Abstract
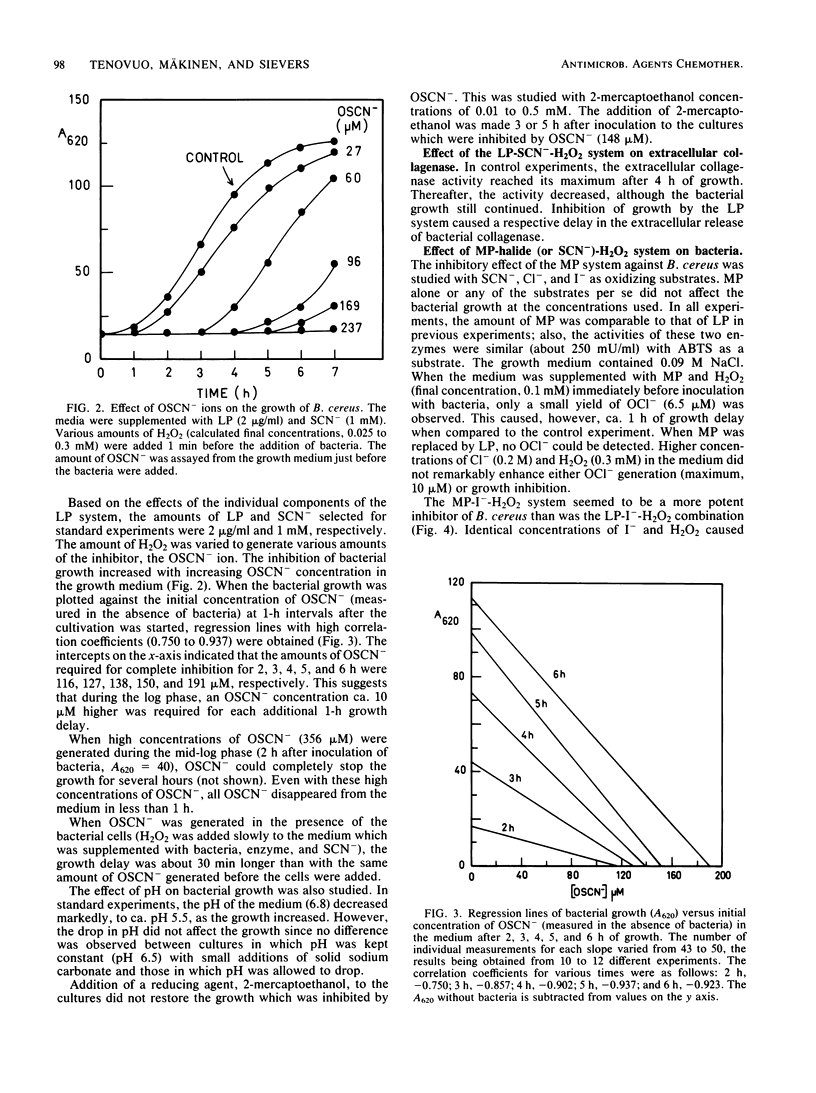
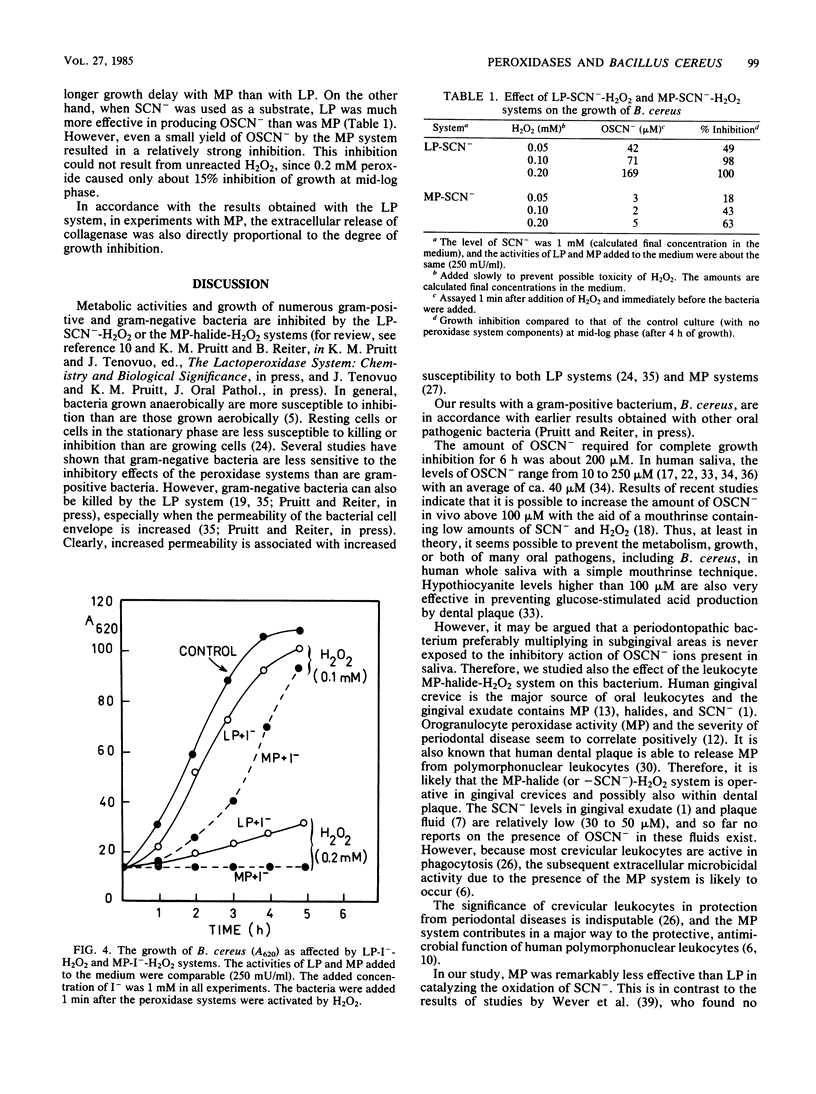
An oral periodontopathic bacterium, Bacillus cereus, was inhibited both by lactoperoxidase (LP) and myeloperoxidase (MP) antimicrobial systems. With the LP-SCN--H2O2 system, the growth inhibition was directly proportional to the amount of OSCN- ions present. The OSCN-, which is the principal oxidation product of the LP (or MP)-SCN--H2O2 system at neutral pH, is a normal component of human saliva. The oxidation products of both peroxidase systems inhibited the growth of the bacteria. This inhibition was associated with reduced extracellular release of collagenase activity from the cells. With LP, the antimicrobial efficiency of the oxidizable substrates was SCN- greater than I-, and with MP, the efficiency was I- greater than Cl- greater than SCN-, respectively. LP did not oxidize Cl-.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttonen T., Tenovuo J. Crevicular thiocyanate and iodide ions: cofactors of the antimicrobial peroxidase system in leucocytes. Proc Finn Dent Soc. 1981;77(6):318–323. [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L. Oxidation of protein sulfhydryls by products of peroxidase-catalyzed oxidation of thiocyanate ion. Biochemistry. 1978 Mar 21;17(6):1005–1010. doi: 10.1021/bi00599a010. [DOI] [PubMed] [Google Scholar]
- Bakkenist A. R., Wever R., Vulsma T., Plat H., van Gelder B. F. Isolation procedure and some properties of myeloperoxidase from human leucocytes. Biochim Biophys Acta. 1978 May 11;524(1):45–54. doi: 10.1016/0005-2744(78)90101-8. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Iwami Y., Yamada T. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect Immun. 1983 Apr;40(1):70–80. doi: 10.1128/iai.40.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. F., Hsu S. D., Baum B. J., Bowen W. H., Sierra L. I., Aquirre M., Gillespie G. Specific and nonspecific immune factors in dental plaque fluid and saliva from young and old populations. Infect Immun. 1981 Mar;31(3):998–1002. doi: 10.1128/iai.31.3.998-1002.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Tellefson L. M. Effect of human saliva on glucose uptake by Streptococcus mutans and other oral microorganisms. Infect Immun. 1981 Feb;31(2):598–607. doi: 10.1128/iai.31.2.598-607.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEBANOFF S. J., LUEBKE R. G. THE ANTILACTOBACILLUS SYSTEM OF SALIVA. ROLE OF SALIVARY PEROXIDASE. Proc Soc Exp Biol Med. 1965 Feb;118:483–486. doi: 10.3181/00379727-118-29882. [DOI] [PubMed] [Google Scholar]
