Abstract
In the mouse, cross-presentation is an exclusive property of the CD8α+ subset of dendritic cells (DC) but the basis for this selectivity remains unclear. Here we report that splenic CD8α+ DC are much superior to other DC subsets in internalizing dying cells in vitro. In contrast, CD8α+, CD8α−CD4+ and CD8α−CD4− DC subsets phagocytose bacteria or latex beads to a similar extent. Although CD8α+ DC are better than CD4+ DC at presenting ovalbumin (OVA)-loaded splenocytes to naïve OT-I T lymphocytes, CD4+ DC are better at presenting OVA-expressing Escherichia coli to the same T cells. In both cases, presentation is abrogated by lactacystin. These results show that both splenic CD8α+ and CD8α− DC can present exogenous antigens on major histocompatibility complex (MHC) class I via a proteasome-dependent pathway and suggest that the specialized cross-presenting function of CD8α+ DC is a result of their ability to endocytose dying cells rather than a unique pathway for handling endosomal contents.
Introduction
Cross-presentation of cell-associated antigens is thought to play an important role in the maintenance of peripheral tolerance, as well as in immunity to some tumours and certain infections.1,2 Although cross-presentation has long been known to require a dedicated bone marrow-derived antigen-presenting cell (APC),3 the identity of the cross-presenting APC in vivo has remained elusive. Recent experiments in the mouse suggest that cross-presenting activity is restricted to a unique subset of CD11cbright CD11bdull dendritic cells (DC) that express CD8α, DEC-205 and CD36.4–6 This subset of DC has been shown to be able to internalize cells in vivo and re-present cellular antigens to both major histocompatibility complex (MHC) class I- and MHC class II-restricted T cells.4,7 Similarly, CD8α+ DC are superior to other DC subsets in their ability to cross-present splenocyte-associated antigens to MHC class I-restricted T cells in vitro.5
Exogenous antigens are generally excluded from the MHC class I presentation pathway. It has been argued that the unusual ability of CD8α+ DC to cross-present cell-associated antigens on MHC class I reflects their unique ability to shuttle endosomal contents into the cytosol.4,8 In contrast to this notion, here we show that the ability of CD8α+ DC to cross-present cell-associated antigens on MHC class I probably reflects their unique ability to phagocytose dying cells. Indeed, we show that CD8α+ DC excel in the internalization of a variety of cell types induced to undergo apoptosis, while CD8α− DC effectively lack the ability to take up dying cells. However, CD8α− DC are able to phagocytose latex beads or bacteria, and CD8α− CD4+ DC are more efficient than CD8α+ DC in presenting bacterially expressed antigens on MHC class I. Thus, cross-presentation of cell-associated antigens is restricted to CD8α+ DC, but both CD8α+ and CD8α− DC are able to present exogenous antigens on MHC class I provided these antigens have access to the endocytic compartment.
Materials and methods
Animals
Female C57BL/6 mice were purchased from Charles River (Margate, UK). BALB/c mice and OT-I mice,9 on a RAG-1−/− background (gift from Dr D. Kioussis, National Institute for Medical Research, Mill Hill, London, UK), were bred at the animal facility of Cancer Research UK (Clare Hall, South Mimms, UK) under specific pathogen-free conditions. All mice were used at 6–10 weeks of age.
Reagents
The ovalbumin (OVA) peptide 257–264 (SIINFEKL) was made by the Cancer Research UK peptide synthesis service. OVA protein and polyethylene glycol 1000 were obtained from CN Biosciences (UK) Ltd. (Nottingham, UK). Lactacystin was purchased from Sigma (Dorset, UK). OVA-E. coli and OVA/LLO-E. coli,10 two lines of recombinant Escherichia coli transformed with a plasmid encoding OVA, with or without a second plasmid encoding listeriolysin O (LLO), respectively, were a gift from G. Vassaux (Molecular Therapy Laboratory, Cancer Research UK, Hammersmith Hospital, UK), obtained with permission from Dr D. E. Higgins (Harvard Medical School, Boston, MA). Fluoresbrite™ Yellow Green (YG) carboxylate microspheres (0·5 µm) were from Polysciences Europe GMBH (Eppelheim, Germany).
