Abstract
Cell volume and cytosolic Ca2+ concentration ([Ca2+]i) were measured in rabbit macula densa (MD) cells loaded with calcein and Fura Red using confocal microscopy. [Ca2+]i was also analysed with Indo-1 and fura-2. We used isolated microperfused thick ascending limbs with attached glomerulus. The results showed that when the luminal NaCl concentration (‘NaCl’) was decreased from 35 to 10 mm, the cell volume decreased by 10.4%, and [Ca2+]i increased by 9.5%. This increase was inhibited in Ca2+-free solution. When luminal [NaCl] was changed from 35 to 135 mm, the cell volume increased by 15.1%, and [Ca2+]i did not change. The cell volume alterations were not different in Ca2+-free solutions. Using Indo-1, basal [Ca2+]i in MD cells was 107.8 nm. When luminal [NaCl] was changed from 135 to 10 mm, [Ca2]i increased by 23.5 nm. Using fura-2, the basal [Ca2+]i in MD cells was 115.3 nm, and when luminal [NaCl] was changed from 135 or 35 to 10 mm, [Ca2+]i change was 30.1 or 10.6 nm, respectively. An increase in [NaCl] caused no change in [Ca2+]i. In Ca2+-free solution, no change in [Ca2+]i occurred. A stepwise decrease in luminal [NaCl] resulted in a sigmoid increase in [Ca2+]i in MD cells. The steepest part of the curve was between 70 and 10 mm. In conclusion, we found that MD cells have cell volume regulation, and that [Ca2+]i elevation caused by decreased luminal [NaCl] is independent of the cell volume.
Macula densa (MD) cells are the specialized epithelial cells at the end portion of the thick ascending limb. They are able to sense alterations in the luminal NaCl concentration ([NaCl]), and thereby regulate glomerular arteriolar resistance through tubuloglomerular feedback and control of renin release (Vander, 1967; Briggs et al. 1984; Skott & Briggs, 1987). The Na+–K+–2Cl− cotransporters are involved in these signal transmissions between the MD and its target cells (Schlatter et al. 1989; Obermuller et al. 1996). The next step is not yet clear. Possible mediators and modulators of the information transmitted between the MD and its target cells have been suggested, and recently ATP and/or adenosine release have been suggested as likely candidates. (Salomonsson et al. 1991; Briggs & Schnermann, 1996; Kurtz et al. 1998; Peti-Peterdi & Bell, 1999; Brown et al. 2001). In the information transfer from NaCl concentration in the lumen at the MD site to an altered tubuloglomerular feedback response and/or renin release, most previous investigations found that the NaCl concentration is important and not the osmolarity.
It has been found that changes in cell volume and in the cytosolic Ca2+ concentration ([Ca2+]i) are important factors in the regulation of cell function, especially in kidney cells (Yamaguchi et al. 1989; Wong et al. 1990; Montrose-Rafizadeh & Guggino, 1991). It has been reported that alterations of the luminal [NaCl] can result in changes in cell volume observed by direct measurement of the length of the cells (Kirk et al. 1985; Gonzalez et al. 1988) and in changes of [Ca2+]i (Salomonsson et al. 1991; Peti-Peterdi & Bell, 1999) in the MD cells. In many other types of cells, the changes in [Ca2+]i are usually accompanied by a regulatory volume decrease (RVD) (Haas & Forbush, 2000; Tinel et al. 2000). But in MD cells, these events are not clear. The use of confocal microscopy made a quantitative simultaneous analysis of cell volume and [Ca2+]i possible.
