Abstract
Although ATP is important for intercellular communication, little is known about the mechanism of endogenous ATP release due to a dearth of suitable models. Using PC12 cells known to express the P2X2 subtype of ATP receptors and to store ATP with catecholamines inside dense-core vesicles, we found that clusters of PC12 cells cultured for 3–7 days generated small transient inward currents (STICs) after an inward current elicited by exogenous ATP. The amplitude of STICs in individual cells correlated with the peak amplitude of ATP-induced currents. STICs appeared as asynchronous responses (approximately 20 pA average amplitude) for 1–20 s and were investigated with a combination of patch clamping, Ca2+ imaging, biochemistry and electron microscopy. Comparable STICs were produced by focal KCl pulses and were dependent on extracellular Ca2+. STICs were abolished by the P2X antagonist PPADS and potentiated by Zn2+, suggesting they were mediated by P2X2 receptor activation. The highest probability of observing STICs was after the peak of intracellular Ca2+ increase caused by KCl. Biochemical measurements indicated that KCl application induced a significant release of ATP from PC12 cells. Electron microscopy studies showed narrow clefts without ‘synaptic-like’ densities between clustered cells. Our data suggest that STICs were caused by quantal release of endogenous ATP by depolarized PC12 cells in close juxtaposition to the recorded cell. Thus, STICs may be a new experimental model to characterize the physiology of vesicular release of ATP and to study the kinetics and pharmacology of P2X2 receptor-mediated quantal currents.
During the last decade extracellular ATP has been recognized as an important signalling molecule for intercellular communication (Lazarowski et al. 2003; Burnstock, 2004). Intracellular concentration of free ATP is usually high (mm range) and appears to be even higher in synaptic or neurosecretory vesicles (Njus et al. 1986). However, despite clear evidence for co-storing of ATP with classical neurotransmitters and hormones (Burnstock, 2004), the mechanism for ATP release remains uncertain. On the one hand, there are slow mechanisms for release of intracellular ATP such as ATP binding cassette transporters, connexin hemichannels and mitochondrial porins (reviewed by Lazarowski et al. 2003). On the other hand, there is evidence in favour of quantal (vesicular) release of ATP (in a Ca2+-dependent fashion) together with classical neurotransmitters (Burnstock, 2004). For example, sniffer patches containing ionotropic P2X receptors for ATP have revealed fast currents generated by Ca2+-dependent release of ATP (with ACh) from stimulated motor nerve terminals (Silinsky & Redman, 1996). Likewise, quantal events generated via P2X1 receptors activated by endogenously released ATP have been found in smooth muscle (Bennett et al. 1995; Brain et al. 2002). Nevertheless, despite growing evidence about release of endogenous ATP in the nervous system (Edwards et al. 1992; Evans et al. 1992; Pankratov et al. 2002), there is only one report on spontaneous quantal events generated by endogenous ATP on medial habenular neurones (Edwards et al. 1992).
Chromaffin cells and the closely related PC12 cells are classical models to study basic mechanisms of neurosecretion. In chromaffin cells concentrations of catecholamines and ATP are remarkably high: dense core vesicles of chromaffin or PC12 cells contain catecholamines and ATP at the concentration of 550 and 125 mm, respectively (Njus et al. 1986). While catecholamine release can be detected with fast amperometry (Leszczyszyn et al. 1991; Chow et al. 1992), this method is unsuitable to monitor extracellular ATP, thus leaving open the question concerning the basic properties of the ATP release process and its effects on target membranes. Heterologous expression of ATP P2X1 receptors in rat chromaffin cells has revealed ATP-mediated transient currents thought to correspond to vesicular ATP release (Hollins & Ikeda, 1997). However, in such a study, the observed currents possessed unusually slow kinetics (Hollins & Ikeda, 1997) which could have been due to post-translational modifications. Furthermore, this subtype of P2X receptor is subjected to fast, strong desensitization (Rettinger & Schmalzing, 2004) and is not normally found in these cells. Hence, it seemed useful to study the properties of vesicular ATP release from and its actions on PC12 cells natively expressing P2X2 ionotropic ATP receptors (Brake et al. 1994) which, unlike P2X1 receptors, are little prone to desensitization (North, 2002) and could thus mediate ATP signals more typical for crosstalk between ATP-releasing cells.
The present study shows that clusters of rat PC12 cells represent an advantageous system to reliably detect quantal events mediated by discrete release of endogenous ATP. Thus, such a preparation offers a new experimental tool to explore the physiological and pharmacological properties of purinergic mechanisms at secretory and target level.
Methods
Cell culturing and patch clamp recording
Rat PC12 cells, obtained from the SISSA cell bank, were prepared as previously described (Khiroug et al. 1997; Skorinkin et al. 2003). Briefly, cells were plated on poly l-lysine-coated 35 mm Petri dishes (2.5 × 105 cells per dish; on average the cell density was 260 cells mm−2) and cultured for 7 days under an atmosphere containing 5% CO2. Monolayers of single PC12 cells gradually organized themselves into clusters in which cells retained their individual round shape indicative of a non-differentiated phenotype (Rudy et al. 1987; Fig. 1E and F). For experiments designed to assess the viability of PC12 cells after several days in culture, cells were cultured in the continuous presence of a saturating concentration (30 μm) of pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (tetrasodium salt; PPADS), a broad spectrum P2X antagonist (North, 2002). Aliquots of this inhibitor were added to the culture medium on day 1, 3 and 5 in culture. On a daily basis, three Petri dishes were used to collect cells and count their number in a grid chamber. Sister dishes (without PPADS) were used as controls.
Figure 1. Correlation between growth and functional properties of PC12 cells in culture.
A–D, time profile (days) for increase in cell number in control or in the presence of PPADS (A), amplitude of intracellular Ca2+ transients induced by K+ pulse; (B, number of cells ranged from 7 to 78); size of membrane currents elicited by 1 mm ATP (C, number of recorded cells ranged from 17 to 86) and per cent of cells generating small transient inward currents (STICs) to 1 mm ATP application (D). E and F, examples of morphology of PC12 cells that, despite cell clustering, maintained spherical shape without processes. Cells were visualized on the basis of their Ca2+ signals before (E) and after (F) K+ pulse (day 4 after plating). Calibration bar = 12 mm
Whole-cell currents were recorded from single cells (12–20 μm diameter) continuously superfused with control solution containing (mm): NaCl 152, KCl 5, MgCl2 1, CaCl2 2, glucose 10, Hepes 10; pH was adjusted to 7.4 with NaOH. Patch pipettes had a resistance of 3–4 MΩ and were filled with (mm): CsCl 130, Hepes 20, MgCl2 1, Mg2ATP3 3, EGTA 5 (the latter omitted in experiments designed not to buffer intracellular Ca2+); pH was adjusted to 7.2 with CsOH. External Ca2+-free solution had Ca2+ replaced by an equimolar concentration of Mg2+ plus 5 mm EGTA.
