCs that could be blocked by both the AM251 and Manning compound, an oxytocin/vasopressin receptor antagonist. This indicates that somatodendritic peptide release and action on previously identified autoreceptors facilitates the release of endocannabinoids that act as mediators of presynaptic inhibition.
Each neurone in the CNS receives thousands of excitatory and inhibitory synaptic inputs, mostly onto an arbor of fine, branching processes – the dendrites. Over a century ago, Ramón y Cajal proposed that information flows unidirectionally from the dendrites to soma to axon (Ramón y Cajal, 1891). This concept, known as the ‘theory of dynamic polarization’, has now been challenged by the realization that dendrites can also relay information back to their own extremities, where they can release both classical transmitters and neuropeptides (Ludwig & Pittman, 2003). These can act as retrograde signals to modulate afferent synapses and to autoregulate the activity of the neurones from which they were released. Thus, dendritic release endows upon these neurones a unique framework for both short-and long-term plasticity to regulate the efficacy of synaptic responses to meet physiological demands.
In the supraoptic nucleus (SON), there is now good evidence that the neurohypophysial peptides arginine vasopressin (AVP) and oxytocin (OXT) are released from the somatodendritic compartment of magnocellular neurones (reviewed in Ludwig, 1995; Morris et al. 2000). A series of physiological and pharmacological studies have shown that OXT acts locally on OXT autoreceptors to elevate intracellular Ca2+ (Lambert et al. 1994; Ludwig et al. 2002). In keeping with this action, immunocytochemical studies have localized OXT receptors to OXT magnocellular neurones (Freund-Mercier et al. 1994; Adan et al. 1995). The locally released OXT both facilitates the activity of OXT magnocellular neurones and increases the axonal and dendritic release of the peptide (Moos et al. 1984; Neumann et al. 1996). In addition, we have shown that both exogenously applied and endogenously released OXT causes a reduction in glutamate-mediated excitatory postsynaptic currents (EPSCs) onto magnocellular neurones through an apparent action at a presynaptic terminal (Kombian et al. 1997). Similar results have been obtained for a presynaptic action of OXT upon GABAergic inhibitory postsynaptic currents (IPSCs) (de Kock et al. 2003). Given the known postsynaptic location of OXT receptors (Lambert et al. 1994; Freund-Mercier et al. 1994) we have tested the hypothesis that dendritically released OXT acts on the postsynaptic magnocellular neurone to indirectly inhibit glutamate release via an intermediary, retrograde messenger.
Recent studies, largely in hippocampus and cerebellum, but also in several other brain areas, have implicated endocannabinoids in retrograde signalling. Depolarization (Kreitzer & Regehr, 2001a,b; Ohno-Shosaku et al. 2001; Wilson & Nicoll, 2001; Trettel & Levine, 2003), an elevation in intracellular Ca2+ (Wilson & Nicoll, 2001; Brenowitz & Regehr, 2003), or activation of postsynaptic metabotropic receptors (Varma et al. 2001; Maejima et al. 2001; Di et al. 2003) cause the release of endocannabinoids that act at presynaptic terminals to inhibit either excitatory or inhibitory transmitter release through the activation of G-protein-coupled CB1 receptors.
There is indirect evidence for the action of cannabinoids on SON function. For over half a century it has been known that marihuana intake in man is associated with diuresis, suggestive of reduced AVP release (Tayleur Stockings, 1947; Ames, 1958), and a similar effect is seen in rats treated with the cannabinoid, ▵9 tetra-hydro-cannabinol (▵9 THC) (Sofia & Barry, 1977). Similarly the administration of hashish (Frischknecht et al. 1980) or ▵9 THC (Borgen et al. 1971) to either lactating rats or mice is associated with reduced pup weight and a lack of milk transfer to the stomach of the pups, an effect consistent with reduced OXT release. In keeping with these observations, ▵9 THC has been reported to reduce the activity of the SON neurones, as indicated by an accumulation of neurosecretory material in their axons (Biswas & Ghosh, 1975). These early observations suggesting the inhibitory effects of cannabinoids in the SON prompted us to ask if they could alter synaptic transmission in the SON and also to determine whether they might be mediators of OXT action. In the present study, we show for the first time that endocannabinoids act as retrograde messengers in the SON and that CB1 receptors are localized on presynaptic terminals. In addition, we have found that the release and autocrine action of OXT subsequent to depolarization of SON magnocellular neurones plays an important role in inducing cannabinoid release.
Methods
All experiments were carried out in accordance with the guidelines established by the Canadian Council on Animal Care and were approved by the University of Calgary Animal Care Committee. We were careful to use only the number of animals necessary to produce reliable results.
Slice preparation
Adult male Sprague-Dawley rats (100–150 g) were decapitated under deep halothane anaesthesia, the brain was removed and 250–300 μm thick coronal slices containing the SON were generated at 0–2°C in a low Ca2+, low Na+-containing buffer solution of composition (mm): NaCl (87), KCl (2.5), NaH2PO4 (1.25), MgCl2 (7), CaCl2 (0.5), NaHCO3 (25), glucose (25) and sucrose (20). Slices were incubated at 32–34°C for an hour and then at room temperature (22°C) in artificial cerebrospinal fluid (ACSF) of the following composition (mm): NaCl (126), KCl (2.5), NaH2PO4 (1.2), MgCl2 (1.2), CaCl2 (2.4), NaHCO3 (18) and glucose (11). Both solutions were continuously bubbled with a mixture of O2 (95%) and CO2 (5%).
Electrophysiological recording
ACSF-submerged slices were visualized by a DIC-IR microscope, and nystatin-perforated patch whole-cell recording (series/access resistance: 10–40 MΩ) was performed at 30–32°C using electrodes with a tip resistance of 3–7 MΩ. The internal recording solution contained (mm): potassium gluconate (120), MgCl2 (5), EGTA (10) and Hepes (40). Nystatin was dissolved in DMSO with Pluronic F127 and added to the internal solution to yield a final concentration of 450 μg ml−1. The pH was adjusted to 7.3. All experiments were done on neurones voltage clamped at −80 mV using an Axopatch 1D amplifier and pCLAMP 9 software (Axon Instruments). Membrane currents were recorded without series resistance compensation, filtered at 1 kHz and digitized at 5–10 kHz and stored for off-line analysis. A 20 mV hyperpolarizing pulse lasting for 75 ms was applied regularly throughout each experiment, and the steady-state current and decay rate (τ) of the capacitance transient were monitored as measures of input resistance and series/access resistance, respectively. Cells that showed more than 15% change in these parameters at the end of each experiment were excluded from further analysis. Magnocellular neurones were identified based on the delayed onset to action potential generation in response to positive current injection and all recordings in this paper were from magnocellular neurones. In some cells, AVP or OXT identity was ascertained in voltage clamp by current responses to hyperpolarizing voltage steps from –40 mV (Hirasawa et al. 2003a).