Methods
Experimental preparation
Individual cortical thick ascending limbs (cTAL) with attached glomeruli were dissected and perfused as previously described (Liu et al. 2002a, b). In short, female New Zealand White rabbits weighing 1.0–1.5 kg were killed with cervical dislocation, and the left kidney was removed and cut into several 1.5–3 mm transverse slices. These slices were placed in chilled 35 mm NaCl buffer solution containing (mm): 35 NaCl, 1.3 CaCl2, 1 MgSO4, 1.6 KH2PO4, 5 glucose and 20 Hepes, with pH adjusted to 7.4, and osmolality adjusted to 290 mosmol with sucrose. Glomeruli with attached cTAL and containing the MD plaque were isolated by microdissection at 4°C under a dissection microscope, and then transferred to a chamber fixed to the stage of a Nikon microscope attached either to a Noran Odyssey laser confocal system (Noran, USA; Fura Red and Indo-1-loaded samples) or an Applied Imaging QC-700 system (Applied Image Co., Sunderland, England; fura-2-loaded samples). The cTAL was cannulated and perfused with the 35 mm NaCl buffer solution. The preparation was continuously bathed in a 135 mm NaCl buffer solution containing (mm): 135 NaCl, 1.3 CaCl2, 1 MgSO4, 1.6 KH2PO4, 5 glucose and 20 Hepes, with pH adjusted to 7.4, and osmolality adjusted to 290 mosmol with sucrose).
Fluorescence loading and measurements
The fluorescent Ca2+ indicator Fura Red was used to measure the cytosolic Ca2+ concentration ([Ca2+]i), and simultaneously, calcein was used to measure the cell volume changes using a confocal system. MD cells were loaded in the low-NaCl buffer solution from the luminal side using 20 μm Fura Red with 0.2% pluronic acid for 40–60 min, and 5 μm calcein dissolved in dimethyl sulfoxide (DMSO) from the lumen for 10–15 min. The ratiometric Ca2+ indicators Indo-1 and fura-2 were used, the former for confocal microscopy and the latter for the video imaging system. MD cells were loaded with 20 μm Indo-1 with 0.2% pluronic acid for 40–60 min, or with 10 μm fura-2 AM in 1% DMSO for 30 min, in both cases at room temperature. A Nikon ×60/1.2 water-immersion objective lens was used to visualize MD cells. The image size was set to 640 × 480 pixels. The confocal slit was set at a width of 25 nm. Photobleaching was kept to a minimum by maintaining laser intensity at below 30% of the maximum, and using a shutter so that the preparation was exposed to laser light only during the collection of images. Data collection, with the Noran Odyssey confocal system, was controlled by a Silicon Graphics workstation. Image acquisition was limited to 30 frames s−1 and, when necessary, image noise was reduced by averaging or summing 16–32 individual images. The sampling time for each pixel was 100 ns. Fura Red and calcein were excited at 488 nm with the argon-ion laser, while emitted fluorescence was recorded at wavelengths of 530 nm for calcein and >600 nm for Fura Red simultaneously. Square-shaped regions of interest (ROIs) were set inside the cytoplasmic area of MD cells, and the mean intensity within the ROIs was recorded every 5 s. Calculations were based on the following equations:
| (1) |
Where Vc1 is cell volume and Cc1 is concentration of calcein under resting conditions; Vc2 and Cc2 are changed cell volume and changed concentration of calcein, respectively. The concentration of calcein is proportional to its fluorescence intensity, and thus the ratio of concentrations is equal to the ratio of intensities. Assuming that Vc1 is 1, the changed cell volume can be expressed from the changed calcein intensity (Fc2) and the intensity at basal level (Fc1).
| (2) |
Relative changes in cell volume can be calculated as:
| (3) |
The relative change in the amount of calcium-bound Fura Red (FRb), which is an indicator of [Ca2+]i, in MD cells can be calculated as the changed cell volume (Vr2) multiplied by the changed concentration (Cr2) of FRb divided by the basal level of cell volume (Vr1) multiplied by the concentration (Cr1) of FRb.
| (4) |
Since Vr2 and Vc2 are the same, and Vr1 is regarded as 1, the Cr2/Cr1 can be expressed as the ratio of FRb intensities in the changed conditions to those (Fr2) in the basal level (Fr1). The relative changes in FRb can be expressed as:
| (5) |
Since the amounts of FRb and [Ca2+]i have a reverse relationship, the relative changes in [Ca2+]i measured with Fura Red can be calculated by:
| (6) |
Eqn (3) and eqn (6) were used to calculated the cell volume and [Ca2+]i of MD cells in the present study.
