Abstract
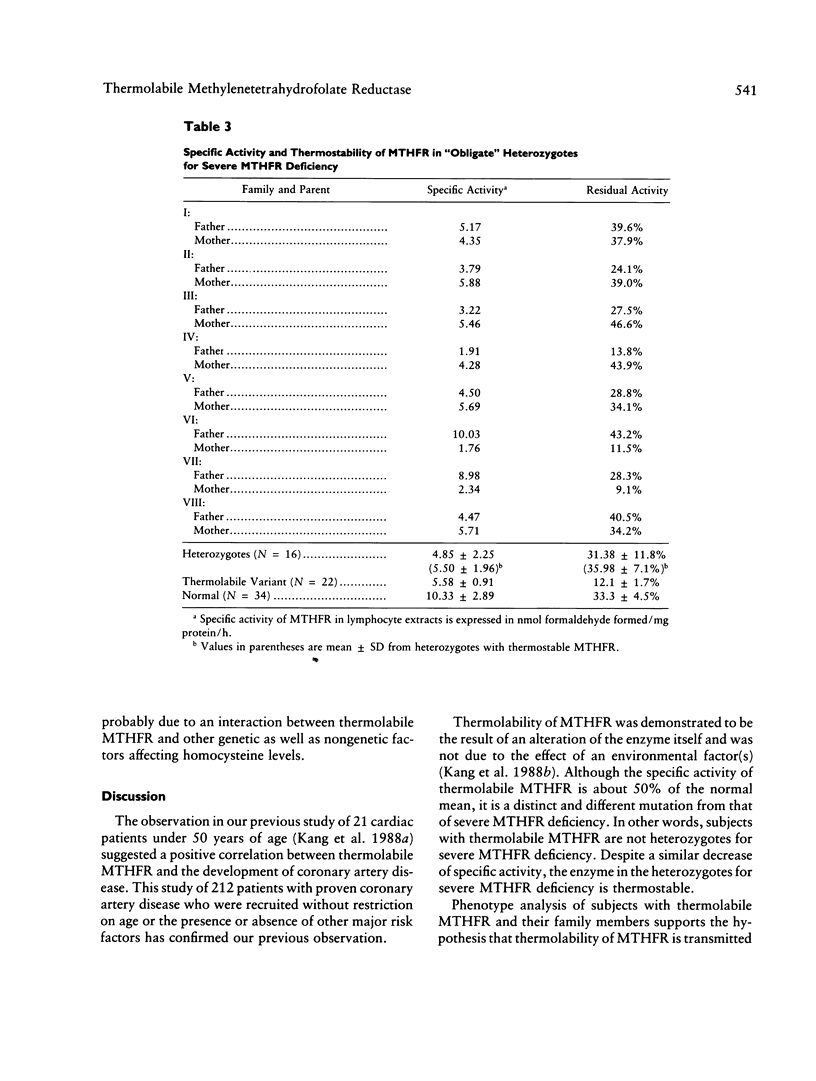
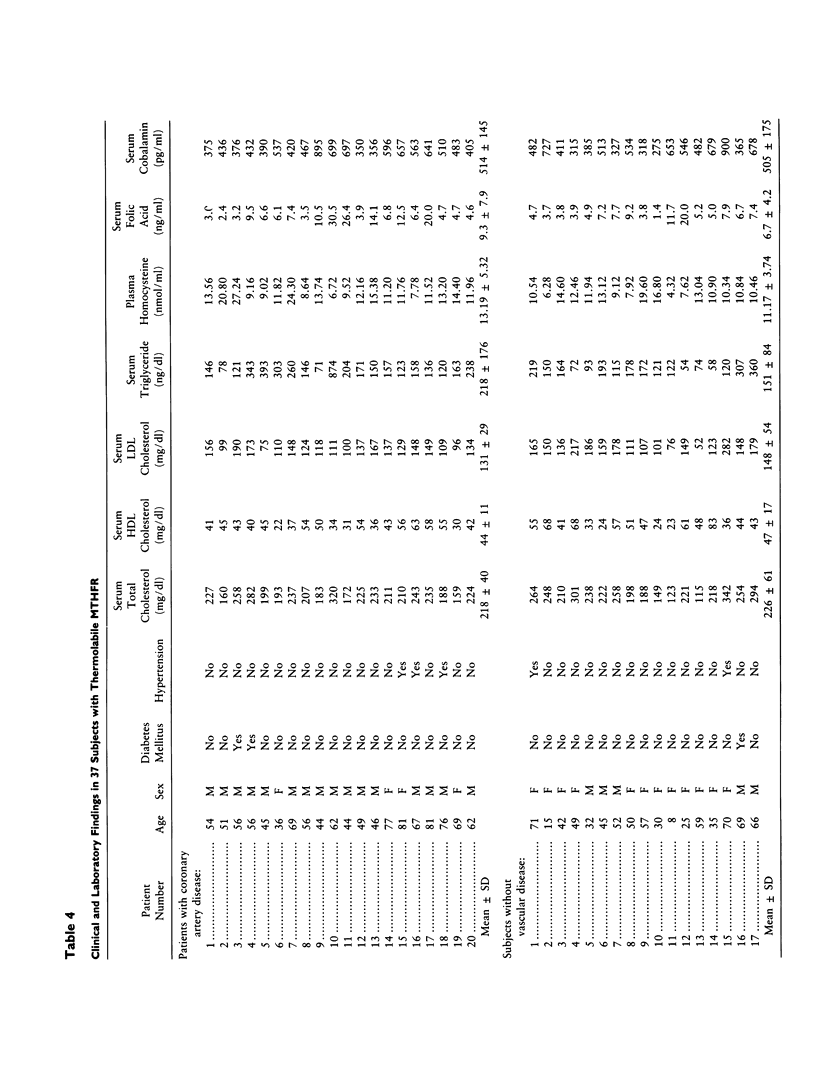
Severe methylenetetrahydrofolate reductase (MTHFR) deficiency with less than 2% of normal enzyme activity is characterized by neurological abnormalities, atherosclerotic changes, and thromboembolism. We have discovered a "new" variant of MTHFR deficiency which is characterized by the absence of neurological abnormalities, an enzyme activity of about 50% of the normal value, and distinctive thermolability under specific conditions of heat inactivation. In this study, lymphocyte MTHFR specific activities in the thermolabile variant and control groups were 5.58 +/- 0.91 and 10.33 +/- 2.89 nmol formaldehyde formed/mg protein/h, respectively. The difference was significant (P less than .01). However, there was overlap among the individual values from the two groups. On the other hand, residual MTHFR activity after heat inactivation was 11.2 +/- 1.43% in the thermolabile variant and 36.3 +/- 5.18% in the controls. There was no overlap. Enzyme studies in 10 subjects with thermolabile MTHFR and their family members support the hypothesis that thermolabile MTHFR is inherited as an autosomal recessive trait. To elucidate the association of thermolabile MTHFR with the development of coronary artery disease, we determined the thermostability of lymphocyte MTHFR in 212 patients with proven coronary artery disease and in 202 controls without clinical evidence of atherosclerotic vascular disease. Thermolabile MTHFR was found in 36 (17.0%) cardiac patients and 10 (5.0%) controls. The difference in incidence between the two groups was statistically significant (P less than .01). The average age at onset of clinical coronary artery disease in 36 patients with thermolabile MTHFR was 57.3 +/- 7.6 years (35-72 years). The mean total plasma homocysteine concentration in patients with thermolabile MTHFR was 13.19 +/- 5.32 nmol/ml and was significantly different from the normal mean of 8.50 +/- 2.80 nmol/ml (P less than .05). There was no association between thermolabile MTHFR and other major risk factors. We conclude that thermolabile MTHFR is a variant(s) of MTHFR deficiency which is inherited as an autosomal recessive trait. In addition, it is positively associated with the development of coronary artery disease. Determination of in vitro thermostability of lymphocyte MTHFR is a reliable method for identifying subjects with this abnormality.