Synaptic currents (50–70% of maximal amplitude) were evoked with a bipolar stimulating electrode placed in the hypothalamic region dorsomedial to the SON, close to the optic tract. For all experiments, picrotoxin (50 μm) was added to ACSF to block GABAA receptor-mediated chloride currents and give pharmacologically isolated EPSCs. EPSCs were mediated by non-NMDA receptors as they were sensitive to 10 μm DNQX, a non-NMDA receptor antagonist.
To monitor paired pulse ratio (PPR), a pair of EPSCs was evoked with an interval of 50 ms and the ratio was calculated as EPSC2/EPSC1. To isolate miniature EPSCs (mEPSCs), tetrodotoxin (1 μm) was also added to the ACSF to eliminate action potential-driven EPSCs. Previously it has been shown that in coronal SON slice preparation, virtually all spontaneous events are action potential independent (Kombian et al. 2000), enabling us to monitor miniature events and evoked response concurrently, by omitting tetrodotoxin in some of the experiments. The results obtained with or without tetrodotoxin were not significantly different, thus the data were combined.
Control data were collected for 3–5 min prior to drug application. Baseline was calculated as the mean of the values obtained during the control period. mEPSCs were detected using MiniAnalysis software (Synaptosoft Inc.) and counted if amplitude was larger than 4–5 times the RMS noise with fast rise times (0.4–3 ms measured 10–90% from baseline to peak) and exponential decay. All values are expressed as mean ± s.e.m. Statistical comparisons were performed by using appropriate tests, i.e. Student's paired and unpaired t tests, ANOVA and the Kolmogorov-Smirnov test. P < 0.05 was considered significant.
All substances were prepared as × 1000 stock solutions, and diluted to their final concentration in ACSF just before use. The source of all chemicals and drugs was Sigma except for the following: oxytocin and Manning compound (Bachem), WIN55,212-2 and AM251 (Tocris Cookson), tetrodotoxin (Alomone Laboratories).
Immunohistochemistry
Rats (n = 4) were deeply anaesthetized with pentobarbital sodium (65 mg kg−1 i.p.; Somnotol, MTC Pharmaceuticals, Cambridge, ON, Canada), and fixed by intracardiac perfusion with 100 ml physiological saline followed by 500 ml 4% paraformaldehyde (pH 7.3). Brains were removed and fixed overnight in 4% paraformaldehyde at 4°C. They were then transferred to 20% sucrose in 0.1 m phosphate buffer at 4°C overnight. Coronal sections of brain at levels containing the SON were cut at 50 μm and floating sections were washed for 3 × 10 min in phosphate-buffered saline (PBS) containing 0.1% Triton X-100 and then incubated in a rabbit anti-CB1 receptor antibody (1 : 1000) (Tsou et al. 1998a; Ong & Mackie, 1999; Van Sickle et al. 2003) or a rabbit anti-fatty acid amide hydrolase antibody (FAAH, 1 : 200) (Tsou et al. 1998b) at 4°C for 48 h. Specificity was confirmed by preincubation of the antibodies (24 h at 4°C) with the peptides used to raise the antibodies (10 nm in diluted antibody). This procedure completely abolished labelling in all cases. Sections were washed with 0.1% Triton X-100 in PBS and incubated for 1–2 h at room temperature in the secondary antisera (donkey anti-rabbit conjugated to CY3; 1 : 100, Biocan Scientific, Mississauga, ON, Canada) followed by washing (3 × 10 min in PBS) and mounting in bicarbonate-buffered glycerol (pH 8.6).
Confocal microscopy
Samples were viewed on an Olympus Fluoview FV300 microscope system using krypton–argon and helium–neon lasers. Differential visualization of the fluorophores FITC (excitation 490 nm and emission 520 nm), and CY3 (excitation 552 nm and emission 565 nm) was accomplished through the use of specific filter combinations. Samples were scanned sequentially if double-labelled, to avoid any potential for bleed-through of fluorophores. Images of 1024 pixels × 1024 pixels were obtained under identical exposure conditions (pinhole aperture, laser strength, scan speed, Kalman averaging × 2) and were processed identically using Fluoview software and then CorelDraw 11. Confocal micrographs are digital composites of Z-stack scans through 1 μm optical sections, as described in the figure legends.
Electron microscopy
Rats (n = 3) were deeply anaesthetized with halothane and perfused through the heart with a mixture of 4% paraformaldehyde and 0.2% glutaraldehyde in 0.1 m PBS, pH 7.4. Brains were then removed and postfixed overnight in 4% paraformaldehyde at room temperature and incubated in 14% glycerol and 30% sucrose in PBS until further processing. Frozen sections (40 μm) were first preincubated for 15 min in 1% sodium borohydride in PBS and then in a blocking buffer containing 0.1% Tween 20 and 5% donkey serum in PBS. Primary antibodies against CB1 receptors (as above) were used at 1 : 500 dilution in PBS + 0.1% Tween 20 and incubated for 48 h at 4°C. After extensive washing with PBS, the slices were incubated with a 1/500 dilution of a biotinylated goat anti-rabbit IgG (Santa Cruz) for 90 min at room temperature and then for 45 min in a 1/500 dilution of the Vectastain ABC kit (Vector Laboratories) followed by DAB (Vector Laboratories) for 8 min at room temperature. Tissues were then postfixed with 2.5% glutaraldehyde in PBS for 30 min, then with 1% osmium tetroxide in PBS for 1 h at 4°C and incubated in 70% ethanol overnight at 4°C. Dehydration was performed in graded alcohol and then after propylene oxide treatment the tissues were embedded in a mixture of Araldite and Epon resins. Silver ultrathin sections were obtained using a Reichert ultramicrotome and collected on copper grids. Pictures were taken with a digital camera (AMT) mounted on a Hitachi H 7500 electron microscope.
Results
Cannabinoid action on excitatory transmission
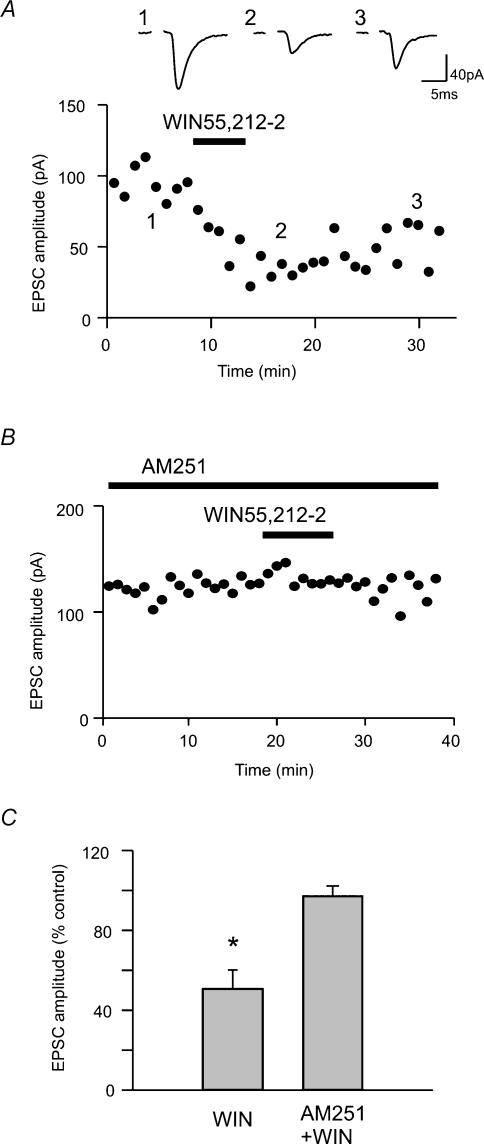
The role of endocannabinoids in synaptic excitation of magnocellular neurones was investigated by testing the effect of a cannabinoid receptor agonist, WIN55,212-2 (1 μm) on evoked EPSCs in SON. WIN55,212-2 induced a significant, long-lasting, partially reversible inhibition of EPSCs to 50.6 ± 9.5% of control (n = 8, Fig. 1A and C). Included in this population were both AVP and OXT cells, identified by standard electrophysiological approaches. This effect was prevented by preincubation with a CB1 receptor antagonist, AM251 (1 μm; 97.0 ± 5.1% of control, P > 0.05, n = 5; Fig. 1B and C). AM251 alone had a highly variable, but not significant, effect on the EPSC amplitude (146.1 ± 22.5% of control, P > 0.05, n = 7).