Cells were voltage clamped at −70 mV with series resistance usually compensated by 80%. Currents were filtered at 1 kHz and acquired on an IBM PC by means of pCLAMP 8.2 software (Axon Instruments, Foster City, CA, USA).
Modified solutions and drugs were applied by a rapid superfusion system (Rapid Solution Changer RSC-200, Bio-Logic Science Instruments, Grenoble, France) placed 100–150 μm away from the recorded cell. Time for solution exchange was about 30 ms. Fresh solutions of ATP were prepared on the day of the experiments and their pH was adjusted to 7.4 with NaOH. ATP and KCl applications were 2 s long (if not otherwise indicated). All chemicals, including enzymes for cell culture, were from Sigma (Milan, Italy) except PPADS which was purchased from Tocris (Bristol, UK). Culture media were obtained from Gibco BRL (Life Technologies, Milan, Italy).
Calcium imaging
For imaging changes in intracellular Ca2+ levels ([Ca2+]i) in the visible light range we used the Ca2+-sensitive dye fluo-3 (AM ester cell-permeable compound; 5 μm; Molecular Probes, Eugene, OR, USA) as reported earlier (Khiroug et al. 1997). Fluorescence emission of the Ca2+-sensitive dye was excited at a fixed wavelength (488 nm) with a monochromator and detected with a fast CCD camera (Coolsnap HQ; Roper Scientific, USA); for emitted light two filters were used (dichroic 525 nm, emission 545 nm). Single cell images were acquired with 300 ms exposure time and analysed with the Metafluor software (Metafluor Imaging Series 6.0, Universal Imaging Corporation, USA). [Ca2+]i transients were expressed as fractional amplitude increase (ΔF/F0, where F0 is the baseline fluorescence level and ΔF is the rise over baseline). The action of 100 mm KCl pulses on [Ca2+]i transients was compared when KCl was dissolved in standard extracellular solution or in a modified solution which had NaCl concentration proportionally reduced for osmotic compensation KCl. No significant difference in [Ca2+]i was found (n = 12 cells).
Luciferin–luciferase bioluminescence assay
Assay of ATP was performed as recently described (Sokolova et al. 2004). Culture medium (800 μl) was collected under various experimental conditions, kept on ice and 50 μl aliquots used for measuring ATP concentration with a bioluminescence method using a kit from Promega (Madison, WI, USA). A 50 μl aliquot of the luciferin–luciferase reaction medium was added to each cuvette. The resulting light signal was immediately measured with an LB 9501 Lumat luminometer (Berthold GmbH, Bad Wilbad, Germany). A calibration curve was generated for each luciferase assay using serial dilutions of the ATP standard solution.
To measure extracellular ATP concentrations following application of KCl (with or without Zn2+), cultured cells were first bathed in a standard extracellular buffer solution (BS) for 10 min and their medium collected to measure baseline ATP concentration. Thereafter, cells were stimulated with 100 mm KCl alone or 100 mm KCl plus 30 μm ZnCl2 for 5 min (osmotic compensation was effected by proportional reduction in NaCl) and the medium sample collected for ATP analysis. Owing to the small concentration of extracellular ATP, shorter application times gave value near or below the sensitivity threshold of the assay. To normalize these results, protein concentration was quantified by the bicinchoninic acid method (Pierce, Rockford, IL, USA).
Electron microscopy
Each plastic Petri dish containing a monolayer of PC12 cells grown for 5 days was superfused for 10 min with buffered saline. Cells were fixed by fully replacing the medium with a solution containing 2% glutaraldehyde and 1% paraformaldehyde in phosphate buffer (54 mm NaCl, 2.7 KCl, 8.2 mm Na2HPO4, 1 mm K2HPO4). Cell fixation was continued for 1 h at room temperature. Cells were post-fixed with 1% OsO4 for 1 h, dehydrated in graded ethanol, and embedded in Araldite/Embed 812 (Electron Microscopy Sciences, Fort Washington, PA, USA). At the same time four or five gelatine capsules (5 mm outer diameter), previously filled with the same Araldite/Embed 812 material, were mounted (open end downwards) over the cell layer. The Petri dish containing the assembly of cells plus capsules was transferred to an oven to be polymerized at 60°C for 48 h. Capsules with attached PC12 cells were then detached from the Petri dish and mounted horizontally. Ultrathin (80 nm) cross sections were cut with a diamond knife in a Reichert-Jung Ultracut microtome. From each Petri dish specimens from only one capsule were used for electron microscopy (EM) analysis. Sections collected on uncoated grids were stained with uranyl acetate, and examined with a Zeiss EM109 transmission electron microscope. To measure the intercellular gap between adjacent PC12 cells, 10 pairs of cells were taken at random and the cell gap was measured (20 000 × magnification) as 1.8 ± 0.1 μm in length. Digital images were acquired at EM column magnification (× 12 000) using a Cool SNAP Digital Monochrome CCD camera (Roper Scientific) mounted in the 35 mm port of the electron microscope.
Data analysis
Recorded electrophysiological events stored on a PC were analysed off-line to calculate mean values of peak amplitude, rise time (10–90% amplitude), and decay time constant (τ). Usually 20–64 events were averaged to obtain mean values. Such events were detected using amplitude threshold set as a multiple (4–5 times) of the s.d. of the noise. Each event was also visually inspected to exclude artifactually noisy components. Currents were analysed off-line using Clampfit (Axon Instruments, Union City, CA, USA, version 9.0) or Mini Analysis (Synaptosoft Inc.).
All data are presented as means ± s.e.m. (n is the number of cells) with statistical significance assessed with a paired (or unpaired) t test (for parametric data) or Mann-Whitney test (for non-parametric data). Linear regression analysis was performed with Origin Microcal Software (version 6.0). Statistical significance of data was assessed using SigmaStat (Jandel Scientific; version 2.0). A value of P < 0.05 was accepted as indicative of statistically significant difference.
Results
Clusters during PC12 cells ‘maturation’
The number of PC12 cells grew steadily during 1 week in culture (Fig. 1A). This growth was characterized by the appearance of cell clusters presumably derived from one parent cell. Clusters comprising 2–15 individual cells in close juxtaposition (see later EM images) included the majority of cells by days 5–7. When cells were cultured in the presence of the P2X antagonist PPADS (30 μm; North, 2002), there was no significant difference in cell numbers (Fig. 1A) or clusters. Figure 1E and F shows typical clusters of PC12 cells (4 days after plating, visualized after cell loading with the membrane-permeable dye Fluo-3 AM) in control (Fig. 1E) and after stimulation of Ca2+ influx by a 2 s application of 100 mm KCl (Fig. 1F). The amplitude of KCl-induced Ca2+ transients was increased during culturing (Fig. 1B) except by day 7, when reduced responsiveness was observed in many cells. Likewise, the amplitude of membrane currents induced by a saturating 1 mm concentration of ATP (applied for 2 s; Skorinkin et al. 2003) increased linearly with the time in culture (correlation coefficient (r) = 0.92; P = 0.002; Fig. 1C). Thus, during the first week in culture there was a parallel increase in cell density and clusterization together with enhanced Ca2+ transients (presumably reflecting the growing number of voltage-activated Ca2+ channels) and ATP-mediated responses (primarily due to P2X2 channels that are the predominant subtype of ionotropic ATP receptors expressed by these cells; Brake et al. 1994). It is worth noting that, despite cell clustering, the shape of cells remained spherical (as exemplified in Fig. 1E and F) without processes.