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner R., Wick H., Ohnacker H., Probst A., Maurer R. Vascular lesions in two patients with congenital homocystinuria due to different defects of remethylation. J Inherit Metab Dis. 1980;3(3):101–103. doi: 10.1007/BF02312541. [DOI] [PubMed] [Google Scholar]
- Boers G. H., Smals A. G., Trijbels F. J., Fowler B., Bakkeren J. A., Schoonderwaldt H. C., Kleijer W. J., Kloppenborg P. W. Heterozygosity for homocystinuria in premature peripheral and cerebral occlusive arterial disease. N Engl J Med. 1985 Sep 19;313(12):709–715. doi: 10.1056/NEJM198509193131201. [DOI] [PubMed] [Google Scholar]
- Brattstrom L. E., Hardebo J. E., Hultberg B. L. Moderate homocysteinemia--a possible risk factor for arteriosclerotic cerebrovascular disease. Stroke. 1984 Nov-Dec;15(6):1012–1016. doi: 10.1161/01.str.15.6.1012. [DOI] [PubMed] [Google Scholar]
- Castelli W. P., Garrison R. J., Wilson P. W., Abbott R. D., Kalousdian S., Kannel W. B. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986 Nov 28;256(20):2835–2838. [PubMed] [Google Scholar]
- Dahlen G. H., Guyton J. R., Attar M., Farmer J. A., Kautz J. A., Gotto A. M., Jr Association of levels of lipoprotein Lp(a), plasma lipids, and other lipoproteins with coronary artery disease documented by angiography. Circulation. 1986 Oct;74(4):758–765. doi: 10.1161/01.cir.74.4.758. [DOI] [PubMed] [Google Scholar]
- GIBSON J. B., CARSON N. A., NEILL D. W. PATHOLOGICAL FINDINGS IN HOMOCYSTINURIA. J Clin Pathol. 1964 Jul;17:427–437. doi: 10.1136/jcp.17.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T., Garcia-Palmieri M. R., Kagan A., Kannel W. B., Schiffman J. Differences in coronary heart disease in Framingham, Honolulu and Puerto Rico. J Chronic Dis. 1974 Sep;27(7-8):329–344. doi: 10.1016/0021-9681(74)90013-7. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. S., Wong P. W., Bock H. G., Horwitz A., Grix A. Intermediate hyperhomocysteinemia resulting from compound heterozygosity of methylenetetrahydrofolate reductase mutations. Am J Hum Genet. 1991 Mar;48(3):546–551. [PMC free article] [PubMed] [Google Scholar]