Indo-1 was excited at 364 nm with a UV laser. Emission was measured at 405 and 485 nm and transmitted to photomultiplier tubes. A 380 nm primary dichroic mirror and a 405/30, 485/25 nm secondary dichroic mirror (Chroma, Brattleboro, VT, USA) were used to achieve appropriate recordings of wavelength. [Ca2+]i was calculated from the following equation (Grynkiewicz et al. 1985):
where Kd is the dissociation constant of Indo-1 (230 nm), R is the ratio between fluorescence at 405 and 485 nm, and Rmin and Rmax are the ratios for unbound and bound forms, respectively, of the Indo-1/Ca2+ complex in MD cells, respectively. Fo/Fs is the ratio of fluorescence at 485 nm in the absence of Ca2+ and at saturating Ca2+ concentrations, respectively. To obtain Rmax, 3 μm ionomycin was added to both the lumen and bath solution containing 1.3 mm Ca2+, whereas to obtain Rmin, Ca2+-free solution containing 5 mm EGTA was perfused from both the lumen and bath sides (Bassani et al. 1995).
In other studies conventional video imaging techniques were used to measure MD [Ca2+]i. Fura-2, loaded into MD cells was alternately excited with light at 340 and 380 nm, and emitted fluorescence was obtained at 510 nm using the Applied Imaging QC-700 system. The fluorescence ratio (340/380 nm) was converted to [Ca2+]i, and digital imaging of [Ca2+]i was displayed using standard pseudo-colour techniques. This system was calibrated using cell-free solutions (Calibration Kit from Molecular Probes). NaCl solutions of 10 mm (containing (mm): 10 NaCl, 1.3 CaCl2, 1 MgSO4, 1.6 KH2PO4, 5 glucose and 20 Hepes, pH adjusted to 7.4, and osmolality adjusted to 290 mosmol with sucrose), 35 mm and 135 mm were perfused from the lumen. Experiments were performed at 37°C with continuous perfusion in a bath with a 135 mm NaCl buffer solution at a rate of 6–7 ml min−1. The perfusion time for any [NaCl] solution was 10 min before a change to the different [NaCl] solutions. In the Ca2+-free solution, CaCl2 was replaced by 5 mm EGTA.
In a small second series of experiments everything was performed as in the first series, but NaCl concentration was kept constant at 40 mm, while osmolarity was reduced from 800 to 120 mosmol l−1 using different concentrations of sucrose.
Fura Red, Indo-1, Fura-2 and calcein were from Molecular Probes. All other chemicals were from Sigma.
Statistics
A paired t test (two-tail) was used where appropriate. The level of significance was set at P < 0.05. Data are presented as means ± standard error of mean.
Results
Figure 1A shows a representative fluorescence intensity response curve from an MD cell when the luminal [NaCl] was decreased from 35 to 10 mm. Figure 1B illustrates the changes in cell volume and in [Ca2+]i corrected for cell volume changes. The result shows a simultaneous cell volume decrease and [Ca2+]i increase. From the averaged data it was seen that when the luminal solution was changed from 35 to 10 mm[NaCl], the cell volume decreased initially by 10.4 ± 0.8% and subsequently increased by 2.4 ± 0.9%; this increase is called a regulatory volume increase (RVI). [Ca2+]i was increased by 9.5 ± 2.2% (n = 27) (P < 0.05), and this increase was inhibited when Ca2+-free solution was used in the bath and lumen (0.6 ± 1.1%, n = 12, P > 0.05) compared with that in 1.3 mm CaCl2 solutions. When the solution was changed from 35 to 135 mm[NaCl], the cell volume increased by 15.1 ± 0.6% (P < 0.05) and then showed an RVD of 3.4 ± 1.6%. No significant changes in [Ca2+]i accompanied these change in cell volume (0.8 ± 1.2%, n = 27, P > 0.05). When the luminal [NaCl] was changed, the cell volume changes did not differ significantly in bath and lumen Ca2+-free solutions (n = 9) from those in solutions with 1.3 mm CaCl2 (Fig. 2).
Figure 1. Simultaneously recorded fluorescence intensities of calcein and Fura Red of macula densa (MD) cells.
Luminal NaCl concentration was changed from 35 to 10 mm. A, the intensity of calcein increased and the intensity of Fura Red decreased. B, the calculated cell volume decreased and cytosolic Ca2+ concentration increased.
Figure 2. Simultaneous changes in cell volume and cytosolic Ca2+ concentration.
