Abstract
BACKGROUND—Intestinal barrier dysfunction concomitant with high levels of reactive oxygen metabolites (ROM) in the inflamed mucosa have been observed in inflammatory bowel disease (IBD). The cytoskeletal network has been suggested to be involved in the regulation of barrier function. Growth factors (epidermal growth factor (EGF) and transforming growth factor α (TGF-α)) protect gastrointestinal barrier integrity against a variety of noxious agents. However, the underlying mechanisms of oxidant induced disruption and growth factor mediated protection remain elusive. AIMS—To determine: (1) if oxidation and disassembly of actin (a key cytoskeletal component) plays a major role in ROM induced epithelial monolayer barrier dysfunction; and (2) if growth factor mediated protection involves prevention of theses alterations. METHODS—Caco-2 monolayers were preincubated with EGF, TGF-α, or vehicle before incubation with ROM (H2O2 or HOCl). Effects on cell integrity, barrier function, and G- and F-actin (oxidation, disassembly, and assembly) were determined. RESULTS—ROM dose dependently and significantly increased F- and G-actin oxidation (carbonylation), decreased the stable F-actin fraction (index of stability), and increased the monomeric G-actin fraction (index of disassembly). Concomitant with these changes were disruption of the actin cytoskeleton and loss of the monolayer barrier function. In contrast, growth factor pretreatment decreased actin oxidation and enhanced the stable F-actin, while in concert prevented actin disruption and restored normal barrier function of monolayers exposed to ROM. Cytochalasin-D, an inhibitor of actin assembly, not only caused actin disassembly and barrier dysfunction but also abolished the protective action of growth factors. Moreover, an actin stabilising agent, phalloidin, mimicked the protective actions of the growth factors. CONCLUSIONS—Oxidation, disassembly, and instability of the actin cytoskeleton appears to play a key role in the mechanism of oxidant induced loss of intestinal barrier integrity. In contrast, organisation and stabilisation of actin through promotion of its assembly plays a critical role in the mechanism of growth factor mediated protection. Keywords: western immunoblotting; F-actin; G-actin; Caco-2 cells; barrier function; growth factors; dinitrophenylhydrazine immunoreactivity
Full Text
The Full Text of this article is available as a PDF (229.3 KB).
Figure 1 .
Disruptive effects of oxidants on barrier integrity of Caco-2 monolayers, as determined by fluorescein sulphonic acid (FSA) clearance. Apical chambers were loaded with FSA before exposure to graded concentrations of H2O2 and HOCl in the range 0.01-5 mM. Barrier integrity was determined as apical to basolateral flux of probe divided by the concentration of FSA in the apical chamber, expressed as clearance. *p<0.05 compared with control (0 mM oxidant or isotonic saline) (n=9 per group). H2O2, hydrogen peroxide; HOCl, hypochlorous acid.
Figure 2 .

Protective effect of epidermal growth factor (EGF) and transforming growth factor α (TGF-α) preincubation on the barrier integrity of Caco-2 monolayers, assessed by fluorescein sulphonic acid (FSA) clearance. Monolayers were pretreated with growth factor 10 ng/ml before subsequent exposure to 0.5 or 5 mM of H2O2 or HOCl. Clearance was assessed as described in fig 1. *p<0.05 v control or growth factor pretreatment; †p<0.05 v 0.5 or 5 mM of H2O2 or HOCl alone (n=6 per group).
Figure 3 .

Blocking effects of epidermal growth factor receptor monoclonal antibody (anti-EGFR) on the protective action of growth factors on Caco-2 monolayer barrier function, as assessed by fluorescein sulphonic acid (FSA) clearance. Conditions as in fig 2 except that anti-EGFR 1 µg/ml was pre-loaded before the growth factors. Clearance was measured as described in fig 1.
Figure 4 .