Quantal events evoked by exogenous ATP
As exemplified in Fig. 2A, we recorded membrane currents from a single cell within a cluster following fast application of 1 mm ATP. ATP generated a large, slowly desensitizing membrane current that quickly returned to baseline upon washout (see thick black trace in Fig. 2Aa). During the washout phase (20 s) there was the appearance of small, transient inward currents (STICs; Fig. 2Aa). The average STIC for the cell shown in Fig. 2Aa is shown in Fig. 2Ab (amplitude = −21 pA, rise time = 10 ms, monoexponential decay time constant = 24 ms from 64 events). The mean amplitude of STICs measured from 31 cells (during 20 s time window after ATP pulse) was −17.8 ± 1.6 pA, with a rise time of 9.3 ± 0.6 ms and decay time constant of 27 ± 1.4 ms. The occurrence of STICs varied between 1 and 18 during the 20 s observation period. STICs were never observed on PC12 cells during the first 2 days after plating (n = 40 cells) while the probability of detecting STICs was increased to 30% at the 6th day in culture (Fig. 1D).
Figure 2. Small transient inward currents (STICs) evoked by ATP and their sensitivity to Zn2+.
Aa, application of ATP (1 mm; 2 s) evoked large membrane current followed by STICs after current decay during washout (black trace). In the same cell washout of ATP with 30 μm Zn2+ increased the number, amplitude, rise time and duration of STICs (grey trace). Ab, superimposed records to show the effect of Zn2+ on average STIC. Note monoexponential decay both in control (decay time constant, τ = 24 ms; n = 64 events) and in the presence of Zn2+ (τ = 104 ms; n = 48 events). Examples of cumulative distribution of amplitude (B) and decay (C) of STICs before (open circles) and after addition of 30 μm Zn2+ (grey circles). D, distribution of STIC latency (from the end of ATP application) in control (open bars) and in the presence of Zn2+ (grey bars). E, the amplitude, rise time and decay time constant of STICs in Zn2+ solution (30 μm) normalized to control (dashed line). Data from 9 cells. *P < 0.05, **P < 0.001.
Under the same experimental conditions STICs were never found when recording from isolated cells (n = 58) regardless of their time in culture. Since a lack of STIC occurrence might have been due to intracellular Ca2+ buffering, we repeated experiments on cells patch-clamped with an intracellular solution lacking EGTA on the 3rd–5th day after plating. In such a case, STICs were observed in only 3 out of 57 cells (5%) which were very sensitive to 1 mm ATP (−1162 ± 122 pA inward current amplitude). In those three cells STICs had an average amplitude of −26 ± 6 pA, 33 ± 3 ms decay time constant and were 3.8 ± 1.9 per 20 s epoch. In the remaining 95% of cells ATP generated inward currents (−280 ± 38 pA) significantly smaller (P < 0.01) than those recorded from control cluster cells dialysed with EGTA (see Fig. 1C). Even including data from the STIC-generating isolated cells (n = 57), the average ATP response of this population remained significantly (P < 0.01) lower (−328 ± 41 pA) than the one of the cluster population (see above).
We also explored the possibility that applying a strong and large depolarizing pulse (cf. Hollins & Ikeda, 1997) to isolated PC12 cells might have been sufficient to evoke STICs. This protocol (depolarizing command from −70 to 0 mV; 2 s long) was tested on 24 cells recorded without EGTA in the pipette, and failed to produce STICs with the exception of the three cells with unusually high sensitivity to ATP as reported above. In this case the depolarization-induced STICs had −30 ± 7 pA average amplitude, 33 ± 4 ms decay time constant, and they were 1.8 ± 0.4 per 20 s epoch.
It is therefore clear that PC12 cell clusterization strongly favoured the occurrence of STICs.
Effect of Zn2+ on STICs
The fast time-course of STICs suggested that they were mediated by ionotropic receptors rather than by G-protein-coupled receptors activated, for instance, by catecholamines released from PC12 cells. We therefore considered that STICs could be membrane events generated by release of endogenous ATP. To explore the nature of STICs we first applied Zn2+ which is known to strongly enhance P2X2 receptor activity in PC12 cells (Koizumi et al. 1995; North, 2002). Figure 2Aa demonstrates that washing out ATP with a solution containing 30 μm Zn2+ largely potentiated STICs in terms of number, amplitude, rise time and decay (Fig. 2Ab, B and C). On average, Zn2+ increased the amplitude of STICs by 84 ± 16% (n = 9; P < 0.05) and their rise time by 33 ± 7% (n = 9; P < 0.05; Fig. 1E). The most prominent effect of Zn2+ was on STIC decay, which increased by 233 ± 30% (n = 9; P < 0.001; Fig. 2E). The strong potentiation action by Zn2+ was consistent with the purinergic nature of STICs (Wildman et al. 1998; North, 2002).
The time profile of STICs generation is shown in Fig. 2D: it is interesting that this process was maximally developed 3–4 s after the end of the ATP pulse and was not changed by Zn2+ (Fig. 2D), although Zn2+ increased (25 ± 6%; n = 5; P < 0.05) the number of events.
Characteristics of STICs
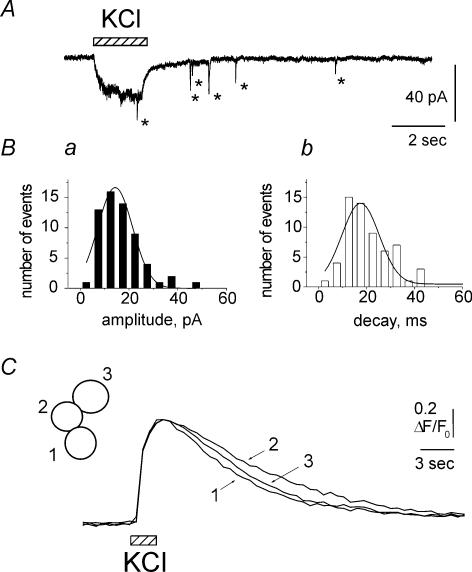
By using another depolarizing agent, namely KCl, it was possible to explore the latency of STIC onset without complications due to ATP receptor occupancy and/or desensitization. Figure 3A indicates that pulse (2 s) application of 100 mm KCl produced an inward current during which a single event only was detected. However, during KCl washout, several STICs appeared (*; Fig. 3A) consistent with a slow mechanism for their release onset. Figure 3Ba and b shows that both amplitude and decay of KCl-induced STICs were fitted by single Gaussian curves, suggesting a mainly monoquantal release process. In four cells the characteristics of STICs evoked by either KCl (−17 ± 5 pA amplitude and 20 ± 4 ms decay time constant) or ATP (−19 ± 3 pA amplitude and 22 ± 5 ms decay time constant) were very similar (P > 0.05). In the same cells comparing the delay of STIC appearance after KCl or ATP showed overlapping time profiles peaking 3–4 s after the agonist pulse (not shown).