'35–10' indicates that the luminal [NaCl] was changed from 35 to 10 mm, and the other numbers indicate analogous changes. When the luminal [NaCl] was decreased, the cell volume decreased and the [Ca2+]i increased, both significantly. When the luminal [NaCl] was increased, the volume of the MD cells increased significantly, but there was no significant change in [Ca2+]i. There was no significant difference in changes in cell volume between experiments with normal Ca2+ solution and those with Ca2+-free solution (Ca2+ free). In Ca2+-free solution, the increase in [Ca2+]i that occurred when [NaCl] was changed from 35 to 10 mm was inhibited.
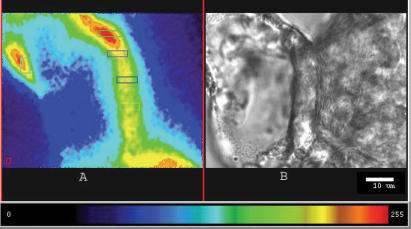
In Indo-1-loaded samples, the basal [Ca2+]i in the MD cells was 107.8 ± 12.5 nm. When the luminal solution was changed from 135 to 10 mm[NaCl], the [Ca2+]i in the MD increased by 23.5 ± 2.2 nm (n = 5) (Fig. 3).
Figure 3. Indo-1-loaded MD cells.
A, the MD cells are loaded with Indo-1. B, laser-transmitted light image.
In fura-2-loaded experiments, the basal [Ca2+]i in the MD cells was 115.3 ± 9.2 nm. When the luminal solution was changed from 135 or 35 mm NaCl to 10 mm NaCl, [Ca2+]i increased by 10.6 ± 3.9 (n = 24) or 30.1 ± 2.4 nm (n = 87), respectively (P < 0.05). When the solution was changed from 10 or 35 mm NaCl to 135 mm NaCl, there were no significant changes in the [Ca2+]i (1.3 ± 3.5 (n = 67) or 0.4 ± 2.6 nm (n = 24), respectively; P > 0.05). In Ca2+-free solution, the [Ca2+]i changes caused by alterations of luminal [NaCl] were inhibited, 1.2 ± 1.7 nm when [NaCl] was changed from 35 to 10 mm (n = 8), and 0.9 ± 1.4 nm when it was changed from 135 to 10 mm (n = 15) (P > 0.05) (Fig. 4).
Figure 4. Changes in cytosolic Ca2+ concentration caused by alterations of luminal NaCl concentration.
When luminal [NaCl] was decreased, [Ca2+]i in the MD cells increased significantly, and this Ca2+ increase was inhibited in Ca2+-free solution. There were no significant changes in [Ca2+]i when luminal [NaCl] was increased.
As shown in Fig. 5, there was a sigmoid increase in [Ca2+]i in the MD cells in response to a stepwise decrease in luminal [NaCl] from 135 to 70 mm, from 70 to 35 mm, from 35 to 10 mm, and from 10 to 0 mm. The steepest part of the curve occurred between 70 and 10 mm[NaCl].
Figure 5. Cytosolic Ca2+ concentration in response to a stepwise decrease in luminal NaCl concentration.
When luminal [NaCl] was decreased stepwise from 135 to 0 mm, there was a sigmoid increase in [Ca2+]i. The steepest part of the curve occurred between 70 and 10 mm.
From the second series of experiments, the influence of osmolarity was tested by changing the perfusate osmolarity from 800 to 120 mosmol l−1 while keeping NaCl constant at 40 mm. As can be seen in Fig. 6 a reduced osmolarity increased MD cell volume (n = 7) by about 100%, while at the same time MD cell Ca2+ concentration increased from 100 to about 250 nm (n = 5).
Figure 6. MD cell changes in volume and Ca2+ on reduction in osmolarity.
The effect of reduction in osmolarity from 800 to 120 mosmol l−1 at a constant NaCl concentration of 40 mm. A, MD cell volume as a percentage. B, MD cell Ca2+ concentration in nm.