- Kersten H. W., Moorer W. R., Wever R. Thiocyanate as a cofactor in myeloperoxidase activity against Streptococcus mutans. J Dent Res. 1981 Apr;60(4):831–837. doi: 10.1177/00220345810600041201. [DOI] [PubMed] [Google Scholar]
- Klinkhamer J. M., Mitchell M. D. Orogranulocyte peroxidase activity as a measure of inflammatory periodontal disease. J Dent Res. 1979 Jan;58(1):531–534. doi: 10.1177/00220345790580011501. [DOI] [PubMed] [Google Scholar]
- Kowolik M. J., Grant M. Myeloperoxidase activity in human gingival crevicular neutrophils. Arch Oral Biol. 1983;28(4):293–295. doi: 10.1016/0003-9969(83)90070-5. [DOI] [PubMed] [Google Scholar]
- Mandel I. D., Behrman J., Levy R., Weinstein D. The salivary lactoperoxidase system in caries-resistant and -susceptible adults. J Dent Res. 1983 Aug;62(8):922–925. doi: 10.1177/00220345830620081501. [DOI] [PubMed] [Google Scholar]
- Mansson-Rahemtulla B., Pruitt K. M., Tenovuo J., Le T. M. A mouthrinse which optimizes in vivo generation of hypothiocyanite. J Dent Res. 1983 Oct;62(10):1062–1066. doi: 10.1177/00220345830620101101. [DOI] [PubMed] [Google Scholar]
- Marshall V. M., Reiter B. Comparison of the antibacterial activity of the hypothiocyanite anion towards Streptococcus lactis and Escherichia coli. J Gen Microbiol. 1980 Oct;120(2):513–516. doi: 10.1099/00221287-120-2-513. [DOI] [PubMed] [Google Scholar]
- Morrison M., Schonbaum G. R. Peroxidase-catalyzed halogenation. Annu Rev Biochem. 1976;45:861–888. doi: 10.1146/annurev.bi.45.070176.004241. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K. Characteristics of the hydrolysis of 4-phenyl-azobenzyloxycarbonyl-l-prolyl-l-leucyl-glycyl-l-prolyl-d-arginine (a collagenase substrate) by enzyme preparations derived from carious dentine. Acta Odontol Scand. 1970 Aug;28(4):485–497. doi: 10.3109/00016357009028240. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K., Tenovuo J. Observations on the use of guaiacol and 2,2'-azino-di(3-ethylbenzthiazoline-6-sulfonic acid) as peroxidase substrates. Anal Biochem. 1982 Oct;126(1):100–108. doi: 10.1016/0003-2697(82)90114-2. [DOI] [PubMed] [Google Scholar]