(A) Effect of graded concentrations of H2O2 or HOCl and of pretreatment with epidermal growth factor (EGF) on the integrity of Caco-2 cells, assessed by the fluorescent probe calcein-AM and expressed as percentage of cells injured. Protective EGF 10 ng/ml was added to the monolayers 10 minutes before subsequent exposure to deleterious oxidant for 30 minutes. *p<0.05 v control (0 mM oxidant or isotonic saline) or EGF pretreatment; †p<0.05 v 0.1-5 mM H2O2 or HOCl alone (n=9 per group). (B) Effect of graded doses of H2O2 or HOCl on the cell integrity of Caco-2 monolayers determined by ethidium homodimer-1, expressed as percentage of cells dead.
Figure 5 .
Percentage of Caco-2 cells displaying normal actin cytoskeleton, determined by high resolution laser scanning confocal microscopy. Monolayers were exposed to damaging H2O2 or HOCl concentrations or pretreated with protective epidermal growth factor (EGF) before immediate exposure to injurious oxidant for 30 minutes. †p <0.05 v control (0 mM oxidant or isotonic saline) or EGF pretreatment; *p<0.05 v 0.1-5 mM oxidant alone (n=6 per group).
Figure 6 .

Fluorescent staining of actin cytoskeleton by fluorescein conjugated phalloidin in intestinal monolayers following incubation with isotonic saline (control) (A), 0.5 mM H2O2 (B), pretreated with epidermal growth factor (EGF) before exposure to 0.5 mM H2O2 (C), control (D), 0.5 mM HOCl (E), and those preincubated with EGF before exposure to 0.5 mM HOCl (F). Control monolayers demonstrate a normal, continuous, smooth distribution of actin (ring or cortex) at areas of cell-cell contact whereas oxidant exposed monolayers show areas of actin disruption, condensation, beading, and fragmentation. Actin in EGF pretreated cells resembles the morphology detected in the controls and appears normal. Bar, 25 µM.
Figure 7 .

Quantitative western immunoblotting analysis of polymerised F-actin (S2 fraction, index of stabilisation) and monomeric G-actin (S1 fraction, index of disruption) after treatment with epidermal growth factor (EGF), transforming growth factor α (TGF-α) 10 ng/ml, or vehicle before exposure to oxidants in Caco-2 cells. Percentage polymerisation of actin pool = ((S2)/total actin pool (S2+S1)). †p<0.05 v control or pretreatment with EGF or TGF-α.; *p<0.05 v H2O2 or HOCl alone (n=6 per group).
Figure 8 .
Western immunoblot micrograph of the F-actin (S2, triton insoluble) extracts following treatments. F-actin fractions were analysed by SDS-PAGE and western immunoblots using monoclonal antiactin as primary antibody followed by horseradish peroxidase conjugated-secondary antibody and subsequently processed for x ray film exposure. The lanes (corresponding optical densities) from left to right are: actin standard (10 951); 0.5 mM H2O2 (7452); 5 mM H2O2 (5611); isotonic saline (control, 10 734); EGF+5 mM H2O2 (9924); EGF alone (9865); TGF-α+5 mM H2O2 (9752); TGF-α alone (10 568).
Figure 9 .

Graphical representation of western immunoblotting of anti-dinitrophenylhydrazone (DNP) immunoreactivity showing oxidation (carbonylation) of the polymerised F-actin fraction in Caco-2 cells after various treatment regimens. Monolayers were pretreated with epidermal growth factor (EGF), transforming growth factor α (TGF-α), phalloidin, or vehicle before exposure to H2O2 or HOCl. Carbonyl formation (anti-DNP immunoreactivity) was expressed as the ratio of carbonyl formation (oxidation) in the treatment group divided by oxidised actin standard. †p<0.05 v control, EGF, TGF-α, or phalloidin pretreatment;*p<0.05 v H2O2 or HOCl alone (n=6 per group).
Figure 10 .