Discussion
Many cell types have developed a complex system of cell volume regulatory mechanisms. Upon exposure to hypotonic or hypertonic conditions, most cells initially behave as osmometers, and thereafter readjust their volume toward the initial value, thus producing a RVD or RVI (Lang et al. 1998; Tinel et al. 2000; Haas & Forbush, 2000). Confocal microscopy has the advantage of having much better spatial and temporal resolutions than conventional microscopy. We found that MD cells are also capable of volume regulation. Similar RVD and RVI responses were observed in this study when the luminal [NaCl] was changed (see Fig. 2). When the luminal [NaCl] was increased, the MD cell swelled and this was followed by RVD. Conversely, when the luminal [NaCl] decreased, the MD cell shrank and RVI occurred. MD cells are exposed to high osmotic stress, which is mainly due to high Na+ and Cl− reabsorption and low water permeability in the apical sides of these cells. It has been found that Na+–K+–2Cl− cotransporter NKCC2 isoform is expressed in these sides (Russell, 2000; Okada et al. 2001). From the second series of experiments, the effect of a large osmotic change on the MD cell volume with constant NaCl (40 mm) was documented. At a reduction in osmolarity from 800 to 120 mosmol l−1, MD cell volume increased by 100%.
Ca2+ plays an important role in cell volume regulation, and it might be a second messenger in MD cell signal transmission. In the present study, the cell volume changes and regulatory volume changes in MD cells in Ca2+-free solution were not significantly different from those in 1.3 mm CaCl2 solution, indicating that cell volume regulation was not dependent on the extracellular Ca2+ concentration and [Ca2+]i. In Ca2+-free solution, the [Ca2+]i increase caused by changes in luminal [NaCl] was inhibited, which means that this Ca2+ increase takes place through the membrane Ca2+ channels and not through the intracellular Ca2+ stores (McCoy et al. 1999; Watanabe & Endoh, 1999; Doi et al. 2000; Mignen & Shuttleworth, 2000). Our results showed that a decrease in luminal [NaCl] led to a significant increase in [Ca2+]i in MD cells. This is in agreement with an earlier report (Salomonsson et al. 1991), but in disagreement with another (Peti-Peterdi & Bell, 1999). The reasons for these differences are not clear, but may depend on the use of a photometer (Peti-Peterdi & Bell, 1999) which does not allow effective control of movement during the experiments. From the present experiments, it is also clear that a large change in osmolarity of the tubular perfusate from 800 to 120 mosmol l−1 releases a large Ca2+ change, thus a decrease in osmolarity could increase the Ca2+ concentration of MD cells.
In several types of cells, osmotic swelling leads to [Ca2+]i elevation, whereas omission of Ca2+ from the extracellular medium or buffering of [Ca2+]i by membrane-permeable Ca2+ chelators partially or completely inhibits RVD (Davis & Finn, 1987; McCarty & O'Neil, 1990; Montrose-Rafizadeh & Guggino, 1991). However, in lymphocytes, [Ca2+]i is not related to the rate of RVD (Grinstein et al. 1982; Grinstein & Smith, 1990). Electrophysiological experiments with [Ca2+]i buffers have shown that the [Ca2+]i elevation is not required for activation of volume-sensitive K+ and anion channels (Szucs et al. 1996). Hyposmotic or hyperosmotic solution has been used in most of the published research concerning volume regulation (Lang et al. 1998; Tinel et al. 2000; Haas & Forbush, 2000). In the present study in series 1, the osmolarity of all solutions was kept constant. The changes in MD cell volume were only caused by changes in luminal [NaCl]. Thus cell volume regulation caused by changes in the NaCl concentration might differ from that due to changes in the initial osmotic concentration. When the luminal [NaCl] is increased, the NKCC2 could be activated and more reabsorbed Na+ and Cl− could enter the MD cell, and could draw more water from the basolateral side into the cells and cause it to swell (Okada et al. 2001; Russell, 2000). Such an event could be quite different from the case of changes in the osmolality of the solution, which would firstly draw water to or exclude water from the cells and then stimulate the active transfer mechanism. (Lange, 2000; Russell, 2000; Tinel et al. 2000; Okada et al. 2001). In series 2 experiments we observed that a decrease in osmolarity gave rise to a large increase in cell volume and an increase in Ca2+ concentration. With this we can see that the initial step of decreasing the luminal [NaCl] in series 1 has similarities to the process of RVD in the other experiments using hypotonic solutions from series 2. We believe that the [Ca2+]i elevation is not a result of the cell volume changes, but might be dependent on the activation of ion transporters and/or cotransporters or some other mechanism.