Monoclonal antibodies (mAbs) used were as follows: HL3, a hamster immunoglobulin G (IgG) mAb against CD11c; 53-6.7, RM4-5 and RA3-6B2, rat IgG2a mAbs against CD8α, CD4 and B220, respectively; 2.4G2 and RB6-8C5, rat IgG2b mAbs against FcγR III/II and Gr-1, respectively. All mAbs were from PharMingen (Becton-Dickinson, Oxford, UK).
Cells
CD11cbright splenic DC and DC subsets were isolated as previously described.5 First, DC-enriched splenocytes were prepared by magnetic selection using anti-CD11c MACS™ beads (Miltenyi Biotec Ltd, Bisley, UK). CD11c-enriched cells (80–90% DC) were then stained with phycoerythrin (PE)-labelled anti-CD11c, fluorescein isothiocyanate (FITC)-labelled anti-CD8α, and Cychrome™-labelled anti-CD4 and sorted into subsets on a MoFlo cytometer (Cytomation, Fort Collins, CO). The purity of each subset was routinely > 95%.
RAW264.7 cells were obtained from the American Type Culture Collection (ATTC; Rockville, MD). OT-I T cells were isolated from lymph nodes of OT-I × RAG-1−/− mice and depleted of APC by negative selection using magnetic beads. Briefly, cells were stained with a cocktail of biotinylated mAbs including anti-FcγR, anti-CD4, anti-CD11c, anti-Gr-1, and anti-B220, washed and incubated with streptavidin-MACS™ beads (Miltenyi Biotec). Labelled cells were removed on a MACS™ depletion column and the flow-through fraction was collected.
In vitro cross-presentation assay
DC subsets (1–2 × 105 cells) and OT-I T cells (105 cells) were co-cultured either with irradiated MHC-mismatched splenocytes preloaded with OVA by osmotic shock, as described previously,5 or with OVA-expressing E. coli, which had been fixed by treatment with paraformaldehyde (PFA; 0·5%, 30 min, room temperature). Triplicate cultures were set up in 96-well flat-bottom culture plates in RPMI-1640 supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (100 µg/ml), glutamine (2 mm) and 2-mercaptoethanol (5 × 10−5m). Two days after culture initiation the cells were pulsed with [3H]thymidine ([3H]TdR) (1 µCi/well; Amersham International, Amersham, UK), harvested the following day onto glass-fibre filtermats (Wallac, Newbury, UK) and [3H]TdR incorporation was measured in a beta plate counter (Wallac).
Phagocytosis assays
CD11c-enriched cells (C57BL/6; 5–10 × 105 per well) were prestained with APC-conjugated anti-CD11c, FITC-conjugated anti-CD8α and cychrome™-conjugated anti-CD4 and subsequently cultured in 24-well plates in 1 ml of medium together with fluorescent test particles. Sodium azide (0·1%) was added to some cultures to prevent uptake and allow differentiation between cell-surface binding of particles and internalization. Cultures were harvested 3 hr later in phosphate-buffered saline (PBS) containing 5 mm EDTA, 1% FCS and 0·02% sodium azide [fluorescence-activated cell sorter (FACS) buffer], and the association between particles and CD11cbright cells was measured by using flow cytometry. Events were collected on a FACS Calibur cytometer (Becton-Dickinson, Mountain View, CA) and analysed using FlowJo software (Treestar, San Carlos, CA).