Figure 1. Cannabinoid receptor agonist inhibits EPSCs through CB1 receptors.
A, evoked EPSCs before (1), during (2) and after (3) the application of WIN 55,212-2 (1 μm). The time–effect plot of evoked EPSCs indicates the times (shown by the numbers) when the traces were taken. WIN55,212-2 was bath applied during the time indicated by the horizontal bar. B, a representative time–effect plot of evoked EPSC amplitude. Pretreatment with AM251 (1 μm) blocked the inhibitory effect of WIN55,212-2. C, summary graph showing the change in EPSC amplitude by WIN55,212-2 alone or AM251 and WIN55,212-2. * P < 0.05 versus control.
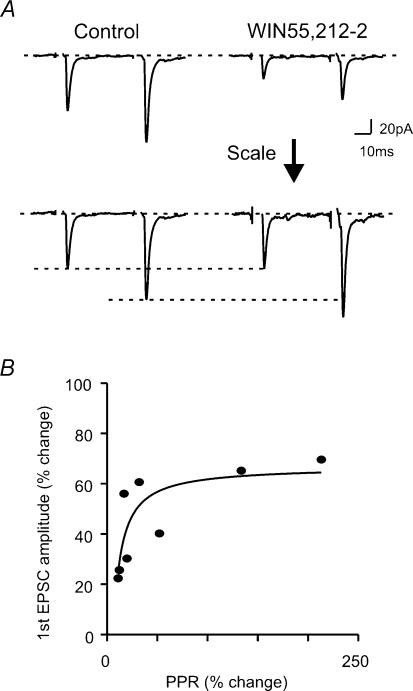
To characterize the locus of WIN55,212-2 action, i.e. pre- or postsynaptic, the effect of WIN55,212-2 on PPR was monitored. As can be seen in Fig. 2, the effect of WIN55,212-2 was greater on the first than on the second of the two EPSCs, thereby resulting in an increased PPR. The change in the first EPSC amplitude was positively related to PPR (Fig. 2; R2 = 0.65); that is the greater the reduction in the size of the first EPSC, the greater was the change in PPR. The presynaptic action of a drug can also be reflected in mEPSC frequency. WIN55,212-2 significantly decreased the frequency of mEPSCs (45.5 ± 5.2% of control, P < 0.05, n = 6; Fig. 3A and C). The effect of WIN55,212-2 on both PPR and mEPSC frequency indicates that a decrease in transmitter release probability was most likely underlying the decrease in evoked EPSC amplitude.
Figure 2. WIN55,212-2 increases paired pulse ratio (PPR).
A: top, representative paired pulse traces during control (left) and in the presence of WIN55,212-2 (right); bottom, the traces are scaled to the size of the first EPSC. B, WIN55,212-2-induced PPR change plotted against the change in the first EPSC amplitude. Each filled circle represents a cell.
Figure 3. Miniature EPSCs are inhibited by WIN55,212-2.
Aa, sample traces showing basal mEPSCs (control) and in the presence of WIN55,212-2 as indicated. Ab and c, cumulative plots of interevent intervals (Ab) or amplitudes (Ac) of mEPSCs recorded from the same cell as shown in Aa. For A and B: black line, control; grey line, WIN55,212-2. Ba, sample traces showing mEPSCs induced by nifedipine (10 μm) and in the presence of both nifedipine and WIN55,212-2. Bb and c, cumulative plots of interevent interval (Bb) or amplitude (Bc) of mEPSCs generated from the same cell as shown in Ba. C, summary of the effect of WIN55,212-2 on the frequency of basal and nifedipine-induced mEPSCs. D, summary graph depicting the effect of WIN55,212-2 on the amplitude of basal and nifedipine-induced mEPSCs. * P < 0.05 versus control.
In contrast, WIN55,212-2 had no effect on the amplitude of mEPSCs (101.7 ± 3.5% of control, P > 0.05, n = 6; Fig. 3D), suggesting that the compound had little effect on the postsynaptic response to glutamate. Furthermore, cannabinoid receptor activation seemed to have no effect on the passive membrane properties of the postsynaptic magnocellular neurones, because WIN55,212-2 did not change the holding current or the current response to voltage ramp ranging from −120 to −40 mV (data not shown). Thus, the effect of WIN55,212-2 on EPSCs seems to be exclusively presynaptic.
Spontaneous presynaptic release of transmitter can be influenced by intraterminal Ca2+ level, which can fluctuate due to the influx or release of Ca2+ from internal stores. Alternatively, spontaneous release can be modulated by mechanism(s) downstream of intraterminal Ca2+ level. To differentiate amongst these possibilities, one can utilize agents that directly facilitate the release process downstream of Ca2+ entry or release and determine how CB1 receptor activation influences transmitter release activated by such agents. We used nifedipine, a secretagogue that induces spontaneous glutamate release independently of Ca2+ influx or release from the internal stores (Hirasawa & Pittman, 2003b). Nifedipine (10 μm) induced a 14.1(± 3.2)-fold increase in mEPSC frequency (n = 5). WIN55,212-2 inhibited nifedipine-induced mEPSC frequency to 40.1 ± 5.0% of the nifedipine effect (P < 0.05, n = 5; Fig. 3B and C) but was without effect on the amplitude of mEPSCs (99.9 ± 6.4% of nifedipine effect, P > 0.05, n = 5; Fig. 3B and D). Thus, WIN55,212-2 seems to be able to modulate a release process independently of Ca2+ influx or release from intracellular stores.