- Pruitt K. M., Adamson M., Arnold R. Lactoperoxidase binding to streptococci. Infect Immun. 1979 Jul;25(1):304–309. doi: 10.1128/iai.25.1.304-309.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt K. M., Mansson-Rahemtulla B., Tenovuo J. Detection of the hypothiocyanite (OSCN-) ion in human parotid saliva and the effect of pH on OSCN- generation in the salivary peroxidase antimicrobial system. Arch Oral Biol. 1983;28(6):517–525. doi: 10.1016/0003-9969(83)90184-x. [DOI] [PubMed] [Google Scholar]
- Pruitt K. M., Tenovuo J., Andrews R. W., McKane T. Lactoperoxidase-catalyzed oxidation of thiocyanate: polarographic study of the oxidation products. Biochemistry. 1982 Feb 2;21(3):562–567. doi: 10.1021/bi00532a023. [DOI] [PubMed] [Google Scholar]
- Purdy M. A., Tenovuo J., Pruitt K. M., White W. E., Jr Effect of growth phase and cell envelope structure on susceptibility of Salmonella typhimurium to the lactoperoxidase-thiocyanate-hydrogen peroxide system. Infect Immun. 1983 Mar;39(3):1187–1195. doi: 10.1128/iai.39.3.1187-1195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revis G. J. Immunoelectrophoretic identification of peroxidase in human parotid saliva. Arch Oral Biol. 1977;22(3):155–158. doi: 10.1016/0003-9969(77)90147-9. [DOI] [PubMed] [Google Scholar]
- Scully C. Phagocytic and killing activity of human blood, gingival crevicular, and salivary polymorphonuclear leukocytes for oral streptococci. J Dent Res. 1982 May;61(5):636–639. doi: 10.1177/00220345820610050301. [DOI] [PubMed] [Google Scholar]
- Sips H. J., Hamers M. N. Mechanism of the bactericidal action of myeloperoxidase: increased permeability of the Escherichia coli cell envelope. Infect Immun. 1981 Jan;31(1):11–16. doi: 10.1128/iai.31.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowey R. R., Eidelman S., Klebanoff S. J. Antibacterial activity of the purified peroxidase from human parotid saliva. J Bacteriol. 1968 Sep;96(3):575–579. doi: 10.1128/jb.96.3.575-579.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderling E., Paunio K. U. Conditions of production and properties of the collagenolytic enzymes by two Bacillus strains from dental plaque. J Periodontal Res. 1981 Sep;16(5):513–523. [PubMed] [Google Scholar]