Test particles were YG latex beads (0·5 µm, 1 : 1000), bacteria (PFA-fixed E. coli, 5–10 × 107/well unless indicated otherwise) or eukaryotic cells. Both bacteria and eukaryotic cells were labelled with 3 µm of the lipophilic fluorescent dye PKH26 (Sigma) for 5 min at room temperature and washed prior to co-culture with DC. Eukaryotic cells included splenocytes that had been osmotically shocked and γ-irradiated (1350 rads) to mimic the conditions used in the cross-presentation assays or RAW264.7 cells exposed to ultraviolet (UV) irradiation (50 J/m2) to induce apoptosis (used at 5 × 106 cells per well unless indicated otherwise). In some experiments, UV-treated RAW cells were cultured for 3 hr and then stained with FITC–annexin V (Sigma) and propidium iodide and sorted into propidium iodide− annexin V+ and propidium iodide− annexin V− fractions. These fractions were fixed in 1% PFA (10 min, room temperature) and quenched in 0·1 m glycine (10 min, room temperature) to prevent further progression along the apoptotic pathway.
Video microscopy
CD8α+ DC were purified by cell sorting using Alexa488-streptavidin/biotin-anti-CD8α, TriColor-anti-CD4 and APC anti-CD11c and were incubated with PKH26-labelled, UV-irradiated RAW264.7 cells in glass-bottom microwell dishes (MatTek, Ashland, MA). Cells were allowed to settle for 30 min at 37° before imaging on a Zeiss Axiovert 135 TV microscope (Carl Zeiss Ltd, Welwyn Garden City, UK). Images were acquired every 15 seconds in three separate channels (phase contrast, Alexa488 fluorescence, and PKH26 fluorescence) using a 20 × NA 0·5 objective, an Orca ER CCD camera (Hammamatsu Photonics UK Ltd, Enfield, UK) and a multiple dichroic in conjunction with motorized excitation and emission filter wheels (Sutter Instruments, Novato, CA). Image acquisition was controlled using Acquisition Manager (Kinetic Imaging Ltd, Wirral, UK). The microscope body was housed in a Perspex environment chamber accurately maintained at 37° and the cells were enclosed within a glass-topped, steel-encased humidity chamber. Films were edited using Adobe ImageReady™ and Adobe Photoshop™ software (Adobe Systems UK, Uxbridge, UK).
Results
Selective uptake of apoptotic cells by CD8α+ DC
We and others have previously shown that cell-associated OVA protein can be cross-presented to CD8+ OT-I T cells by CD8α+ DC (Fig. 1a and refs 4–6). In contrast, cross-presentation of the same inoculum by CD4+ DC, the most abundant subtype among the CD8α− DC population,11 was practically undetectable although both CD8α+ and CD4+ DC had a similar capacity to present OVA peptide to OT-I (Fig. 1a). During osmotic shock and irradiation, many splenocytes are triggered to undergo apoptosis. To examine the basis of selective cross-presentation by CD8α+ DC, we studied the ability of DC to internalize dying cells. Various cell lines were treated with UV irradiation to induce apoptosis, labelled with PKH26 and co-cultured with splenic DC. As shown in Fig. 1(b) for a representative cell line, a large fraction of CD11cbright DC acquired PKH26 label following a short period of co-culture with labelled RAW264.7 cells. This was inhibitable by azide (Fig. 1b) and correlated with the extent of internalization of labelled cells, as determined by examination using microscopy (see below), indicating that it reflected true uptake of dying cells by DC. Similar results were obtained using osmotically shocked irradiated splenocytes as the phagocytic meal (data not shown). When RAW264.7 cells were UV treated and separated into annexin V+ and annexin V− fractions, the former was internalized to a twofold greater extent by DC (Fig. 1b).
Figure 1.