Cannabinoid mediation of oxytocin action
The inhibitory effect on evoked EPSCs by CB1 receptor activation (Fig. 1) was similar to the previously reported effect of OXT on SON excitatory transmission. Thus we tested the hypothesis that OXT exerts its effect through endocannabinoids. The effect of OXT on evoked EPSCs was tested in the presence of a CB1 receptor antagonist. While OXT (1 μm) alone induced a significant decrease in EPSC amplitude (65.2 ± 2.5% of control, P < 0.05, n = 14; Fig. 4A and D), the effect was blocked by pretreatment with AM251 (103.7 ± 3.5% of AM251 alone, P > 0.05, n = 6; Fig. 4B and D). Furthermore, the OXT effect was occluded in the presence of WIN55,212-2 (96.8 ± 4.5% of WIN55,212-2 alone, P > 0.05, n = 3; Fig. 4C and D). These results indicate that OXT inhibits EPSCs through the activation of CB1 receptors, by the release of endocannabinoids.
Figure 4. Oxytocin inhibits EPSCs by inducing endocannabinoid release.
A, oxytocin (OXT; 1 μm) inhibits EPSC amplitude in a typical cell. B, AM251 (1 μm) blocks oxytocin effect. C, WIN55,212-2 (1 μm)-induced EPSC inhibition occludes oxytocin effect. D, summary of the effect of oxytocin on EPSC amplitude. The effect of oxytocin is normalized to the value prior to oxytocin application. * P < 0.05 versus control. E, Manning compound does not block WIN 55,212-2 effect. The inhibitory effect of WIN55,212-2 is reversed by AM251. F, summary graph of the effect of WIN55,212-2 on EPSC amplitude. * P < 0.05 versus control. The presence of Manning compound (MC) has no significant effect (n.s.).
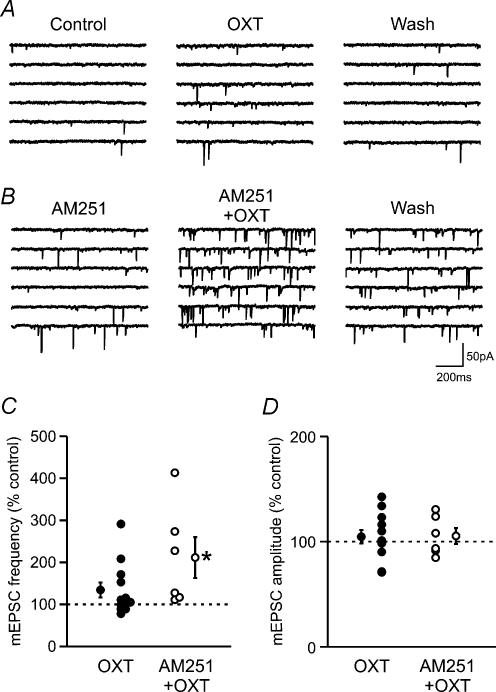
If endocannabinoids mediate the OXT effect, the action of OXT and cannabinoids has to be the same. However, our previous study has shown that OXT has no effect on mEPSCs (Hirasawa et al. 2001), which is in contrast to the inhibition of mEPSCs by the CB1 receptor agonist (Fig. 3). The discrepancy may be explained if OXT has multiple, opposing effects. In order to investigate if OXT has CB1 receptor-independent effects on mEPSCs, we tested OXT in the presence of AM251. In this condition, OXT induced a significant increase in mEPSC frequency in all the cells tested (206.9 ± 41.3% of control, P < 0.05, n = 6; Fig. 5B and C). On the other hand, OXT alone induced a diverse effect: out of 12 cells, 5 responded with an increase, 4 with a decrease and the remaining 3 showed no response (Fig. 5A and C). Overall, the response was statistically not significant compared to control (134.4 ± 18.1% of control, P > 0.05, n = 12; Fig. 5A and C). This result indicates that OXT has at least two opposing effects on mEPSC frequency: an inhibitory effect mediated by endocannabinoids and an excitatory effect independent of CB1 receptors. In contrast to its effect on mEPSC frequency, OXT had no significant effect on the amplitude of mEPSCs both in the absence or presence of AM251 (OXT alone: 104.6 ± 6.4% of control, P > 0.05, n = 12; AM251 + OXT: 105.4 ± 7.6% of control, P > 0.05, n = 6; Fig. 5D). However, the statistical analysis of individual cells that had been tested for the electrophysiological fingerprints of OXT or AVP cells indicated that cells showing the characteristics of OXT cells responded with an increase in mEPSC amplitude in 60% of cases (6/10 cells; remaining 40% showed no response), while 75% of putative AVP cells showed a decrease in amplitude (3/4 cells; remaining 25% showed no response). This result is consistent with our previous finding suggesting that OXT modulates postsynaptic AMPA receptors.
Figure 5. Oxytocin has an endocannabinoid-independent excitatory effect on miniature EPSCs.
A, example of a cell that responded to OXT with an increase in mEPSC frequency. B, sample traces showing the effect of OXT in the presence of AM251. C, effect of OXT on mEPSC frequency in the absence or presence of AM251. Each circle represents a cell. Mean and error bars are shown beside each group. * P < 0.05 versus control. D, effect of OXT on mEPSC amplitude in the absence or presence of AM251. Symbols as in C.
The induction of endogenous transmitter release from the magnocellular neurones was attempted by brief postsynaptic depolarization, and its effect on excitatory transmission was tested. The holding potential was stepped from –80 mV to 0 mV for 1 s. In keeping with our previous finding, depolarization induced a transient decrease in evoked EPSCs (n = 8; Fig. 6A). As we have previously described (Kombian et al. 1997), this depression was completely blocked by Manning compound (10 μm), an OXT/V1a receptor antagonist (n = 4; Fig. 6B), indicating that neurohypophysial peptides are released in response to postsynaptic depolarization and mediate the synaptic depression. However, when CB1 receptors were blocked by AM251, a similar blockade was seen, i.e. postsynaptic depolarization did not inhibit EPSCs in the presence of the CB1 receptor antagonist (n = 4; Fig. 6C).
Figure 6. Postsynaptic depolarization induces inhibition of evoked EPSCs.
Aa, depolarization of the postsynaptic magnocellular neurone (0 mV, 1 s) induces a transient depression of evoked EPSCs. In Aa, Ba and Ca, arrows indicate when the cells were depolarized. In Aa, Ba and Ca, all values were normalized to control in each cell. Control value was calculated as an average of 3–5 min prior to depolarization. * P < 0.05 versus control. Ba, the inhibitory effect of postsynaptic depolarization was blocked by Manning compound (10 μm), a OXT/V1a receptor antagonist. Ca, the inhibitory effect of postsynaptic depolarization was blocked by AM251 (1 μm). Ab, Bb, Cb, representative current traces recorded from three different cells.
The blockade by both Manning compound and by AM251 raises the possibility that Manning compound, although a highly specific antagonist of OXT/V1a receptors (Kruszynski et al. 1980), may have a previously unrecognized action of blocking cannabinoid receptors. However, WIN55,212-2 applied in the presence of Manning compound induced a reduction (56.4 ± 1.3% of control, n = 3; Fig. 4E and F) in EPSCs that was similar to that induced by WIN55,212-2 alone (P > 0.05).