Disruptive effect of cytochalasin-D (CD) and protective action of phalloidin on the barrier integrity of Caco-2 monolayers, as determined by fluorescein sulphonic acid (FSA) clearance. Monolayers were preincubated with actin modulators, cytochalasin-D, or phalloidin, and then exposed to H2O2 or HOCl in the presence or absence of epidermal growth factor (EGF) or transforming growth factor α (TGF-α) pretreatment. Clearance was determined as described in fig 1. †p<0.05 v corresponding cytochalasin-D or oxidant alone; *p<0.05 v control (isotonic saline) or corresponding EGF (or TGF-α) + H2O2 (or HOCl), or corresponding phalloidin treatment (n=6 per group).
Figure 11 .
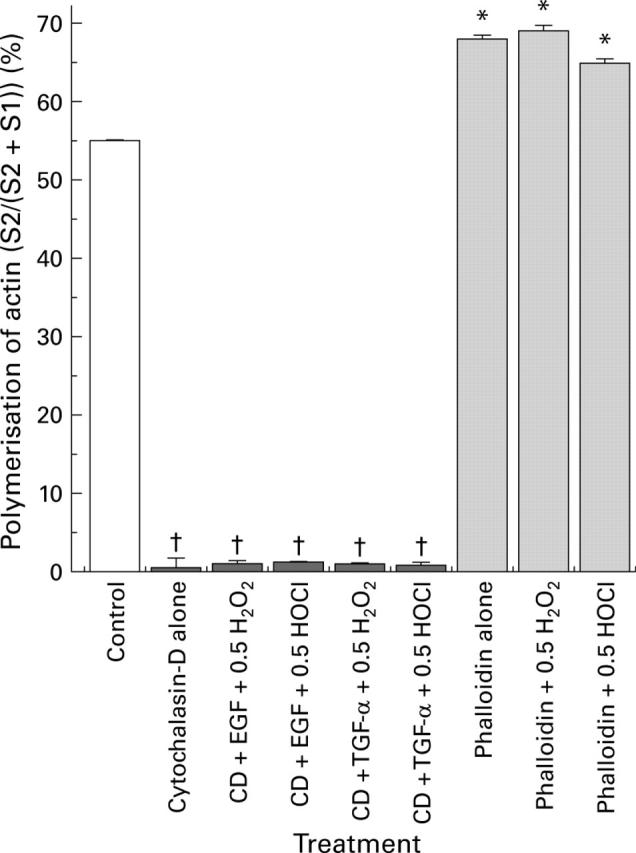
Cytochalasin-D (CD) induced actin depolymerisation and phalloidin induced actin assembly, as determined by western immunoblotting analysis of the distribution of polymerised F-actin (S2, index of stabilisation) and monomeric G-actin (S1, index of disruption) in Caco-2 cultures. Monolayers were pretreated with cytochalasin-D or phalloidin before incubation with or without epidermal growth factor (EGF) (or transforming growth factor α (TGF-α)) followed by exposure to H2O2 or HOCl. Percentage polymerisation of actin pool=((S2)/total actin pool (S2+S1)). †p<0.05 v control or phalloidin alone or pretreated groups; *p<0.05 v cytochalasin-D alone or pretreated groups (n=6 per group).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banan A., McCormack S. A., Johnson L. R. Polyamines are required for microtubule formation during gastric mucosal healing. Am J Physiol. 1998 May;274(5 Pt 1):G879–G885. doi: 10.1152/ajpgi.1998.274.5.G879. [DOI] [PubMed] [Google Scholar]
- Banan A., Smith G. S., Rieckenberg C. L., Kokoska E. R., Miller T. A. Protection against ethanol injury by prostaglandin in a human intestinal cell line: role of microtubules. Am J Physiol. 1998 Jan;274(1 Pt 1):G111–G121. doi: 10.1152/ajpgi.1998.274.1.G111. [DOI] [PubMed] [Google Scholar]
- Banan A., Wang J. Y., McCormack S. A., Johnson L. R. Relationship between polyamines, actin distribution, and gastric healing in rats. Am J Physiol. 1996 Nov;271(5 Pt 1):G893–G903. doi: 10.1152/ajpgi.1996.271.5.G893. [DOI] [PubMed] [Google Scholar]
- Blikstad I., Carlsson L. On the dynamics of the microfilament system in HeLa cells. J Cell Biol. 1982 Apr;93(1):122–128. doi: 10.1083/jcb.93.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode C., Kugler V., Bode J. C. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987 Feb;4(1):8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- Chang J. H., Gill S., Settleman J., Parsons S. J. c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J Cell Biol. 1995 Jul;130(2):355–368. doi: 10.1083/jcb.130.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner K. R., Anderson N. H., Rowlands B. J., Barbul A. Colitis and colonic mucosal barrier dysfunction. Gut. 1995 Oct;37(4):530–535. doi: 10.1136/gut.37.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T., Le Bivic A., Quaroni A., Rodriguez-Boulan E. Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. J Cell Biol. 1991 Apr;113(2):275–288. doi: 10.1083/jcb.113.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Fukuda T., Sugiyama M., Tarnawski A. Direct protective action of epidermal growth factor on isolated gastric mucosal surface epithelial cells. J Physiol Pharmacol. 1992 Dec;43(4):323–332. [PubMed] [Google Scholar]