Figure 3. High K+ increased intracellular Ca2+ and generated STICs in clustered cells.
A, application of 100 mm KCl (2 s) generated single STIC (*) during KCl-induced inward current and several STICs (*) during washout. Ba, distribution of amplitude of KCl-induced STICs. Bb, distribution of decay of STICs. Note that both distributions could be fitted by a single Gaussian curve, suggesting a mainly monoquantal release process. C, representative records of the time course of intracellular Ca2+ transients ([Ca2+]i) induced by KCl (100 mm; 2 s) in a cell cluster (schematized in inset) loaded with Fluo-3AM. Signals were scaled to the Ca2+ transient from cell no. 1 (to which calibration applies) to compare their time course. Note almost synchronous generation of Ca2+ transients in all three cells of this cluster.
To investigate the cause for the delayed appearance of STICs, we monitored the time course of intracellular Ca2+ transients ([Ca2+]i) induced by KCl depolarization in cell clusters loaded with Fluo-3. Figure 3C shows that 100 mm KCl (2 s) induced almost synchronous generation of Ca2+ transients in all three cells of this cluster which peaked 2.4 ± 0.07 s from the start of application and had a half-decay time of 6.0 ± 1.6 s. These data were replicated on 45 cells, suggesting that generation of STICs had an intrinsically slow release onset, apparently not due to simple delay in [Ca2+]i changes. Unlike KCl- or ATP-evoked release, spontaneous STICs were rare. On average, their mean frequency at rest was 0.06 s−1 (n = 8).
In full accordance with the classical transmitter release process (Augustine, 2001), appearance of STICs was abolished in Ca2+-free solution. Figure 4A shows one example of the fact that removal of Ca2+ from physiological solution reversibly abolished KCl-induced [Ca2+]i transients (similar results were obtained from 7 intact PC12 cells). Figure 4B shows that, on patched cells, removal of Ca2+ did not change the KCl-induced inward current which, however, was not followed by STICs; STICs were detected again when the test was repeated in control solution. This observation was confirmed on a sample of five cells (Fig. 4C). Similarly, removal of extracellular Ca2+ almost completely blocked STIC appearance after a single application of 1 mm ATP (n = 8; Fig. 4D). The small remaining fraction (16%) of STICs was probably mediated by metabotropic P2Y receptors coupled to IP3 and internal Ca2+ release by PC12 cells (Moskvina et al. 2003). Return to physiological solution completely restored initial release probability (Fig. 4D). These data clearly indicate the Ca2+-dependent nature of STICs regardless of the triggering pulse.
Figure 4. STICs were generated in a Ca2+-dependent manner.
A, test showing that removal of Ca2+ from physiological solution reversibly abolished KCl-induced [Ca2+]i transients. KCl application was 2 s long. B, STICs (*) generated by KCl pulse were reversibly abolished after removal of Ca2+. C, average data for generation of STICs by KCl in control solution, Ca2+-free media and upon return to normal saline (n = 5; *P < 0.05). D, average data for generation of STICs by 1 mm ATP in control solution, Ca2+-free media and upon return to normal saline (n = 8; *P < 0.05).
STICs were generated by release of endogenous ATP
Confirmation that STICs evoked by KCl or ATP were indeed generated by activation of purinergic receptors was obtained by applying the P2X receptor antagonist PPADS (North, 2002). Figure 5A shows that application of 10 μm PPADS significantly reduced the amplitude of the large ATP-induced current (which in control was followed by several STICs; inset to Fig. 5Aa) and abolished STICs (inset to Fig. 5Ab). This result was replicated in five clusters of PC12 cells. Consistent with the ATP-mediated nature of STICs, PPADS also abolished this type of activity evoked by 100 mm KCl (10 s; n = 4 cells; see example in Fig. 5B).
Figure 5. STICs were abolished by PPADS, an antagonist of P2X receptors.
A, membrane currents induced by 1 mm ATP (2 s) in control (a) and after 3 min application of 10 μm PPADS (b). Note that PPADS reduced ATP-induced current and abolished STICs (compare inset to 5Aa with inset to 5Ab). B, STICs generated by 10 s pulse of KCl (a) were abolished in 10 μm PPADS solution (b).
On a large sample of cells (n = 25), we compared the amplitude of currents evoked by 1 mm ATP and the amplitude of subsequent STICs. The amplitude of STICs was closely correlated with the peak current generated by exogenous ATP on the same cell as demonstrated in the example of Fig. 6A, in which one cell (Fig. 6Aa) produced a relatively high (−1480 pA) amplitude of ATP current and large average amplitude of STICs (−29 pA). In another cell (Fig. 6Ab) the amplitude of the ATP-induced current was much smaller (−315 pA) and STICs had average amplitude of −11 pA only. For 25 cells there was a strong correlation between these two parameters (r = 0.86; P < 0.001). Interestingly, no correlation (r = 0.06; P = 0.8) was found between STICs decay time constant and ATP current amplitude (Fig. 6Bb). In summary, these data are consistent with the purinergic nature of STICs.
Figure 6. Correlation between amplitude of ATP-induced membrane current and of STICs.
Aa, recording from cell in which 1 mm ATP evoked large membrane current (−1480 pA, left trace) followed by average STICs with relatively large amplitude (−29 pA, 42 events, right trace). Ab, recording from another cell with smaller amplitude of ATP current (−315 pA) followed by STICs with average amplitude of −11 pA (55 events). Ba, the amplitude of currents evoked by 1 mm ATP was plotted against the amplitude of subsequent STICs (n = 25). Note strong correlation between these two parameters (r = 0.86; P < 0.001). Bb, similar plot for the amplitude of membrane current and STICs decay. Note lack of correlation in this case (r = 0.06; P = 0.8).