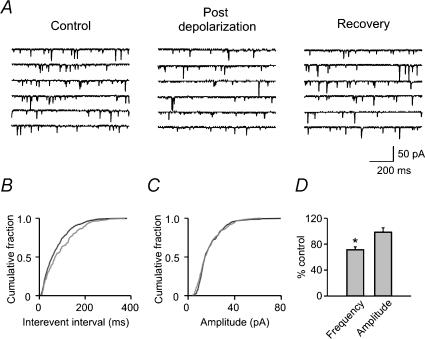
In addition to the effect on evoked EPSCs, depolarization induced a reduction in the frequency of mEPSCs (71.6 ± 4.4% of control, P < 0.05, n = 8, Fig. 7D), without affecting their amplitude (98.7 ± 6.8% of control, P > 0.05, n = 8). This result is similar to the effect of WIN55,212-2 on mEPSCs (see Fig. 3).
Figure 7. Postsynaptic depolarization induces inhibition of miniature EPSCs.
A, sample traces recorded before (Control), within 1 min after depolarization (Post depolarization) and 4 min later (Recovery). B, cumulative plot of mEPSC interevent interval. For B and C: black line, control; grey line, post depolarization. The data were derived from the same cell as A. C, cumulative plot of mEPSC amplitude. D, summary graph showing the change in frequency and amplitude of mEPSCs after postsynaptic depolarization.
Immunohistochemistry
Immunohistochemical localization of the cannabinoid degradative enzyme FAAH is depicted in Fig. 8A1. Magnocellular cell bodies displayed immunoreactivity for the enzyme. In contrast, CB1 receptor immunoreactivity appears to be an inverse of FAAH localization (Fig. 8A2). At electron microscopic level, the presence of CB1 receptor immunoreactivity was clearly localized to axon terminals forming asymmetric or symmetric synapses on magnocellular dendrites (Fig. 8B–F). However, immunoreactive product was not found in postsynaptic structures.
Figure 8. CB1 receptor is in presynaptic processes.
A1, confocal fluorescence micrographs of FAAH immunoreactivity (FAAH, 12 optical sections). A2, CB1 receptor immunoreactivity (CB1R, 20 optical sections). Scale bars for A1 and A2, 50 μm. B, electron micrographs displaying a bundle of axons (a) surrounded by glial processes (asterisks) and dendrites (d). One of the axons is labelled for CB1 receptors (arrowhead; dark deposit). Scale bars in B–F, 300 nm. C and D, asymmetric synapse with CB1 receptor-labelled synaptic terminal (st) contacting a postsynaptic cell. Arrows, postsynaptic density; ds, dendritic spine. E and F, symmetric synapse between a labelled synaptic terminal and a dendrite.
Discussion
The present results provide persuasive electrophysiological and immunohistochemical evidence that exogenous cannabinoids can act at presynaptic inhibitory receptors on afferent excitatory terminals in the SON. Furthermore, depolarization of magnocellular neurones causes release of endocannabinoids that act as a retrograde messenger. Of possibly greatest interest is the fact that dendritically released neuropeptides, which have previously been thought to act at the presynaptic excitatory terminal (Kombian et al. 1997) now appear to act via release/action of endocannabinoids. Nonetheless, neuropeptide release seems to be obligatory for inducing endocannabinoid action arising from depolarization of OXT neurones in the slice.
Presynaptic action of cannabinoids on excitatory transmission
Regulation of magnocellular neuronal activity is accomplished not only through the modulation of intrinsic conductances (Bourque et al. 1993), but also through excitatory and inhibitory afferent inputs. Glutamatergic inputs comprise the majority of excitatory inputs onto these cells (Theodosis, 1988; Van den Pol et al. 1990) and presynaptic receptors on these terminals are now recognized to be major loci for the control of SON (Pittman, 1999). Di et al. (2003) have previously reported in paraventricular nucleus that the presynaptic effects of glucocorticoids are mediated by the release of endocannabinoids from postsynaptic cells. Our demonstration that WIN55,212-2 reduces both evoked and mEPSCs indicates that cannabinoid receptors can also modulate excitatory glutamatergic inputs in the SON. Both PPR and mEPSC frequency, but not amplitude, are altered, indicating that the presynaptic terminal is the target of modulation. These effects are most likely mediated by CB1 receptors, because the WIN55,212-2 effect is blocked by AM251, a CB1 receptor antagonist, which is consistent with CB1 receptors being the major cannabinoid receptors in the central nervous system (Onaivi et al. 2002). In order to localize CB1 receptors, immunohistochemical studies were performed with an antibody that was highly specific for CB1 receptors. The immunoreactive material at the light microscopic level appeared to surround the magnocellular neurones. At the electron microscopic level, this immunoreactivity was clearly localized to axon terminals that form asymmetrical or symmetrical synapses with dendrites of magnocellular neurones, which indicates that CB1 receptors are expressed at both the excitatory and inhibitory presynaptic terminals in the SON.
The mechanism by which CB1 receptor activation reduces glutamatergic EPSCs was investigated with the use of the secretagogue, nifedepine, that is thought to directly target the release process (Hirasawa & Pittman, 2003b). The observation that CB1 receptor activation was effective in reducing nifedepine-stimulated glutamate secretion suggests an action of cannabinoids at the release process. As nifedepine-stimulated mEPSCs are insensitive to Ca2+ channel blockers or to internal Ca2+ depletion (Hirasawa & Pittman, 2003b), this would indicate that the effect can occur downstream from Ca2+ entry. Similar results have been reported elsewhere (Diana et al. 2002; Azad et al. 2003). However, there is also evidence that cannabinoids can inhibit voltage-dependent Ca2+ and K+ conductances (Mackie et al. 1995; Twitchell et al. 1997; Sullivan, 1999; Kreitzer & Regehr, 2001b; Diana et al. 2002). Indeed, the OXT effect in the SON can be occluded by N- and P/Q-type Ca2+ channel blockers (Hirasawa et al. 2001) suggesting that CB1 receptors may also modulate these Ca2+ channels in the SON.
There have been reports that some CB1 receptor antagonists, including AM251, can act as inverse agonists when CB1 receptors are overexpressed in transfected cell lines (MacLennan et al. 1998; New & Wong, 2003). However, in our study, the antagonist (at an effective blocking concentration of 1 μm) was without significant effect upon evoked EPSCs, thereby arguing against this possibility. Nonetheless, despite the lack of a significant effect overall, in some cells AM251 did clearly increase the evoked EPSC, as is indicated by the large variation in the response (146.1 ± 22.5% of control). Thus, it is possible that there is ongoing endocannabinoid activity at the glutamatergic synapse in the SON slice or that, in some synapses, AM251 may have inverse agonist activity.