- Keshavarzian A., Sedghi S., Kanofsky J., List T., Robinson C., Ibrahim C., Winship D. Excessive production of reactive oxygen metabolites by inflamed colon: analysis by chemiluminescence probe. Gastroenterology. 1992 Jul;103(1):177–185. doi: 10.1016/0016-5085(92)91111-g. [DOI] [PubMed] [Google Scholar]
- Kirsner J. B., Shorter R. G. Recent developments in "nonspecific" inflammatory bowel disease (first of two parts). N Engl J Med. 1982 Apr 1;306(13):775–785. doi: 10.1056/NEJM198204013061304. [DOI] [PubMed] [Google Scholar]
- Lawrence J. P., Brevetti L., Obiso R. J., Wilkins T. D., Kimura K., Soper R. Effects of epidermal growth factor and Clostridium difficile toxin B in a model of mucosal injury. J Pediatr Surg. 1997 Mar;32(3):430–433. doi: 10.1016/s0022-3468(97)90598-4. [DOI] [PubMed] [Google Scholar]
- Luck M. S., Bass P. Effect of epidermal growth factor on experimental colitis in the rat. J Pharmacol Exp Ther. 1993 Feb;264(2):984–990. [PubMed] [Google Scholar]
- Luna E. J., Hitt A. L. Cytoskeleton--plasma membrane interactions. Science. 1992 Nov 6;258(5084):955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- McKenzie S. J., Baker M. S., Buffinton G. D., Doe W. F. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996 Jul 1;98(1):136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. D., Mooseker M. S. An in vitro model for the analysis of intestinal brush border assembly. I. Ultrastructural analysis of cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci. 1993 Jun;105(Pt 2):445–460. doi: 10.1242/jcs.105.2.445. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Riegler M., Sedivy R., Sogukoglu T., Castagliuolo I., Pothoulakis C., Cosentini E., Bischof G., Hamilton G., Teleky B., Feil W. Epidermal growth factor attenuates Clostridium difficile toxin A- and B-induced damage of human colonic mucosa. Am J Physiol. 1997 Nov;273(5 Pt 1):G1014–G1022. doi: 10.1152/ajpgi.1997.273.5.G1014. [DOI] [PubMed] [Google Scholar]
- Riegler M., Sedivy R., Sogukoglu T., Cosentini E., Bischof G., Teleky B., Feil W., Schiessel R., Hamilton G., Wenzl E. Epidermal growth factor promotes rapid response to epithelial injury in rabbit duodenum in vitro. Gastroenterology. 1996 Jul;111(1):28–36. doi: 10.1053/gast.1996.v111.pm8698221. [DOI] [PubMed] [Google Scholar]
- Romano M., Polk W. H., Awad J. A., Arteaga C. L., Nanney L. B., Wargovich M. J., Kraus E. R., Boland C. R., Coffey R. J. Transforming growth factor alpha protection against drug-induced injury to the rat gastric mucosa in vivo. J Clin Invest. 1992 Dec;90(6):2409–2421. doi: 10.1172/JCI116132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno N., Menconi M. J., Smith M., Aguirre D. E., Fink M. P. Hyperpermeability of intestinal epithelial monolayers is induced by NO: effect of low extracellular pH. Am J Physiol. 1997 May;272(5 Pt 1):G923–G934. doi: 10.1152/ajpgi.1997.272.5.G923. [DOI] [PubMed] [Google Scholar]