Transient currents evoked by pressure application of exogenous ATP
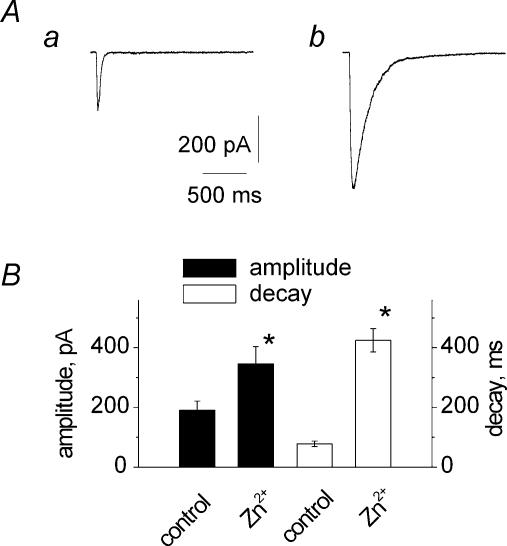
Unlike steady-state membrane currents evoked by ATP superfusion, STICs were presumably based on non-equilibrium agonist transients. We therefore generated faster ATP responses with short (10 ms) pulses of pressure-applied 1 mm ATP (Fig. 7Aa) which yielded peak amplitudes corresponding to 15% of steady-state currents observed with a 2 s application of 1 mm ATP. Zn2+ (30 μm) increased the amplitude of the fast ATP current and slowed down its decay which remained monoexponential (see example in Fig. 7Ab). In four cells Zn2+ significantly (P < 0.05) increased the amplitude and prolonged the decay of transient ATP currents (Fig. 7B). In contrast, the amplitude of maximal, steady-state currents induced by 2 s pulses of 1 mm ATP on the same cells was not changed by 30 μm Zn2+ (6 ± 4%, n = 7; P > 0.05), consistent with previous data (Wildman et al. 1998; Vorobjev et al. 2003). Thus, the Zn2+-induced potentiation of transient currents evoked by exogenous ATP was very similar to the change in the amplitude and duration of STICs in the presence of Zn2+.
Figure 7. Zn2+ strongly augmented the amplitude and prolonged the decay of currents induced by brief puffer application of ATP.
A, fast ATP current elicited by 10 ms pulse of pressure-applied 1 mm ATP in control (a) and in the presence of 30 μm Zn2+ (b). B, histograms showing potentiating action of 30 μm Zn2+ on the amplitude or decay of ATP-induced currents (n = 4). *P < 0.05.
EM study of PC12 cells
In general, PC12 cells within a cluster had a mean volume of 675 ± 55 μm3 and possessed standard morphological characteristics, including dense cytoplasm, round nucleus with one or two nucleoli in the central part, and chromatin condensed on the internal surface of the nuclear membrane. Their dense cytoplasm contained mitochondria and rough endoplasmic reticulum while the Golgi apparatus occupied a small part of the cell only.
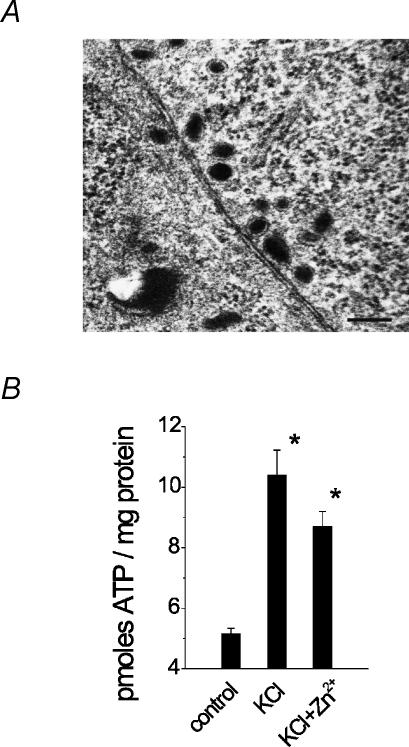
As far as the ultrastructure of contacts was concerned, Fig. 8A shows an example of closely apposed membranes of two PC12 cells in a cell cluster. In this example the mean distance between the two cell membranes was 9.2 ± 0.6 nm (range 6–12.5 nm). On average, in areas of close contact between cells the gap was 11.5 ± 0.55 nm (n = 10 cell pairs). Although NGF-treated PC12 cells can generate synapse-like formations (Ruzzier et al. 1988; Tao-Cheng et al. 1995), in the present experiments (without NGF application) at the stage when we detected STICs, no synaptic-like, asymmetrical specializations between neighbour cells were observed. Cells contained predominantly large, dense core vesicles (known to contain ATP; Njus et al. 1986) which, when they had an elongated appearance, had 129.5 ± 4.3 nm smaller diameter and 176.8 ± 6.4 nm larger diameter (n = 110) in accordance with Yamakuni et al. (2002).
Figure 8. Cell contacts, intracellular vesicles and amount of released ATP.
A, EM micrograph with example of cross section of clustered PC12 cells showing narrow intercellular gap and group of dense core vesicles (dark organelles) in perimembrane regions. Note lack of any ‘synapse-like’ densities between apposed cells. Scale bar, 200 nm. B, quantification of ATP release into the extracellular medium using luciferin–luciferase assay at rest, after stimulation of release with 100 mm KCl, and KCl plus 30 μm Zn2+. n = 3 dishes. *P < 0.05.
Biochemical determination of released ATP
Our electrophysiological experiments have suggested that, under resting conditions, basal ATP release was presumably very low owing to the low occurrence of STICs. The very sensitive luciferin–luciferase assay was next used to quantify ATP into the extracellular medium under various experimental conditions that facilitated the appearance of STICs. The ambient concentration of ATP was 5.0 ± 0.2 pmol mg−1 of protein (N = 3 dishes), and it more than doubled with application of 100 mm KCl or KCl plus 30 μm Zn2+ (Fig. 8B), indicating that ATP release was strongly dependent on cell depolarization and that the action of Zn2+ on purinergic mechanisms did not involve changes in ATP release.
Discussion
This study is the first report of quantal ATP events generated by activation of native P2X2 receptors on PC12 cells, a classical model to study dense core vesicle exocytosis. Since the quantitative description of quantal events generated by vesicular transmitter release plays a principal role in understanding synaptic physiology (Del Castillo & Katz, 1954; Korn & Faber, 1991), it is suggested that the simple model described in the present study provides novel data on the properties of ATP release and its interaction with native P2X2 receptors. This information might therefore help to further our understanding of the ATP-operated synapses found in the central nervous system (Burnstock, 2004).
Basic properties favouring occurrence of STICs
After plating, the density of PC12 cells progressively increased and was accompanied by: (1) a higher number of cell clusters (see also Baldwin et al. 1996), (2) a larger response to extracellular ATP, and (3) a stronger increase in [Ca2+]i to KCl depolarization. The probability of observing STICs was clearly dependent on these three factors. In accordance with this notion there was a close correlation between the amplitude of STICs and the amplitude of ATP-induced currents indicating very low probability to detect STICs in clustered cells generating maximal ATP currents < 300 pA.