Endocannabinoids mediate oxytocin action on excitatory transmission
The present demonstration that the OXT-induced reduction in evoked EPSC amplitude is antagonized by the CB1 receptor antagonist (and occluded by a cannabinoid agonist) could be accounted for through several mechanisms. One possibility is that the CB1 receptor antagonist is capable of blocking a presynaptic OXT receptor. We believe this to be unlikely; the CB1 receptor (Matsuda et al. 1990) and the OXT receptor (Kimura et al. 1992) have both been cloned and although both are G-protein coupled, they show little homology. Furthermore, the structural basis of ligand binding for neurohypophysial receptors has been well described (Barberis et al. 1998) and it appears highly unlikely that the diarylpyrazole, AM251, that is structurally very different from the neurohypophysial peptides, would interfere with OXT binding (admittedly, this does not negate the possibility that AM251 could interfere with OXT binding through a non-competetive, allosteric interaction). A second possibility is that OXT and CB1 receptors coexist on the same terminal and that second messenger systems downstream to either of the receptors somehow interact with each other. For example, it has been reported in transfected neurones that the CB1 receptor can sequester Gi/o proteins (Vasquez & Lewis, 1999); such sequestration would make them unavailable to other signalling pathways such as those required for OXT signalling. In support of this possibility, a cannabinoid agonist WIN55,212-2 and OXT occluded each other. However, if this were the case, one would expect that the AM251, a CB1 receptor antagonist, would be ineffective against OXT action, but as we have seen, it blocked the OXT effect. A third possibility is a sensitization of OXT receptors by active CB1 receptors, similar to the interaction observed between orexin and CB1 receptors (Hilairet et al. 2003). However, the absence of tonic endocannabinoid tone, which is apparent as a lack of AM251 effect, argues against such a mechanism. Also, WIN55,212-2 should facilitate OXT effect rather than occlude it, if such a mechanism exists. The most likely explanation for our findings is that exogenously applied OXT acts at postsynaptic OXT autoreceptors to release endocannabinoids that then act on the presynaptic terminal to inhibit glutamate release. Consistent with this idea is the fact that activation of postsynaptic OXT receptors has been shown to elevate intracellular Ca2+ (Lambert et al. 1994), a prerequisite for endocannabinoid synthesis and release in many neurones (Wilson & Nicoll, 2001; Brenowitz & Regehr, 2003). As we have demonstrated the presence of FAAH in SON neurones, and the presence of the enzyme is indicative of endocannabinoid signalling (Tsou et al. 1998b; Egertova et al. 1998), this is additional evidence that magnocellular neurones in the SON release endocannabinoids. Thus OXT is one of several metabotropic transmitters that have now been shown to activate endocannabinoid signalling. It is possible also that the activation of other metabotropic receptors on magnocellular neurones could cause endocannabinoid synthesis and release in a manner similar to the one described here for OXT.
Comparison of WIN55,212-2 and OXT action
If OXT action is mediated by endocannabinoids, there ought to be identity of action of OXT and WIN55, 212-2. With respect to the evoked EPSC, this appears to be the case (Kombian et al. 1997; Hirasawa et al. 2001); however, as for mEPSCs, OXT was relatively ineffective (Hirasawa et al. 2001) while WIN55,212-2 inhibited mEPSCs. The present study indicates that this discrepancy derives from mixed excitatory and inhibitory effects of OXT. The inhibitory effect seems to be mediated by CB1 receptors, since it was blocked by the CB1 antagonist. The mechanism of the excitatory effect of OXT in the presence of AM251 is unknown: possibilities include the direct action of OXT on presynaptic terminals inducing Ca2+ release from internal stores, leading to increased spontaneous glutamate release, or involvement of yet unknown excitatory retrograde messengers. It is curious that, whereas mEPSC frequency increased in the presence of AM251, there was no consistent increase in the size of the evoked EPSCs. However, in previous studies we have also observed large alterations in mEPSC frequency without an accompanying change in the size of evoked EPSCs (Kombian et al. 1997; Hirasawa et al. 2001). The discrepancy might arise from different Ca2+ sensitivity for evoked versus spontaneous transmitter release (Ravin et al. 1997).
Another factor that might account for the difference is the postsynaptic effect of OXT. Our previous study has demonstrated modulatory effects of neuropeptides on AMPA-induced postsynaptic currents in both OXT and AVP neurones (Hirasawa et al. 2003a), which would have little influence on the mEPSC frequency but would affect the amplitudes of evoked and miniature EPSCs. However, in the presence of AM251, OXT had no effect on these parameters, i.e. evoked or miniature EPSC amplitudes (Figs 4 and 5). This discrepancy could be due to differential effects of neuropeptides on AVP and OXT neurones (inhibitory effect mediated by V1a receptors and excitatory effect mediated by OXT receptors, respectively), because in the current study we recorded from a mixture of both population of neurones. This could be the case, as OXT may have significant action not only at OXT receptors but also at V1a receptors (Chen et al. 1999). Indeed, the majority of AVP neurones (identified by their electrophysiological fingerprints) showed a decrease in mEPSC amplitude in response to OXT application while most OXT neurones responded with an increase in mEPSC amplitude, which is in agreement with our previous findings.
We still stand by our previous conclusion that OXT inhibits presynaptic Ca2+ channels, possibly via endocannabinoids, because of the fact that the OXT effect was partially occluded by Ca2+ channel blockers (Hirasawa et al. 2001). Future investigation will be required to elucidate whether Ca2+ channel blockers also occlude the cannabinoid effect in a manner similar to OXT. There remains an anomaly in the effects of exogenous OXT and endogenous neuropeptide released by postsynaptic depolarization, i.e. exogenous OXT induced diverse effects on mEPSCs (Fig. 5) whereas endogenously released neuropeptide seemed to induce only inhibitory effects on mEPSCs (Fig. 7). One possible explanation for this discrepancy is that bath-applied OXT may, at the doses we used, have effects that do not exactly mimic those seen with endogenously released OXT that would have a much more localized action.
We have confirmed an action of OXT on both OXT and AVP cells identified by electrophysiological fingerprint in the SON (data not shown) and, in the present study, WIN55,212-2 was effective in every cell tested, whether they showed an OXT or an AVP electrophysiological fingerprint. Thus it is possible that OXT action on OXT cells releases sufficient endocannabinoids to diffuse to neighbouring synapses on both OXT and AVP cells. An alternative possibility is that OXT action at V1a receptors on AVP neurones also releases endocannabinoids. Although the present set of experiments did not address the potential role of AVP in activating endocannabinoid release, the fact that it also elevates intracellular Ca2+ (Gouzenes et al. 1999) makes it likely that it could do so in a manner similar to the one we have described for OXT. Indeed Manning compound is effective in blocking both V1a and OXT receptors, leaving the possibility that the retrograde inhibition is also induced by AVP.