- Kang S. S., Wong P. W., Cook H. Y., Norusis M., Messer J. V. Protein-bound homocyst(e)ine. A possible risk factor for coronary artery disease. J Clin Invest. 1986 May;77(5):1482–1486. doi: 10.1172/JCI112461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. S., Wong P. W., Norusis M. Homocysteinemia due to folate deficiency. Metabolism. 1987 May;36(5):458–462. doi: 10.1016/0026-0495(87)90043-6. [DOI] [PubMed] [Google Scholar]
- Kang S. S., Wong P. W., Zhou J. M., Sora J., Lessick M., Ruggie N., Grcevich G. Thermolabile methylenetetrahydrofolate reductase in patients with coronary artery disease. Metabolism. 1988 Jul;37(7):611–613. doi: 10.1016/0026-0495(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Kang S. S., Zhou J., Wong P. W., Kowalisyn J., Strokosch G. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. 1988 Oct;43(4):414–421. [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Manaligod J. R., Wong P. W. Morphologic studies in a patient with homocystinuria due to 5, 10-methylenetetrahydrofolate reductase deficiency. Pediatr Res. 1976 Jun;10(6):598–609. doi: 10.1203/00006450-197606000-00008. [DOI] [PubMed] [Google Scholar]
- Kostner G. M., Avogaro P., Cazzolato G., Marth E., Bittolo-Bon G., Qunici G. B. Lipoprotein Lp(a) and the risk for myocardial infarction. Atherosclerosis. 1981 Jan-Feb;38(1-2):51–61. doi: 10.1016/0021-9150(81)90103-9. [DOI] [PubMed] [Google Scholar]
- Kutzbach C., Stokstad E. L. Mammalian methylenetetrahydrofolate reductase. Partial purification, properties, and inhibition by S-adenosylmethionine. Biochim Biophys Acta. 1971 Dec 15;250(3):459–477. doi: 10.1016/0005-2744(71)90247-6. [DOI] [PubMed] [Google Scholar]
- Malinow M. R., Kang S. S., Taylor L. M., Wong P. W., Coull B., Inahara T., Mukerjee D., Sexton G., Upson B. Prevalence of hyperhomocyst(e)inemia in patients with peripheral arterial occlusive disease. Circulation. 1989 Jun;79(6):1180–1188. doi: 10.1161/01.cir.79.6.1180. [DOI] [PubMed] [Google Scholar]
- McCully K. S. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969 Jul;56(1):111–128. [PMC free article] [PubMed] [Google Scholar]