When recording from isolated cells cultured under identical condition and patch-clamped without intracellular Ca2+ buffer (to maximize the chance of Ca2+-dependent vesicular release), the probability of detecting STICs was quite low when the depolarizing stimulus was exogenous ATP or membrane depolarization. Interestingly, the few isolated cells generating STICs were those very sensitive to ATP itself (> 1000 pA response amplitude). Although undifferentiated PC12 cells grown in culture without addition of NGF (as in the present study) stably express a variety of voltage-activated Ca2+ channels (Liu et al. 1996), their expression is relatively small (Elhamdani et al. 2000). Furthermore, unlike chromaffin cells, undifferentiated PC12 cells release only a limited number of catecholamine-containing vesicles with considerable differences in their mean content (Westerink et al. 2000). Thus, these properties make it difficult to detect STICs especially when using isolated PC12 cells.
In bovine chromaffin cells (about 804 μm2 average membrane area) the cell region in contact with the culture support is relatively small and can show exocytosis (Plattner et al. 1997). Had the same conditions applied to PC12 cells, STICs might have been expected to be detected in the present experiments on single cells. However, in PC12 cells (with average volume of about 675 μm3) the calculated membrane area is 366 μm2, thus considerably smaller than in chromaffin cells. Hence, limited cell surface contact plus release mechanisms less efficient than in chromaffin cells and generally low sensitivity to ATP can help to explain the difficulty to observe STICs in isolated PC12 cells. Conversely, when PC12 cells formed clusters, this condition provided a larger number of cell contacts potentially contributing to vesicular release onto the recorded cell (with relatively high sensitivity to ATP) and consequently enhanced the chance of detecting STICs.
A previous report on isolated chromaffin cells transfected with P2X1 receptors described abundance of large, quantal-like events (Hollins & Ikeda, 1997). Nevertheless, such events were kinetically and pharmacologically different from those recorded in the present study based on native P2X2 receptors and may reflect not only differing releasing properties between chromaffin cells and PC12 cells (Martin, 2003) but also between recombinant and native receptor systems.
In the present investigation STICs were typically recorded from voltage-clamped cells after application of KCl or ATP which depolarized surrounding cells as demonstrated with Ca2+ imaging. It should also be noted that, as a patch-clamped cell was routinely dialysed with the Ca2+ chelator EGTA, it was unlikely that robust, Ca2+-dependent release of transmitter by itself was the main phenomenon responsible for STICs. The simplest hypothesis is thus that, although STICs could occasionally originate from isolated PC12 cells, in the large majority of cases STICs were the result of intercellular cross-talk via discrete release of transmitter by clustered cells. The amplitude and decay distribution of STICs together with their rapid kinetics suggest these events to be quantal responses, mostly unitary because of their single peak in the amplitude distribution plot. Under resting conditions STICs were very rare (comparable with amperometric measurements of spontaneous release events; Westerink & Vijverberg, 2002), indicating a low probability of release.
Evidence for purinergic nature of quantal events
Despite the fact that depolarized PC12 cells release adrenaline and/or noradrenaline (Greene & Rein, 1977), it seems very unlikely that catecholamines might have interacted with the recorded cells to generate STICs because PC12 cells do not express native adrenoreceptors (Williams et al. 1998). Furthermore, the rise time (< 10 ms) of single STICs seemed to be too fast to account for the typical G-protein-coupled receptor activation induced by catecholamines.
There are several lines of evidence suggesting that STICs were generated by endogenous ATP acting on P2X2 receptors. First, although PC12 cells contain transcripts for various types of ATP receptors (Arslan et al. 2000), it is clear that the main group of functional receptors expressed in high density is the P2X2 one (Arslan et al. 2000; Skorinkin et al. 2003). Second, it is known that ATP is present at high concentration in dense core vesicles of PC12 cells (Njus et al. 1986). Third, we showed that cell depolarization with KCl not only generated STICs in a Ca2+-dependent manner, but it also increased ATP release. Fourth, Zn2+, which is a well-known potentiator of ATP P2X2 receptors (Koizumi et al. 1995; Wildman et al. 1998; North, 2002), enhanced amplitude and decay of STICs as much as that of fast currents evoked by ATP pulses. Since the main action of Zn2+ was on the decay time of STICs, it seems likely that agonist dissociation from P2X2 receptors was the primary target for the modulatory action of Zn2+ (Vorobjev et al. 2003). Fifth, the amplitude of STICs correlated with the intensity of ATP-mediated responses. Finally, the P2X antagonist PPADS abolished both ATP currents and STICs.
Collectively, all observations support quantal release of ATP from depolarized, clustered PC12 cells to induce purinergic STICs. Our proposal can be summarized in the simple scheme of Fig. 9. Application of ATP or KCl is suggested to generate depolarization with Ca2+ influx via P2X2 receptors and voltage-activated Ca2+ channels (VACCs). Raised [Ca2+]i should promote vesicular release (Augustine, 2001) of catecholamines and ATP into the narrow gap between cells. While catecholamines are ineffective on PC12 cells, ATP is thought to bind to P2X2 receptors to generate STICs. The ability to detect STICs may therefore depend on the intensity of depolarization of clustered cells and the consequent rise in [Ca2+]i.
Figure 9. Idealized scheme to account for P2X2 receptor-mediated STICs.
Application of exogenous ATP or KCl (boxes) to intact (not voltage-clamped) cell (top) is supposed to generate depolarization with Ca2+ influx via activated P2X2 receptors and voltage-activated Ca2+ channels (VACC). Raised [Ca2+]i promotes vesicular release of catecholamines (not shown) and endogenous ATP (black circles). When this process occurs within the close gap between two apposed PC12 cells, extracellular ATP can bind P2X2 receptors to generate STICs in patched cell under voltage clamp. For sake of simplicity and because of its rare occurrence, the possibility that the patched cell can release ATP to self-generate STICs is not included.
Latency and time course of STICs
Our data showed that both KCl and ATP could evoke STICs (in a Ca2+-dependent manner) with a comparably slow time course, peaking 3–4 s after termination of the depolarizing pulse. When the latency of STICs and [Ca2+]i changes was compared, it was found that such delayed release was not due to retarded peaking of [Ca2+]i. This result is consistent with the inherently slow release process of PC12 cells measured with amperometry (Wang et al. 2001; Martin, 2003). Likewise, capacitance measurements (Zorec et al. 1991) have identified a sustained, slow component of release attributable to exocytosis from dense core vesicles (Ninomiya et al. 1997; Kasai et al. 1999; Martin, 2003).
The relatively long latency of STICs after the triggering pulse should not obscure the fact that single STICs were actually fast events (< 10 ms rise time). Thus, STICs were five times shorter than events generated by P2X1 receptor activation on chromaffin cells (Hollins & Ikeda, 1997). This difference cannot be explained by a distinct vesicle fusion rate since in PC12 cells the rise time of single amperometric events is 0.2–0.5 ms, which is comparable with the 0.9 ms rise time recorded from chromaffin cells (for review see Martin, 2003). The difference probably reflects the faster agonist dissociation rate from PC12 P2X2 receptors than from chromaffin P2X1 receptors (Rettinger & Schmalzing, 2004).