The cooperative action of OXT and endocannabinoids and its functional implications
We were able to elicit a short-lasting inhibition of evoked and miniature EPSCs by depolarization of the postsynaptic magnocellular neurone, as we have previously reported (Kombian et al. 1997). We also confirmed that this depolarization-induced inhibition could be blocked by Manning compound, an OXT/V1a receptor antagonist (Kombian et al. 1997). In addition, the present study shows that the same inhibition induced by postsynaptic depolarization can be prevented in the presence of a CB1 receptor antagonist, thereby providing evidence for endocannabinoid release. Our first thought was that the OXT/V1a receptor antagonist was non-specifically blocking the CB1 receptor on the presynaptic terminal. However, this was not the case, as it did not block WIN55,212-2 action. Thus the similar blockade by both Manning compound and AM251 suggests that endogenous release of both endocannabinoid and the neurohypophysial peptides are required to inhibit afferent synapses. The most plausible explanation for how this might happen is that depolarization of the magnocellular neurone in itself is insufficient to cause enough endocannabinoid synthesis and release to inhibit the evoked EPSC in the slice. Rather, as depicted in Fig. 9, the depolarization-induced dendritic release of OXT needs to activate local autoreceptors on the same neurone, which in turn causes the synthesis and release of sufficient endocannabinoids to diffuse to the presynaptic terminals and inhibit the release of glutamate. Whether the endocannabinoid synthesis involves Ca2+ release from the internal store induced by OXT receptor activation (Lambert et al. 1994) remains to be elucidated. Meanwhile, Ca2+ release induced by activation of the OXT receptor will further stimulate OXT release creating a feed-forward activation of the system (Moos et al. 1984; Ludwig et al. 2002), which may underlie the prolonged inhibition caused by postsynaptic depolarization (order of minutes). In addition, OXT will also activate receptors (OXT receptors and possibly V1a receptors) expressed on neighbouring cells and induce endocannabinoid signalling at their synapse. Thus, dendritically released OXT may initiate a temporal and spatial spread or expansion of endocannabinoid signalling in the nucleus. Diffusion of endocannabinoids seems to be limited to individual synapses in the paraventricular nucleus; Di et al. (2003) have shown that blockade of G-protein signalling in the postsynaptic neurone prevents endocannabinoid release induced by glucocorticoids and the resulting synaptic inhibition. To understand whether endocannabinoids can diffuse to neighbouring synapses to modulate neurotransmission in the SON will require future investigation.
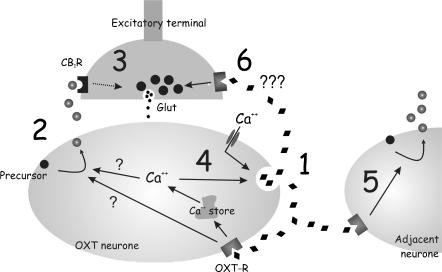
Figure 9. Postulated mechanism for cooperative action of dendritically released OXT and endocannabinoids in the supraoptic nucleus.
OXT initiates the temporal and spatial spread of endocannabinoid signalling. (1) Initial depolarization of an OXT neurone induces OXT release. (2) OXT action on autoreceptors leads to synthesis and release of sufficient endogenous endocannabinoids which may be dependent or independent of Ca2+ release from internal stores. (3) Endocannabinoids diffuse to CB1 receptors on presynaptic terminals and inhibit glutamate release. (4) Ca2+ release from internal stores also causes further release of OXT, generating a feed-forward stimulation of OXT–endocannabinoid signalling. (5) OXT may also diffuse to adjacent neurones and induce endocannabinoid release. (6) OXT has an excitatory effect on spontaneous glutamate release, which may be due to a direct action on the presynaptic terminal.
This neuropeptide–endocannabinoid mechanism provides another synaptic negative feedback loop in this nucleus, in addition to the previously described depression of synaptic transmission due to presynaptic metabotropic glutamate receptor (mGluR) activation by spillover of glutamate (Oliet et al. 2001). The mGluR mechanism would be confined to the level of presynaptic terminals, inhibiting glutamate release independently of the level of postsynaptic activity. In contrast, the neuropeptide–endocannabinoid mechanism described in this study consists of a larger feedback loop involving both presynaptic terminal and activated postsynaptic cell. The sequential action of OXT and endogenous cannabinoids may allow the sustained activation of OXT neurones by dendritically released OXT that has been shown to be necessary for the action of the peptide on milk ejection (Moos et al. 1998). Once sufficient peptide is released from the pituitary, and once the activation of OXT receptors within the SON has risen to a critical level, endocannabinoid synthesis and release will occur to quiet afferent excitation onto OXT cells. Such a mechanism may possibly contribute to the inactivation of the regenerative positive feed-forward loop by OXT to enable replenishment of stores of neuropeptides following intense activity and to the synchronization of neuronal activity.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR, to K.A.S. and Q.J.P.) and the National Institutes of Health (DA00286 and DA11322 to K.M., DA09155 to C.J.H.). M.H. was supported by a CIHR/Heart & Stroke Foundation of Canada Fellowship. S.N. is supported by a Canadian Association of Gastroenterology/CIHR/AstraZeneca Fellowship. K.A.S. and Q.J.P. are Alberta Heritage Foundation for Medical Research Scientists. We thank Winnie Ho and Mio Tsutsui for skilled technical assistance and Drs J. Bains and B. Hu for constructive comments on the manuscript.
References
- Adan RAH, van Leeuwen FW, Sonnemans MAF, Brouns M, Hoffman G, Verbalis JG, Burbach JPH. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: Partial sequence and immunocytochemical localization. Endocrinology. 1995;136:4022–4028. doi: 10.1210/endo.136.9.7649111. [DOI] [PubMed] [Google Scholar]
- Ames F. A clinical and metabolic study of acute intoxication with Cannabis sativa and its role in the model psychoses. J Ment Sci. 1958;104:972–999. doi: 10.1192/bjp.104.437.972. [DOI] [PubMed] [Google Scholar]
- Azad SC, Eder M, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learn Mem. 2003;10:116–128. doi: 10.1101/lm.53303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis C, Mouillac B, Durroux T. Structural bases of vasopressin/oxytocin receptor function. J Endocrinol. 1998;156:223–229. doi: 10.1677/joe.0.1560223. [DOI] [PubMed] [Google Scholar]
- Biswas B, Ghosh JJ. Delta-9-tetrahydrocannabinol and lysergic acid diethylamide: comparative changes in the supraoptic and paraventricular neurosecretory activities in rat hypothalamus. Anat Anz. 1975;138:324–331. [PubMed] [Google Scholar]
- Borgen LA, Davis WM, Pace HB. Effects of synthetic 9-tetrahydrocannabinol on pregnancy and offspring in the rat. Toxicol Appl Pharmacol. 1971;20:480–486. doi: 10.1016/0041-008x(71)90252-3. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Oliet SH, Kirkpatrick K, Richard D, Fisher TE. Extrinsic and intrinsic modulatory mechanisms involved in regulating the electrical activity of supraoptic neurons. Ann N Y Acad Sci. 1993;689:512–519. doi: 10.1111/j.1749-6632.1993.tb55581.x. [DOI] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci. 2003;23:6373–6384. doi: 10.1523/JNEUROSCI.23-15-06373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Shepherd C, Spinelli W, Lai FM. Oxytocin and vasopressin constrict rat isolated uterine resistance arteries by activating vasopressin V1A receptors. Eur J Pharmacol. 1999;376:45–51. doi: 10.1016/s0014-2999(99)00351-9. [DOI] [PubMed] [Google Scholar]