- Taichman N. S., Tsai C. C., Baehni P. C., Stoller N., McArthur W. P. Interaction of inflammatory cells and oral microorganisms. IV. In vitro release of lysosomal constituents from polymorphonuclear leukocytes exposed to supragingival and subgingival bacterial plaque. Infect Immun. 1977 Jun;16(3):1013–1023. doi: 10.1128/iai.16.3.1013-1023.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenovuo J. Formation of the bacterial inhibitor, hypothiocyanite ion, by cell-bound lactoperoxidase. Caries Res. 1979;13(3):137–143. doi: 10.1159/000260393. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Knuuttila M. L. Antibacterial effect of salivary peroxidases on a cariogenic strain of Streptococcus mutans. J Dent Res. 1977 Dec;56(12):1608–1613. doi: 10.1177/00220345770560123301. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Mansson-Rahemtulla B., Pruitt K. M., Arnold R. Inhibition of dental plaque acid production by the salivary lactoperoxidase antimicrobial system. Infect Immun. 1981 Oct;34(1):208–214. doi: 10.1128/iai.34.1.208-214.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenovuo J., Pruitt K. M., Thomas E. L. Peroxidase antimicrobial system of human saliva: hypothiocyanite levels in resting and stimulated saliva. J Dent Res. 1982 Aug;61(8):982–985. doi: 10.1177/00220345820610081301. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Susceptibility of Escherichia coli to bactericidal action of lactoperoxidase, peroxide, and iodide or thiocyanate. Antimicrob Agents Chemother. 1978 Feb;13(2):261–265. doi: 10.1128/aac.13.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Bates K. P., Jefferson M. M. Hypothiocyanite ion: detection of the antimicrobial agent in human saliva. J Dent Res. 1980 Sep;59(9):1466–1472. doi: 10.1177/00220345800590090201. [DOI] [PubMed] [Google Scholar]
- Thomas E. L. Lactoperoxidase-catalyzed oxidation of thiocyanate: equilibria between oxidized forms of thiocyanate. Biochemistry. 1981 May 26;20(11):3273–3280. doi: 10.1021/bi00514a045. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A., Smith K. W., Chwang A. K. Inhibition of Streptococcus mutans by the lactoperoxidase antimicrobial system. Infect Immun. 1983 Feb;39(2):767–778. doi: 10.1128/iai.39.2.767-778.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WUENSCH E., HEIDRICH H. G. ZUR QUANTITATIVEN BESTIMMUNG DER KOLLAGENASE. Hoppe Seylers Z Physiol Chem. 1963;333:149–151. doi: 10.1515/bchm2.1963.333.1.149. [DOI] [PubMed] [Google Scholar]
- Wever R., Kast W. M., Kasinoedin J. H., Boelens R. The peroxidation of thiocyanate catalysed by myeloperoxidase and lactoperoxidase. Biochim Biophys Acta. 1982 Dec 20;709(2):212–219. doi: 10.1016/0167-4838(82)90463-0. [DOI] [PubMed] [Google Scholar]