Ultrastructure of PC12 cells
The EM morphological features of undifferentiated PC12 cells in culture were essentially those expected for cells at this stage of maturation (Kasai et al. 1999). PC12 cells contained a large number of dense core vesicles distributed throughout the cell cytoplasm (including the region near the cell membrane) presumably representing the morphological elements supplying STICs. Although the contact area between cells lacked specialized densities, the intercellular gap was relatively small, indicating that the local concentration of free ATP could be transiently high. Since amperometric measurements revealed 114 300 molecules of catecholamines per release event (Chen et al. 1994), the corresponding value for ATP (assuming a catecholamines/ATP ratio of 4.4; Njus et al. 1986) is expected to be ∼26 000 ATP molecules per event. Nevertheless, since STICs were readily potentiated by Zn2+, which up-regulates ATP currents evoked by submaximal (< 1 mm) agonist concentrations only, it seems likely that the intercellular ATP concentration was insufficient to saturate P2X2 receptors during generation of quantal events. Furthermore, because Zn2+ did not facilitate ATP release measured biochemically, it is unlikely that there was altered vesicular ATP content.
In summary then, morphological results demonstrate a narrow gap between clustered PC12 cells which could make possible a build-up of endogenously released ATP.
Clusters of PC12 cells as a model to study ATP quantal release
Measuring catecholamine release with amperometry provides a fast, reliable and efficient method to study the release of such transmitters from chromaffin and PC12 cells (Leszczyszyn et al. 1991; Wightman et al. 1991; Chow et al. 1992). An equivalent method to measure fast ATP transient is not available. Neurochemical methods have therefore to rely on determining the concentration of ATP in the bulk solution bathing the cells under investigation. This approach has inevitably slow resolution and averages ATP release properties of many cells (Kasai et al. 2001). The present study offers a simple, cheap and reproducible technique to study at single cell level the characteristics of individual ATP-releasing events. It should therefore be possible to employ such a model to investigate the action of drugs designed to modify the release of ATP or its effects on target cells.
Physiological implications
Release of endogenous ATP plays a physiological role in cell crosstalk and propagation of signals from cell to cell over large distances (Lazarowski et al. 2003). Over a short time scale, successful propagation of purinergic excitation to pheochromocytoma or adrenal chromaffin cells may depend on the presence (and level of expression) of P2X receptors (Liu et al. 1999; Afework & Burnstock, 2000). Purinergic signalling could provide a mechanism to synergize the collective behaviour of the adrenal medulla cells and to prompt a large release of catecholamines under the flight-and-fight reaction in addition to the known process of gap junction-mediated recruitment (Martin et al. 2001). The ATP-mediated excitation of clustered PC12 cells might therefore contribute to the non-linear increase in catecholamine secretion in aggregated versus single PC12 cells (Baldwin & Saltzman, 2001). However, ability to release ATP did not influence cell growth and the tendency to generate clusters because culturing cells in the presence of a P2X antagonist produced equivalent cell density and morphology. The present results suggest a local function for purinergic mechanisms triggered by ATP release while blood-born catecholamines represent endocrine signals to remote targets.
Acknowledgments
This work was supported by a FIRB grant from MIUR.
References
- Afework M, Burnstock G. Age-related changes in the localization of P2X (nucleotide) receptors in the rat adrenal gland. Int J Dev Neurosci. 2000;18:515–520. doi: 10.1016/s0736-5748(00)00023-x. [DOI] [PubMed] [Google Scholar]
- Arslan G, Filipeanu CM, Irenius E, Kull B, Clementi E, Allgaier C, Erlinge D, Fredholm BB. P2Y receptors contribute to ATP-induced increases in intracellular calcium in differentiated but not undifferentiated PC12 cells. Neuropharmacology. 2000;39:482–496. doi: 10.1016/s0028-3908(99)00141-0. [DOI] [PubMed] [Google Scholar]
- Augustine GJ. How does calcium trigger neurotransmitter release. Curr Opin Neurobiol. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- Baldwin SP, Krewson CE, Saltzman WM. PC12 cell aggregation and neurite growth in gels of collagen, laminin and fibronectin. Int J Dev Neurosci. 1996;14:351–364. doi: 10.1016/0736-5748(96)00018-4. [DOI] [PubMed] [Google Scholar]
- Baldwin SP, Saltzman WM. Aggregation enhances catecholamine secretion in cultured cells. Tissue Eng. 2001;7:179–190. doi: 10.1089/107632701300062796. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Farnell L, Gibson WG, Karunanithi S. Quantal transmission at purinergic junctions: stochastic interaction between ATP and its receptors. Biophys J. 1995;68:925–935. doi: 10.1016/S0006-3495(95)80268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain KL, Jackson VM, Trout SJ, Cunnane TC. Intermittent ATP release from nerve terminals elicits focal smooth muscle Ca2+ transients in mouse vas deferens. J Physiol. 2002;541:849–862. doi: 10.1113/jphysiol.2002.019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Chen TK, Luo G, Ewing AG. Amperometric monitoring of stimulated catecholamine release from rat pheochromocytoma (PC12) cells at the zeptomole level. Anal Chem. 1994;66:3031–3035. doi: 10.1021/ac00091a007. [DOI] [PubMed] [Google Scholar]
- Chow RH, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Elhamdani A, Brown ME, Artalejo CR, Palfrey HC. Enhancement of the dense-core vesicle secretory cycle by glucocorticoid differentiation of PC12 cells: characteristics of rapid exocytosis and endocytosis. J Neurosci. 2000;20:2495–2503. doi: 10.1523/JNEUROSCI.20-07-02495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Derkach V, Suprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Greene LA, Rein G. Release of (3H)norepinephrine from a clonal line of pheochromocytoma cells (PC12) by nicotinic cholinergic stimulation. Brain Res. 1977;138:521–528. doi: 10.1016/0006-8993(77)90687-4. [DOI] [PubMed] [Google Scholar]
- Hollins B, Ikeda SR. Heterologous expression of a P2X-purinoceptor in rat chromaffin cells detects vesicular ATP release. J Neurophysiol. 1997;78:3069–3076. doi: 10.1152/jn.1997.78.6.3069. [DOI] [PubMed] [Google Scholar]
