Figure 3.
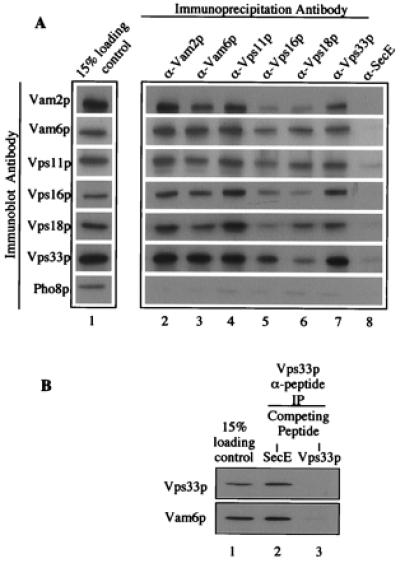
Coimmunoprecipitation of HOPS complex subunits. (A) Detergent-solubilized vacuolar supernatants (500 μl) were prepared from 200 μg of frozen BJ3505 vacuoles as described in Fig. 1 (lane 1). Each vacuole extract was mixed by nutation for 2 h at 4°C with 20 μl (packed volume) of protein A-Sepharose CL-4B beads (Amersham Pharmacia), which had been cross-linked to affinity-purified antibodies to Vam2p (lane 2), Vam6p (lane 3), Vps11p (lane 4), Vps16p (lane 5), Vps18p (lane 6), Vps33p (lane 7), or IgG antibodies to E. coli SecE (lane 8). [Before mixing, beads were mock-eluted with 200 μl of 1% (wt/vol) SDS at 37°C for 10 min followed by sedimentation (10,000 × g, 1 min, 4°C). Beads were washed three times in 500 μl of IP solubilization buffer and then preblocked by a 15-min nutation in 500 μl of IP solubilization buffer containing 2% (wt/vol) BSA (Fraction V; Sigma). Beads were sedimented (10,000 × g, 1 min, 4°C) and then suspended twice in 500 μl of IP solubilization buffer and sedimented.] Beads were collected by sedimentation (10,000 × g, 1 min, 4°C) and washed two times by resuspension in 1 ml of IP solubilization buffer and sedimentation. Bound proteins were eluted with 200 μl of 1% (wt/vol) SDS as above. After sedimentation (10,000 × g, 1 min, 4°C) to remove beads, 180 μl of the eluate was supplemented with 45 μl of 5× SDS/PAGE loading buffer (45). Proteins were analyzed by SDS/PAGE (10% acrylamide) and immunoblotting. (B) Immunoprecipitations were as described in A, except that the antibodies were to Vps33p peptides (see Materials and Methods). After incubating the beads in IP solubilization buffer plus 2% (wt/vol) BSA and before mixing with vacuole detergent extracts, the beads were mixed with a 3:1 molar excess of either SecE peptide (ATV AFA REA ATE VRK VIW PTR QET C; SecE, lane 2) or Vps33 peptides (Vps33p, lane 3) for 1 h at room temperature.