CD8α+ dendritic cells (DC) selectively capture apoptotic cells and cross-present cell-associated antigen on major histocompatibility complex (MHC) class I in vitro. (a) Sorted CD4+ (open bars) or CD8α+ (filled bars) DC subsets (C57BL/6) were co-cultured with OT-I T cells in the presence of irradiated, ovalbumin (OVA)-loaded allogeneic (BALB/c) splenocytes. Results represent the mean [3H]thymidine uptake of triplicate wells. All error bars are shown and represent 1 SD from the mean. c.p.m., counts /min. (b)–(d) Flow cytometry analysis of 3-hr cultures containing prestained, CD11c-enriched cells and PKH26-labelled RAW264.7 cells, as indicated. (b) Ultraviolet (UV)-treated RAW cells were added to DC cultures in the presence or absence of sodium azide (top) or were first cultured and then sorted into annexin V+ or annexin V− (AxV+, AxV−, respectively) fractions and fixed in paraformaldehyde (PFA), as described in the Materials and methods (bottom). Dot-plots represent CD8α expression versus PKH26 fluorescence for DC gated on the basis of high CD11c expression. Numbers show the percentage of cells within each quadrant gate. (c) Histograms showing PKH26 profile for CD8α+ (top), CD4+ (middle) and double negative (DN, bottom) DC subsets from cultures with (thick line) or without (thin line) PKH26-labelled UV-treated RAW cells. (d) Frequencies of PKH26+ DC subsets after incubation with increasing doses of UV-treated, PKH26-labelled RAW cells. Data are representative of (a) six experiments, (b) three experiments, or (c) and (d) two experiments.
Interestingly, in all uptake experiments, a much higher frequency of CD8α+ DC than CD8α− DC internalized PKH26-labelled cells (Fig. 1b). In addition, CD8α+ DC internalized a greater number of targets on a per cell basis, as reflected in a higher mean PKH26 fluorescence for the CD8α+ than the CD8α− DC fractions (Fig. 1b). When DC were further analysed with respect to CD4 expression, it was clear that CD4+ DC were unable to internalize dying cells and that CD4− CD8α− (double negative) DC were able to take up target cells but were inferior to CD8α+ DC in this respect (Fig. 1c). Careful quantification of uptake by all three DC subsets after incubation with different numbers of dying cells revealed that CD4+ DC did not take up target cells, even at the highest particle : DC ratio, whereas up to 60% of CD8α+ DC were able to do so under the same conditions (Fig. 1d). Double-negative DC had only a limited capacity to take up dead cells and were more similar to CD4+ DC than to the CD8α+ subset in this respect (Fig. 1d). The differences in uptake among all three DC subsets correlated exceedingly well with their ability to cross-present cell-associated OVA in vitro.5
Uptake of dying cells by CD8α+ DC was directly visualized by co-culturing purified spleen CD8α+ DC with PKH26-labelled irradiated RAW264.7 cells and recording their interactions by video microscopy. CD8α+ DC moved actively around the field of observation and formed intimate contacts with clusters of dying RAW cells (Fig. 2). Following these interactions, several DC could be seen to leave the clusters carrying fragments of labelled cells (Fig. 2). Some CD8α+ DC carrying labelled material entered other clusters of dying cells and appeared to internalize additional label. We conclude that CD8α+ DC and, to a much lesser extent, double-negative DC are unique among DC subsets in their ability to internalize dead or dying cells.
Figure 2.
Visualization of dead cell uptake by CD8α+ dendritic cells (DC). CD8α+ DC purified by cell sorting were co-cultured with ultraviolet (UV)-treated, PKH26-labelled RAW cells. Images were acquired at 15-second intervals for a period of 3 hr. The image is a composite of four stills taken from a film and is shown as a superimposed phase-contrast and PKH26 fluorescence image; DC appear as phase-dense, unlabelled, irregularly shaped cells, whereas the dying RAW cells appear as round cells labelled red. The DC in this composite is indicated by the white arrowhead in the first frame. Note that labelled material is associated with the DC as it moves away from the cluster of RAW cells. The time in seconds is indicated in each frame; the time on the first frame was arbitrarily set at 0. Excerpts from the films can be seen on the world wide web as supplementary material to the publication of this article on the web.
Both CD8α+ and CD4+ DC can phagocytose particulate antigens and present bacterial antigen on MHC class I
To determine if the superior ability of CD8α+ DC in cellular uptake reflected overall differences in phagocytic ability amongst DC subsets, the cells were compared for the ability to internalize other particles. Both CD8α+ and CD8α− DC subsets internalized latex beads and fixed E. coli bacteria (Fig. 3a), consistent with published data on the phagocytic ability of murine DC subsets.12,13 When CD8α− DC were analysed for CD4 expression, it was clear that CD4+ DC were able to phagocytose E. coli although, on a per cell basis, CD8α+ DC were approximately fivefold more efficient than CD4+ DC in bacterial uptake (Fig. 3b). Double-negative DC had an intermediate capacity to phagocytose E. coli compared to the other two subsets (Fig. 3b).