- Kasai H, Kishimoto T, Liu TT, Miyashita Y, Podini P, Grohovaz F, Meldolesi J. Multiple and diverse forms of regulated exocytosis in wild-type and defective PC12 cell. Proc Natl Acad Sci U S A. 1999;96:945–949. doi: 10.1073/pnas.96.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai Y, Ohta T, Nakazato Y, Ito S. Release of dopamine and ATP from PC12 cells treated with dexamethasone, reserpine and bafilomycin A1. J Vet Med Sci. 2001;63:367–372. doi: 10.1292/jvms.63.367. [DOI] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Talantova M, Nistri A. Role of intracellular calcium in fast and slow desensitization of P2-receptors in PC12 cells. Br J Pharmacol. 1997;120:1552–1560. doi: 10.1038/sj.bjp.0701060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Ikeda M, Inoue K, Nakazawa K, Inoue K. Enhancement by zinc of ATP-evoked dopamine release from rat pheochromocytoma PC12 cells. Brain Res. 1995;673:75–82. doi: 10.1016/0006-8993(94)01404-6. [DOI] [PubMed] [Google Scholar]
- Korn H, Faber DS. Quantal analysis and synaptic efficacy in the CNS. Trends Neurosci. 1991;14:439–445. doi: 10.1016/0166-2236(91)90042-s. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ, Jr, Near JA, Wightman RM. Secretion of catecholamines from individual adrenal medullary chromaffin cells. J Neurochem. 1991;56:1855–1863. doi: 10.1111/j.1471-4159.1991.tb03441.x. [DOI] [PubMed] [Google Scholar]
- Liu M, Dunn PM, King BF, Burnstock G. Rat chromaffin cells lack P2X receptors while those of the guinea pig express a P2X receptors with novel pharmacology. Br J Pharmacol. 1999;128:61–68. doi: 10.1038/sj.bjp.0702790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Felix R, Gurnett CA, De Waard M, Witcher DR, Campbell KP. Expression and subunit interaction of voltage-dependent Ca2+ channels in PC12 cells. J Neurosci. 1996;16:7557–7565. doi: 10.1523/JNEUROSCI.16-23-07557.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AO, Mathieu MN, Chevillard C, Guerineau NC. Gap junctions mediate electrical signaling and ensuing cytosolic Ca2+ increases between chromaffin cells in adrenal slices: a role in catecholamine release. J Neurosci. 2001;21:5397–5405. doi: 10.1523/JNEUROSCI.21-15-05397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TFJ. Tuning exocytosis for speed: fast and slow modes. Biochim Biophys Acta. 2003;1641:157–165. doi: 10.1016/s0167-4889(03)00093-4. [DOI] [PubMed] [Google Scholar]
- Moskvina E, Unterberger U, Boehm S. Activity-dependent autocrine-paracrine activation of neuronal P2Y receptors. J Neurosci. 2003;23:7479–7488. doi: 10.1523/JNEUROSCI.23-20-07479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Kishimoto T, Yamazawa T, Ikeda H, Miyashita Y, Kasai H. Kinetic diversity in the fusion of exocytotic vesicles. EMBO J. 1997;16:929–934. doi: 10.1093/emboj/16.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njus D, Kelley PM, Harnadek GJ. Biogenics of secretory vesicles. Biochim Biophys Acta. 1986;853:237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal O, Verkhratsky A. Ionotropic P2X purinoreceptors mediate synaptic transmission in rat pyramidal neurones of layer II/III of somato-sensory cortex. J Physiol. 2002;542:529–536. doi: 10.1113/jphysiol.2002.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner H, Artalejo AR, Neher E. Ultrastructural organization of bovine chromaffin cell cortex-analysis by cryofixation and morphometry of aspects pertinent to exocytosis. J Cell Biol. 1997;139:1709–1717. doi: 10.1083/jcb.139.7.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G. Desensitization masks nanomolar potency of ATP for the P2X1 receptor. J Biol Chem. 2004;279:6426–6433. doi: 10.1074/jbc.M306987200. [DOI] [PubMed] [Google Scholar]
- Rudy B, Kirschenbaum B, Rukenstein A, Greene LA. Nerve growth factor increases the number of functional Na channels and induces TTX-resistant Na channels in PC12 pheochromocytoma cells. J Neurosci. 1987;7:1613–1625. doi: 10.1523/JNEUROSCI.07-06-01613.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzier F, Lee S, Dryden WF, Miledi R. In vitro reinnervation of adult rat muscle fibres by foreign neurons and transformed chromaffin PC12 cells. Proc R Soc Lond B Biol Sci. 1988;234:1–9. doi: 10.1098/rspb.1988.0035. [DOI] [PubMed] [Google Scholar]
- Silinsky EM, Redman RS. Synchronous release of ATP and neurotransmitter within milliseconds of motor nerve impulse in the frog. J Physiol. 1996;492:815–822. doi: 10.1113/jphysiol.1996.sp021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorinkin A, Nistri A, Giniatullin R. Bimodal action of protons on ATP currents of rat PC12 cells. J General Physiol. 2003;122:33–44. doi: 10.1085/jgp.200308825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova E, Skorinkin A, Fabbretti E, Masten L, Nistri A, Giniatullin R. Agonist-dependence of recovery from desensitization of P2X3 receptors provides a novel and sensitive approach for their rapid up or down regulation. Br J Pharmacol. 2004;141:1048–1058. doi: 10.1038/sj.bjp.0705701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Dosemeci A, Bressler JP, Brightman MW, Simpson DL. Characterization of synaptic vesicles and related neuronal features in nerve growth factor and ras oncogene differentiated PC12 cells. J Neurosci Res. 1995;42:323–334. doi: 10.1002/jnr.490420306. [DOI] [PubMed] [Google Scholar]
- Vorobjev VS, Sharonova IN, Sergeeva OA, Haas HL. Modulation of ATP-induced currents by zinc in acutely isolated hypothalamic neurons of the rat. Br J Pharmacol. 2003;139:919–926. doi: 10.1038/sj.bjp.0705321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CT, Grishanin R, Earles CA, Chang PY, Martin TF, Chapman ER, Jackson MB. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
- Westerink RH, de Groot A, Vijverberg HP. Heterogeneity of catecholamine-containing vesicles in PC12 cells. Biochem Biophys Res Comm. 2000;270:625–630. doi: 10.1006/bbrc.2000.2470. [DOI] [PubMed] [Google Scholar]
- Westerink RH, Vijverberg HP. Ca2+-independent vesicular catecholamine release in PC12 cells by nanomolar concentrations of Pb2+ J Neurochem. 2002;80:861–873. doi: 10.1046/j.0022-3042.2001.00751.x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br J Pharmacol. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NG, Zhong H, Minneman KP. Differential coupling of α1-, α2, and β-adrenergic receptors to mitogen-activated protein kinase pathways and differentiation in transfected PC12 cells. J Biol Chem. 1998;273:24624–24632. doi: 10.1074/jbc.273.38.24624. [DOI] [PubMed] [Google Scholar]
- Yamakuni T, Yamamoto T, Ishida Y, Yamamoto H, Song SY, Adachi E, Hiwatashi Y, Ohizumi Y. V-1, a catecholamine biosynthesis regulatory protein, positively controls catecholamine secretion in PC12D cells. FEBS Lett. 2002;530:94–98. doi: 10.1016/s0014-5793(02)03431-2. [DOI] [PubMed] [Google Scholar]
- Zorec R, Sikdar SK, Mason WT. Increased cytosolic calcium stimulates exocytosis in bovine lactotrophs. Direct evidence from changes in membrane capacitance. J General Physiol. 1991;97:473–497. doi: 10.1085/jgp.97.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]