However, the use of Fura Red, which is a single wavelength excitation dye, precluded absolute measurements of [Ca2+]i (Thomas et al. 2000). Additional studies were therefore performed with use of the ratiometric dyes, Indo-1 and fura-2 with UV confocal microscopy and a conventional imaging system. Using Indo-1 and fura-2, baseline and dynamic changes in [Ca2+]i can be estimated. The basal [Ca2+]i in MD cells was 107.8 ± 12.5 nm when measured with Indo-1 and 115.3 ± 9.2 nm when measured with fura-2. These values are similar to previous reports (Salomonsson et al. 1991; Peti-Peterdi & Bell, 1999). Both in Indo-1-loaded samples using UV confocal microscopy, and in fura-2-loaded samples using the video image system, constant [Ca2+]i elevation caused by a decrease in luminal [NaCl] was observed in the present study. With a stepwise decrease in luminal [NaCl], there was a sigmoid increase in [Ca2+]i in MD cells. The steepest part of the curve was seen between 70 and 10 mm, which is the most sensitive range for changes in the transforming growth factor (TGF) response (Schnermann & Briggs, 1985). It is important to point out that in the ‘in vivo’ situation when decreasing distal delivery of fluid there is a decrease in NaCl concentration as well as a small decrease in fluid osmolarity. Both of these events would be expected to increase macula densa cell Ca2+ concentration. However, the in vivo situation is complicated, and it is not fully known what happens as a consequence of the changes in not only NaCl, but also in other electrolytes and nonelectrolyte substances, and their influence on MD cell volume and Ca2+. More studies are needed to investigate these questions further.
It is well known that a reduction of NaCl at the MD site leads to release of renin. To speculate, we would suggest that an increased MD cell Ca2+ can activate phospholipase A2 to release arachidonic acid, the rate-limiting step in the formation of prostaglandins (Persson et al. 2004). Recent evidence suggests that the prostaglandin produced is PGE2, a potent stimulator of renin release (Peti-Peterdi et al. 2003). Whether or not a decrease in osmolarity of the perfusate leads to an increased renin release is to our knowledge not yet studied at a constant NaCl concentration. From the present data we would suggest it does occur.
In conclusion, we have found that MD cells can regulate their volume, and that the [Ca2+]i elevation caused by a decrease in luminal [NaCl] and osmolarity is independent of the changes in cell volume. Changes in cell volume and in [Ca2+]i might be factors involved in the transmission of signals between MD and its target cells, and may be important for the release of renin. Confocal microscopy and simultaneous measurement of cell volume and [Ca2+]i have made the present study possible.
Acknowledgments
This study was financially supported by the Swedish Medical Research Council (project number K 2003-04X-03522-32 A), The Swedish Heart and Lung Foundation, the Wallenberg Foundation, and the Ingabritt and Arne Lundberg Foundation.