Figure 3.
CD4+ dendritic cells (DC) can phagocytose Escherichia coli and latex beads and present exogenous ovalbumin (OVA) on major histocompatibility complex (MHC) class I. (a) Flow cytometric analysis of 3-hr cultures of DC-enriched splenocytes and PKH26-labelled E. coli (left) or yellow green (YG) latex beads (right). Dot-plots represent gated CD11cbright cells. Numbers show the percentage of cells within each quadrant gate. (b) Quantification of E. coli uptake by double negative (DN), CD8α+ and CD4+ DC subsets. (c) Sorted CD4+ DC (open bars) and CD8α+ DC (filled bars) were cultured with OT-I T cells in the presence of two doses of OVA-E. coli or OVA/LLO-E.coli, as indicated. c.p.m., counts /min. (d) Sorted CD4+ (circles) and CD8α+ (triangles) DC subsets (3 × 104/well) were cultured with OT-I T cells and different doses of OVA-peptide in the absence (open symbols) or presence (filled symbols) of paraformaldehyde (PFA)-treated E. coli (106/well). Results in (c) and (d) represent the mean [3H]thymidine uptake of triplicate wells. All error bars are shown and represent 1 SD from the mean. Results are representative of (a) and (b) three experiments, (c) two experiments, or (d) one experiment, with a full peptide dose range and an additional experiment using selected peptide doses.
It has been argued that the unique ability of CD8α+ DC in presenting cell-associated OVA is the result of a specialized pathway for antigen handling that allows transport of exogenous antigens to the cytosol and access to the conventional MHC class I processing pathway.4,8 However, the selective ability of CD8α+ DC to internalize cellular material seen here could account for the superiority of this subset in cross-presenting cell-associated antigens. To determine if, indeed, CD8α+ DC were unique among DC subsets in presenting exogenous antigen on MHC class I, we examined whether CD4+ DC were able to present OVA expressed in E. coli to OT-I T cells. As shown in Fig. 3(c), both CD4+ and CD8α+ DC were equally able to present fixed OVA-E. coli to OT-I T cells. It was considered possible that this effect was the result of a disproportionate increase in the capacity of CD4+ DC to stimulate naïve T cells following bacterial activation, which resulted in potent OT-I activation, even in the face of weak presentation. This was not the case, as the ability of both CD4+ and CD8α+ DC to present peptide to OT-I T cells was increased to a similar extent by exposure to bacteria (Fig. 3d). When bacterial uptake was taken into account (Fig. 3b), CD4+ DC were actually found to be fivefold superior to CD8α+ DC in MHC class I presentation of fixed OVA-E. coli (Fig. 3c). However, presentation of E. coli-expressed OVA by either subset could be further augmented by co-expression of LLO, a lysosome-disrupting haemolysin from Listeria monocytogenes,14 showing that active promotion of cytosolic access can improve presentation (Fig. 3c). We conclude that, like CD8α+ DC, CD4+ DC can present exogenous antigens on MHC class I, provided those antigens are internalized.
