Abstract
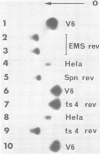
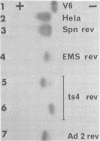
Hpt-13 is a Chinese hamster cell line deficient in hypoxanthine phosphoribosyltransferase (EC 2.4.2.8) and sensitive to a medium containing 10(-4) M hypoxanthine, 5.5 X 10(-6) M aminopterin, and 10(-4) M thymidine. In this cell line there is a high incidence of cells resistant to this selective medium after an incubation with either ethyl methane sulfonate or adenovirus type 2 complete virions or their incomplete particles. The rate of reversion in the presence of these agents was 34-fold higher with ethyl methane sulfonate and 2.5- to 5.6-fold higher with adenovirus particles than the spontaneous rate of reversion. The revertant phenotypes were stable for many generations without selective pressure. All of the revertants tested recovered the hypoxanthine phosphoribosyltransferase activity. Most of them, however, carried an enzyme of lower activity and faster electrophoretic mobility than that of the wild type. The preferential reversion to this type of enzyme was observed among spontaneous revertants as well as among those induced by mutagenesis with ethyl methane sulfonate or exposure to viral particles. Our results suggest that adenovirus particles and ethyl methane sulfonate induce mutations at the hpt locus of Hpt-13 cells through similar mechanisms.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellett A. J., Younghusband H. B. Spontaneous, mutagen-induced and adenovirus-induced anchorage independent tumorigenic variants of mouse cells. J Cell Physiol. 1979 Oct;101(1):33–47. doi: 10.1002/jcp.1041010106. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S., Defendi V. Increased mutation rates in doubly viral transformed Chinese hamster cells. Somatic Cell Genet. 1979 Nov;5(6):887–895. doi: 10.1007/BF01542648. [DOI] [PubMed] [Google Scholar]
- Khittoo G., Weber J. Genetic analysis of adenovirus type 2. VI. A temperature-sensitive mutant defective for DNA encapsidation. Virology. 1977 Aug;81(1):126–137. doi: 10.1016/0042-6822(77)90064-2. [DOI] [PubMed] [Google Scholar]
- Lassam N. J., Bayley S. T., Graham F. L. Tumor antigens of human Ad5 in transformed cells and in cells infected with transformation-defective host-range mutants. Cell. 1979 Nov;18(3):781–791. doi: 10.1016/0092-8674(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Legator M. S. Chemical mutagens. Annu Rev Med. 1972;23:413–428. doi: 10.1146/annurev.me.23.020172.002213. [DOI] [PubMed] [Google Scholar]
- Lehman J. M. Early chromosome changes in diploid Chinese hamster cells after infection with Simian virus 40. Int J Cancer. 1974 Feb 15;13(2):164–172. doi: 10.1002/ijc.2910130203. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. The use of drug-resistant markers to study the hybridization of mouse fibroblasts. Exp Cell Res. 1966 Jan;41(1):190–196. doi: 10.1016/0014-4827(66)90558-1. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey J. K., Green M., Wold W. S., Rigden P. Analysis of human cancer DNA for DNA sequences of human adenovirus type 4. J Natl Cancer Inst. 1979 Jan;62(1):23–26. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak M. I., Varshaver N. B., Shapiro N. I. Induction of gene mutations and chromosomal aberrations by simian virus 40 in cultured mammalian cells. Mutat Res. 1975 Dec;30(3):383–396. [PubMed] [Google Scholar]
- McCann J., Spingarn N. E., Kobori J., Ames B. N. Detection of carcinogens as mutagens: bacterial tester strains with R factor plasmids. Proc Natl Acad Sci U S A. 1975 Mar;72(3):979–983. doi: 10.1073/pnas.72.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M. A., Weber J. Uncoating of adenovirus type 2. J Virol. 1979 May;30(2):462–471. doi: 10.1128/jvi.30.2.462-471.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W. Virus-induced chromosome abnormalities. Annu Rev Microbiol. 1970;24:479–500. doi: 10.1146/annurev.mi.24.100170.002403. [DOI] [PubMed] [Google Scholar]
- Shaw E. I., Hsie A. W. Conditions necessary for quantifying ethyl methanesulfonate-induced mutations to purine-analogue resistance in Chinese hamster V79 cells. Mutat Res. 1978 Aug;51(2):237–254. doi: 10.1016/s0027-5107(78)80020-7. [DOI] [PubMed] [Google Scholar]
- Shaw M. W. Human chromosome damage by chemical agents. Annu Rev Med. 1970;21:409–432. doi: 10.1146/annurev.me.21.020170.002205. [DOI] [PubMed] [Google Scholar]
- Stich H. F. Oncogenic and nononcogenic mutants of adenovirus 12: induction of chromosome aberrations and cell divisions. Prog Exp Tumor Res. 1973;18:260–272. doi: 10.1159/000393170. [DOI] [PubMed] [Google Scholar]
- Stich H. F., Yohn D. S. Mutagenic capacity of adenoviruses for mammalian cells. Nature. 1967 Dec 30;216(5122):1292–1294. doi: 10.1038/2161292a0. [DOI] [PubMed] [Google Scholar]
- Strauss M., Theile M., Eckert R., Geissler E. Detection of antigenically active mutant HGPRT after mutagenesis with Simian virus 40. Mutat Res. 1978 Aug;51(2):297–300. doi: 10.1016/s0027-5107(78)80027-x. [DOI] [PubMed] [Google Scholar]
- Theile M., Scherneck S., Geissler E. Mutagenesis by simian virus 40. I. detection of mutations in Chinese hamster cell lines using different resistance markers. Mutat Res. 1976 Oct;37(1):111–123. doi: 10.1016/0027-5107(76)90059-2. [DOI] [PubMed] [Google Scholar]
- Thirion J. P., Banville D., Noel H. Galactokinase mutants of Chinese hamster somatic cells resistant to 2-deoxygalactose. Genetics. 1976 May;83(1):137–147. doi: 10.1093/genetics/83.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirion J. P., Talbot B. Alcohol dehydrogenase mutants of Chinese hamster somatic cells resistant to allyl alcohol. Genetics. 1978 Feb;88(2):343–356. doi: 10.1093/genetics/88.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshaver N. B., Marshak M. I., Luss E. V., Gorbunova L. V., Shapiro N. I. Spontaneous, chemical and viral mutagenesis in temperature-sensitive glutamine-requiring chinese hamster cells. Mutat Res. 1977 May;43(2):263–278. doi: 10.1016/0027-5107(77)90010-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J., Hassell J. A. Genetic analysis of adenovirus type 2. IX. The physical locations of structural genes. J Gen Virol. 1979 Sep;44(3):639–655. doi: 10.1099/0022-1317-44-3-639. [DOI] [PubMed] [Google Scholar]