References
- Bassani JW, Bassani RA, Bers DM. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J. 1995;68:1453–1460. doi: 10.1016/S0006-3495(95)80318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs JP, Schnermann JB. Whys and wherefores of juxtaglomerular apparatus function. Kidney Int. 1996;49:1724–1726. doi: 10.1038/ki.1996.255. [DOI] [PubMed] [Google Scholar]
- Briggs JP, Schubert G, Schnermann J. Quantitative characterization of the tubuloglomerular feedback response: effect of growth. Am J Physiol Renal Physiol. 1984;247:F808–815. doi: 10.1152/ajprenal.1984.247.5.F808. [DOI] [PubMed] [Google Scholar]
- Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fedholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regnl Integr Comp Physiol. 2001;281:R1362–1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- Davis CW, Finn AL. Interactions of sodium transport, cell volume, and calcium in frog urinary bladder. J Gen Physiol. 1987;89:687–702. doi: 10.1085/jgp.89.5.687. 10.1085/jgp.89.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi S, Damron DS, Horibe M, Murray PA. Capacitative Ca2+ entry and tyrosine kinase activation in canine pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L118–130. doi: 10.1152/ajplung.2000.278.1.L118. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Salomonsson M, Muller-Suur C, Persson AE. Measurements of macula densa cell volume changes in isolated and perfused rabbit cortical thick ascending limb. II. Apical and basolateral cell osmotic water permeabilities. Acta Physiol Scand. 1988;133:159–166. doi: 10.1111/j.1748-1716.1988.tb08395.x. [DOI] [PubMed] [Google Scholar]
- Grinstein S, Dupre A, Rothstein A. Volume regulation by human lymphocytes. Role of calcium. J Gen Physiol. 1982;79:849–868. doi: 10.1085/jgp.79.5.849. 10.1085/jgp.79.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S, Smith JD. Calcium-independent cell volume regulation in human lymphocytes. Inhibition by charybdotoxin. J Gen Physiol. 1990;95:97–120. doi: 10.1085/jgp.95.1.97. 10.1085/jgp.95.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Haas M, Forbush B., III The Na+–K+–Cl− cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- Kirk KL, Bell PD, Barfuss DW, Ribadeneira M. Direct visualization of the isolated and perfused macula densa. Am J Physiol Renal Physiol. 1985;248:F890–894. doi: 10.1152/ajprenal.1985.248.6.F890. [DOI] [PubMed] [Google Scholar]
- Kurtz A, Gotz KH, Hamann M, Wagner C. Stimulation of renin secretion by nitric oxide is mediated by phosphodiesterase 3. Proc Natl Acad Sci U S A. 1998;95:4743–4747. doi: 10.1073/pnas.95.8.4743. 10.1073/pnas.95.8.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F, Busch GL, Volkl H. The diversity of volume regulatory mechanisms. Cell Physiol Biochem. 1998;8:1–45. doi: 10.1159/000016269. 10.1159/000016269. [DOI] [PubMed] [Google Scholar]
- Lange K. Regulation of cell volume via microvillar ion channels. J Cell Physiol. 2000;185:21–35. doi: 10.1002/1097-4652(200010)185:1<21::AID-JCP2>3.0.CO;2-D. 10.1002/1097-4652(200010)185:1<21::AID-JCP2>3.3.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Liu R, Bell PD, Peti-Peterdi J, Kovacs G, Johansson A, et al. Purinergic receptor signaling at the basolateral membrane of macula densa cells. J Am Soc Nephrol. 2002a;13:1145–1151. doi: 10.1097/01.asn.0000014827.71910.39. 10.1097/01.ASN.0000014827.71910.39. [DOI] [PubMed] [Google Scholar]
- Liu R, Pittner J, Persson AE. Changes of cell volume and nitric oxide concentration in macula densa by changes in luminal NaCl concentration. J Am Soc Nephol. 2002b;13:2688–2696. doi: 10.1097/01.asn.0000033275.17169.67. 10.1097/01.ASN.0000033275.17169.67. [DOI] [PubMed] [Google Scholar]
- McCarty NA, O'Neil RG. Dihydropyridine-sensitive cell volume regulation in proximal tubule: the calcium window. Am J Physiol Renal Physiol. 1990;259:F950–960. doi: 10.1152/ajprenal.1990.259.6.F950. [DOI] [PubMed] [Google Scholar]
- McCoy DE, Taylor AL, Kudlow BA, Karlson K, Slattery MJ, et al. Nucleotides regulate NaCl transport in mIMCD-K2 cells via P2X and P2Y purinergic receptors. Am J Physiol Renal Physiol. 1999;277:F552–559. doi: 10.1152/ajprenal.1999.277.4.F552. [DOI] [PubMed] [Google Scholar]