OVA-bearing cells and OVA-E. coli are processed similarly via a proteasome-dependent pathway
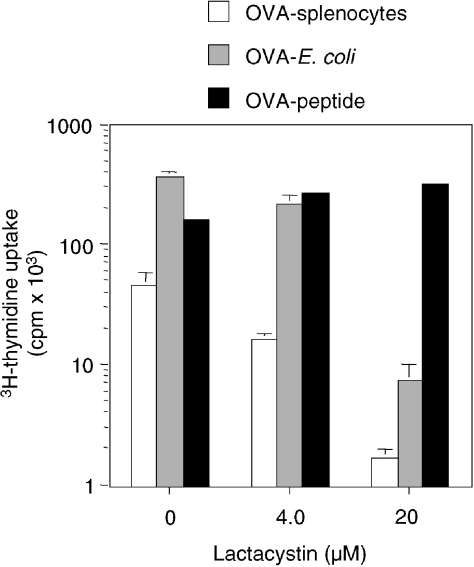
At least two alternative pathways have been described for MHC class I presentation of particulate antigens by macrophages (Mφ).15–19 One of these pathways involves endosomal processing followed by peptide regurgitation and binding to cell-surface MHC class I, and plays a prominent role in the presentation of some bacterial antigens.15,17 Thus, it was considered possible that CD8α+ DC, but not CD4+ DC, possess a specialized pathway for endosome-to-cytosol transport that was not revealed in the experiments using OVA expressed in E. coli. To determine if OVA-E. coli and OVA-bearing cells were processed via different pathways, we examined the susceptibility of presentation to the proteasome inhibitor, lactacystin.20 As shown in Fig. 4, lactacystin inhibited, by more than 95%, DC presentation of either OVA-loaded splenocytes or OVA-E. coli. This result suggests that both bacterially expressed OVA and cell-associated OVA are processed via the endogenous proteasome-dependent pathway for MHC class I presentation and therefore utilize an endosome-to-cytosol pathway.
Figure 4.
Major histocompatibility complex (MHC) class I presentation of ovalbumin (OVA)-bearing splenocytes and OVA-Escherichia coli by dendritic cells (DC) requires proteasomal activity. CD11c-enriched splenocytes and OT-I T cells were cultured with either OVA-loaded splenocytes (5 × 105/well; open bars), OVA-E. coli (1 × 106/well; grey bars) or OVA-peptide (25 pm, filled bars) in the presence or absence of the indicated doses of the proteasome-specific inhibitor lactacystin. Results represent the mean [3H]thymidine uptake of triplicate wells. All error bars are shown and represent 1 SD from the mean. Results are representative of four experiments.
Discussion
The role of DC subsets in immune function remains controversial. It has been proposed that different types of DC may be specialized to induce tolerance versus immunity, T helper 1 (Th1) versus T helper 2 (Th2) responses, or CD8+ versus CD4+ T-cell responses.21 Murine CD8α+ DC appear to be unique among DC subtypes in their ability to cross-present cell-associated antigens to both MHC class I- and MHC class II-restricted T cells.4,7 Here we show that the selective ability of splenic CD8α+ DC to cross-present cell-associated material probably results from their selective ability to internalize dead or dying cells. This is in contrast to a previous report in which both CD8α+ and CD8α− DC were shown to have a similar ability to internalize injected splenocytes in vivo4 and might suggest that DC subsets may behave differently in vitro and in vivo. However, this does not appear to be the case: in a paper published just prior to submission of this manuscript, Iyoda et al. reported that CD8α+ DC are vastly superior to CD8α− DC in their ability to internalize dying cells both in vivo and in vitro.22 These authors further showed that selective uptake of dying cells was not restricted to CD8α+ DC from spleen but could also be observed with those in liver and lymph nodes.22 Our results, using spleen DC fractionated on the basis of both CD8α and CD4, confirm and extend those of Iyoda et al.22 and firmly establish the superiority of CD8α+ DC in the uptake of dead cells compared to the other two CD11cbright DC subsets.