- de Kock CP, Wierda KD, Bosman LW, Min R, Koksma JJ, Mansvelder HD, Verhage M, Brussaard AB. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–2734. doi: 10.1523/JNEUROSCI.23-07-02726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana MA, Levenes C, Mackie K, Marty A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci. 2002;22:200–208. doi: 10.1523/JNEUROSCI.22-01-00200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, Giang DK, Cravatt BF, Elphick MR. A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc R Soc Lond B Biol Sci. 1998;265:2081–2085. doi: 10.1098/rspb.1998.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Stoeckel ME, Klein MJ. Oxytocin receptors on oxytocin neurones: histoautoradiographic detection in the lactating rat. J Physiol. 1994;480:155–165. doi: 10.1113/jphysiol.1994.sp020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht HR, Sieber B, Waser PG. Behavioral effects of hashish in mice. II. Nursing behavior and development of the sucklings. Psychopharmacology (Berl) 1980;70:155–161. doi: 10.1007/BF00435307. [DOI] [PubMed] [Google Scholar]
- Gouzenes L, Sabatier N, Richard P, Moos FC, Dayanithi G. V1a- and V2-type vasopressin receptors mediate vasopressin-induced Ca2+ responses in isolated rat supraoptic neurones. J Physiol. 1999;517:771–779. doi: 10.1111/j.1469-7793.1999.0771s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilairet S, Bouaboula M, Carriere D, Le Fur G, Casellas P. Hypersensitization of the Orexin 1 receptor by the CB1 receptor: evidence for cross-talk blocked by the specific CB1 antagonist, SR141716. J Biol Chem. 2003;278:23731–23737. doi: 10.1074/jbc.M212369200. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Kombian SB, Pittman QJ. Oxytocin retrogradely inhibits evoked, but not miniature, EPSCs in the rat supraoptic nucleus: role of N- and P/Q-type calcium channels. J Physiol. 2001;532:595–607. doi: 10.1111/j.1469-7793.2001.0595e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa M, Mouginot D, Kozoriz MG, Kombian SB, Pittman QJ. Vasopressin differentially modulates non-NMDA receptors in vasopressin and oxytocin neurons in the supraoptic nucleus. J Neurosci. 2003a;23:4270–4277. doi: 10.1523/JNEUROSCI.23-10-04270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa M, Pittman QJ. Nifedipine facilitates neurotransmitter release independently of calcium channels. Proc Natl Acad Sci U S A. 2003b;100:6139–6144. doi: 10.1073/pnas.0936131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356:526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Hirasawa M, Mouginot D, Chen X, Pittman QJ. Short-term potentiation of miniature excitatory synaptic currents causes excitation of supraoptic neurons. J Neurophysiol. 2000;83:2542–2553. doi: 10.1152/jn.2000.83.5.2542. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Mouginot D, Pittman QJ. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001a;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001b;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Kruszynski M, Lammek B, Manning M, Seto J, Haldar J, Sawyer WH. [1-(beta-Mercapto-beta,beta-cyclopentamethylenepropionic acid),2-(O-methyl) tyrosine]arginine-vasopressin and [1-(beta-mercapto-beta, beta-cyclopentamethylenepropionic acid)]arginine-vasopressin, two highly potent antagonists of the vasopressor response to arginine-vasopressin. J Med Chem. 1980;23:364–368. doi: 10.1021/jm00178a003. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Dayanithi G, Moos FC, Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994;478:275–288. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M. Functional role of intrahypothalamic release of oxytocin and vasopressin: Consequences and controversies. Am J Physiol. 1995;268:E537–E545. doi: 10.1152/ajpendo.1995.268.4.E537. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan SJ, Reynen PH, Kwan J, Bonhaus DW. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br J Pharmacol. 1998;124:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol. 1984;102:63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- Moos F, Gouzenes L, Brown D, Dayanithi G, Sabatier N, Boissin L, Rabie A, Richard P. New aspects of firing pattern autocontrol in oxytocin and vasopressin neurones. Adv Exp Med Biol. 1998;449:153–162. doi: 10.1007/978-1-4615-4871-3_18. [DOI] [PubMed] [Google Scholar]
- Morris JF, Christian H, Ma D, Wang H. Dendritic secretion of peptides from hypothalamic magnocellular neurosecretory neurones: a local dynamic control system and its functions. Exp Physiol. 2000;85:131S–138S. doi: 10.1111/j.1469-445x.2000.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Neumann I, Douglas AJ, Pittman QJ, Russell JA, Landgraf R. Oxytocin released within the supraoptic nucleus of the rat brain by positive feedback action is involved in parturition-related events. J Neuroendocrinol. 1996;8:227–233. doi: 10.1046/j.1365-2826.1996.04557.x. [DOI] [PubMed] [Google Scholar]
- New DC, Wong YH. BML-190 and AM251 act as inverse agonists at the human cannabinoid CB2 receptor: signalling via cAMP and inositol phosphates. FEBS Lett. 2003;536:157–160. doi: 10.1016/s0014-5793(03)00048-6. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Leonard CM, Ishiguro H, Zhang PW, Lin Z, Akinshola BE, Uhl GR. Endocannabinoids and cannabinoid receptor genetics. Prog Neurobiol. 2002;66:307–344. doi: 10.1016/s0301-0082(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in the primate spinal cord. J Neurocytol. 1999;28:39–45. doi: 10.1023/a:1007011700677. [DOI] [PubMed] [Google Scholar]
- Pittman QJ. The action is at the terminal. J Physiol. 1999;520:629. doi: 10.1111/j.1469-7793.1999.t01-1-00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Significacion fisiologica de las expansiones protoplasmicas y nerviosas de la sustancia gris. Rev Ciencias Med. 1891;22:23. [Google Scholar]
- Ravin R, Spira ME, Parnas H, Parnas I. Simultaneous measurement of intracellular Ca2+ and asynchronous transmitter release from the same crayfish bouton. J Physiol. 1997;501:251–262. doi: 10.1111/j.1469-7793.1997.tb00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia RD, Barry H., III Comparative activity of delta9-tetrahydrocannabinol, diphenylhydantoin, phenobarbital and chlordiazepoxide on electroshock seizure threshold in mice. Arch Int Pharmacodyn Ther. 1977;228:73–78. [PubMed] [Google Scholar]
- Sullivan JM. Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J Neurophysiol. 1999;82:1286–1294. doi: 10.1152/jn.1999.82.3.1286. [DOI] [PubMed] [Google Scholar]
- Tayleur Stockings G. A new euphoriant for depressive mental states. Br Med J. 1947;1:918–922. doi: 10.1136/bmj.1.4512.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT. Synaptic inputs to oxytocin- and vasopressin-secreting neurons: multiple immunostaining for light and electron microscopy. In: Yoshida S, Share L, editors. Recent Progress in Posterior Pituitary Hormones. Amsterdam: Elsevier; 1988. pp. 33–42. [Google Scholar]
- Trettel J, Levine ES. Endocannabinoids mediate rapid retrograde signaling at interneuron right-arrow pyramidal neuron synapses of the neocortex. J Neurophysiol. 2003;89:2334–2338. doi: 10.1152/jn.01037.2002. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998a;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tsou K, Nogueron MI, Muthian S, Sanudo-Pena MC, Hillard CJ, Deutsch DG, Walker JM. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci Lett. 1998b;254:137–140. doi: 10.1016/s0304-3940(98)00700-9. [DOI] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G566–G576. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez C, Lewis DL. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]