- Mignen O, Shuttleworth TJ. I (ARC), a novel arachidonate-regulated, noncapacitative Ca2+ entry channel. J Biol Chem. 2000;275:9114–9119. doi: 10.1074/jbc.275.13.9114. 10.1074/jbc.275.13.9114. [DOI] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C, Guggino WB. Role of intracellular calcium in volume regulation by rabbit medullary thick ascending limb cells. Am J Physiol Renal Physiol. 1991;260:F402–409. doi: 10.1152/ajprenal.1991.260.3.F402. [DOI] [PubMed] [Google Scholar]
- Obermuller N, Kunchaparty S, Ellison DH, Bachmann S. Expression of the Na+–K+–2Cl− cotransporter by macula densa and thick ascending limb cells of rat and rabbit nephron. J Clin Invest. 1996;98:635–640. doi: 10.1172/JCI118834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, et al. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) J Physiol. 2001;532:3–16. doi: 10.1111/j.1469-7793.2001.0003g.x. 10.1111/j.1469-7793.2001.0003g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson A, Ollerstam A, Liu R, Brown R. Mechanisms for macula densa cell release of renin. Acta Physiol Scand Physiol. 2004;181:471–474. doi: 10.1111/j.1365-201X.2004.01320.x. 10.1111/j.1365-201X.2004.01320.x. [DOI] [PubMed] [Google Scholar]
- Peti-Peterdi J, Bell PD. Cytosolic [Ca2+] signaling pathway in macula densa cells. Am J Physiol Renal Physiol. 1999;277:F472–476. doi: 10.1152/ajprenal.1999.277.3.F472. [DOI] [PubMed] [Google Scholar]
- Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, et al. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Inv. 2003;112:76–82. doi: 10.1172/JCI18018. 10.1172/JCI200318018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JM. Sodium–potassium–chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Salomonsson M, Gonzalez E, Westerlund P, Persson AE. Intracellular cytosolic free calcium concentration in the macula densa and in ascending limb cells at different luminal concentrations of sodium chloride and with added furosemide. Acta Physiol Scand. 1991;142:283–290. doi: 10.1111/j.1748-1716.1991.tb09158.x. [DOI] [PubMed] [Google Scholar]
- Schlatter E, Salomonsson M, Persson AE, Greger R. Macula densa cells sense luminal NaCl concentration via furosemide sensitive Na+–2Cl–K+ cotransport. Pflugers Arch. 1989;414:286–290. doi: 10.1007/BF00584628. 10.1007/BF00584628. [DOI] [PubMed] [Google Scholar]
- Schnermann J, Briggs J. Function of the Juxtaglomerular Apparatus: Local Control of Glomerular Hemodynamics. New York: Raven; 1985. [Google Scholar]
- Skott O, Briggs JP. Direct demonstration of macula densa-mediated renin secretion. Science. 1987;237:1618–1620. doi: 10.1126/science.3306925. [DOI] [PubMed] [Google Scholar]
- Szucs G, Heinke S, Droogmans G, Nilius B. Activation of the volume-sensitive chloride current in vascular endothelial cells requires a permissive intracellular Ca2+ concentration. Pflugers Arch. 1996;431:467–469. doi: 10.1007/BF02207289. 10.1007/s004240050023. [DOI] [PubMed] [Google Scholar]
- Thomas D, Tovey SC, Collins TJ, Bootman MD, Berridge MJ, et al. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 2000;28:213–223. doi: 10.1054/ceca.2000.0152. 10.1054/ceca.2000.0152. [DOI] [PubMed] [Google Scholar]
- Tinel H, Kinne-Saffran E, Kinne RK. Calcium signalling during RVD of kidney cells. Cell Physiol Biochem. 2000;10:297–302. doi: 10.1159/000016375. 10.1159/000016375. [DOI] [PubMed] [Google Scholar]
- Vander AJ. Control of renin release. Physiol Rev. 1967;47:359–382. doi: 10.1152/physrev.1967.47.3.359. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Endoh M. Characterization of the endothelin-1-induced regulation of L-type Ca2+ current in rabbit ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:654–664. doi: 10.1007/s002109900130. 10.1007/s002109900130. [DOI] [PubMed] [Google Scholar]
- Wong SM, DeBell MC, Chase HS. Cell swelling increases intracellular free [Ca] in cultured toad bladder cells. Am J Physiol Renal Physiol. 1990;258:F292–296. doi: 10.1152/ajprenal.1990.258.2.F292. [DOI] [PubMed] [Google Scholar]
- Yamaguchi DT, Green J, Kleeman CR, Muallem S. Characterization of volume-sensitive, calcium-permeating pathways in the osteosarcoma cell line UMR-106–01. J Biol Chem. 1989;264:4383–4390. [PubMed] [Google Scholar]