- McGill J. J., Mettler G., Rosenblatt D. S., Scriver C. R. Detection of heterozygotes for recessive alleles. Homocyst(e)inemia: paradigm of pitfalls in phenotypes. Am J Med Genet. 1990 May;36(1):45–52. doi: 10.1002/ajmg.1320360111. [DOI] [PubMed] [Google Scholar]
- Murai A., Miyahara T., Fujimoto N., Matsuda M., Kameyama M. Lp(a) lipoprotein as a risk factor for coronary heart disease and cerebral infarction. Atherosclerosis. 1986 Feb;59(2):199–204. doi: 10.1016/0021-9150(86)90048-1. [DOI] [PubMed] [Google Scholar]
- Murphy-Chutorian D. R., Wexman M. P., Grieco A. J., Heininger J. A., Glassman E., Gaull G. E., Ng S. K., Feit F., Wexman K., Fox A. C. Methionine intolerance: a possible risk factor for coronary artery disease. J Am Coll Cardiol. 1985 Oct;6(4):725–730. doi: 10.1016/s0735-1097(85)80473-3. [DOI] [PubMed] [Google Scholar]
- Rosenblatt D. S., Erbe R. W. Methylenetetrahydrofolate reductase in cultured human cells. II. Genetic and biochemical studies of methylenetetrahydrofolate reductase deficiency. Pediatr Res. 1977 Nov;11(11):1141–1143. doi: 10.1203/00006450-197711000-00005. [DOI] [PubMed] [Google Scholar]
- SMITH C. A. A test for segregation ratios in family data. Ann Hum Genet. 1956 May;20(4):257–265. doi: 10.1111/j.1469-1809.1955.tb01280.x. [DOI] [PubMed] [Google Scholar]
- Sartorio R., Carrozzo R., Corbo L., Andria G. Protein-bound plasma homocyst(e)ine and identification of heterozygotes for cystathionine-synthase deficiency. J Inherit Metab Dis. 1986;9(1):25–29. doi: 10.1007/BF01813897. [DOI] [PubMed] [Google Scholar]
- Stamler J., Wentworth D., Neaton J. D. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 1986 Nov 28;256(20):2823–2828. [PubMed] [Google Scholar]
- Wilcken D. E., Reddy S. G., Gupta V. J. Homocysteinemia, ischemic heart disease, and the carrier state for homocystinuria. Metabolism. 1983 Apr;32(4):363–370. doi: 10.1016/0026-0495(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Wilcken D. E., Wilcken B. The pathogenesis of coronary artery disease. A possible role for methionine metabolism. J Clin Invest. 1976 Apr;57(4):1079–1082. doi: 10.1172/JCI108350. [DOI] [PMC free article] [PubMed] [Google Scholar]