We attempted to establish if uptake of dead/dying cells is specific for apoptotic cells by fractionating UV-irradiated cells into annexin V+ and annexin V− fractions. We found that although annexin V+ cells were indeed taken up to a greater extent by CD8α+ DC, uptake of annexin V− cells was significant (Fig. 1b). This may be because many annexin V− cells are already committed to undergo apoptosis and display early membrane changes that are recognized by DC and/or because annexin V− cells undergo apoptosis during sorting and subsequent manipulation steps, despite our attempts to inhibit progression along the apoptotic pathway by fixation (see the Materials and methods). Alternatively, in accordance with published data, it is also probable that DC take up live cells.23
The inefficiency of CD8α− DC in cross-presenting cell-associated antigens on MHC class I has been suggested to result from the lack of a pathway that allows transport of endosomal contents to the cytosol.4 That conclusion was supported by a subsequent study in which CD8α− DC were inferior to CD8α+ DC in presenting soluble exogenous OVA protein to OT-I cells after in vivo immunization.8 Thus, CD8α+ DC would appear to resemble bone marrow-derived DC and certain DC cell lines that constitutively present exogenous antigens on MHC class I,24–27 while CD8α− DC would seem to resemble Mφ and B cells, which have a much more limited ability to do so.28 However, the notion of differential presentation by DC subsets has been challenged by a recent report of Yrlid & Wick who showed that both CD8α+ and CD8α− splenic DC could present Salmonella-expressed OVA to OT-I T cells in vitro.13 Similarly, Iyoda et al. concluded that CD8α− DC can present high concentrations of soluble OVA protein on MHC class I,22 in contrast to the earlier findings of Pooley et al.8 Here we confirm that the major CD8α− DC subset, CD4+ DC, is able to present exogenous bacterially expressed proteins on MHC class I and show that this occurs via a proteasome-dependent pathway that can be inhibited with lactacystin. This demonstrates that, provided they can take them up, CD4+ DC are able to translocate exogenous antigens to the cytosol and present them on MHC class I as efficiently as CD8α+ DC. However, we also found that MHC class I presentation of exogenous bacterial antigens by DC can be improved by a lysosomal disrupting protein, such as E. coli-expressed LLO (Fig. 3). We cannot entirely exclude that the increased presentation of OVA/LLO-E. coli could be caused by factors other than LLO, as we have not been able to selectively inactivate the haemolysin in those bacteria and determine its consequence for presentation (O. Schulz et al., unpublished observations). However, the fact that OVA/LLO-bacteria express lower levels of OVA protein than OVA-E. coli (data not shown), and are nevertheless presented more efficiently, may suggest that the endosome-to-cytosol pathway is a rate-limiting step in DC, as reported for other cell types.18 Altogether, these data argue against a specialization in handling of exogenous antigen by different DC subsets and suggest that the basis of selective cross-presentation of cell-associated antigens by CD8α+ DC rests with expression of specific receptor(s) for apoptotic cell uptake, the identity of which remains unclear.5
Acknowledgments
This study was supported by Cancer Research UK. We thank Georges Vassaux and Darren Higgins for the gift of OVA-expressing bacteria. We thank Colin Gray for assistance with video microscopy, and Cathy Simpson, Ayad Eddaoudi and Gary Warnes for cell sorting. We are grateful to Facundo Batista, Sandra Diebold and Roman Spörri for helpful comments on the manuscript and members of the Immunobiology Laboratory, Cancer Research UK, for discussions.
Abbreviations
- DC
dendritic cells
- LLO
listeriolysin O
- Mφ
macrophages
- OVA
ovalbumin
- PFA
paraformaldehyde
- YG
yellow green
Supplementary Material
Clips of the films from which Fig. 2 was taken can be found at the following web address: http://www.blackwell-science.com/products/journals/suppmat/IMM/IMM1513/IMM1513sm.htm
References
- 1.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 2.den Haan JM, Bevan MJ. Antigen presentation to CD8+ T cells: cross-priming in infectious diseases. Curr Opin Immunol. 2001;13:437–41. doi: 10.1016/s0952-7915(00)00238-7. [DOI] [PubMed] [Google Scholar]
- 3.Bevan MJ. Class discrimination in the world of immunology. Nature. 1987;325:192–4. doi: 10.1038/325192b0. [DOI] [PubMed] [Google Scholar]
- 4.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–96. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz O, Pennington D, Hodivala-Dilke K, Febbraio M, Reis e Sousa C. CD36 or αvβ3 and αvβ5 integrins are not essential for MHC class I cross-presentation of cell-associated antigen by CD8α+ murine dendritic cells. J Immunol. 2002;168:6057–65. doi: 10.4049/jimmunol.168.12.6057. [DOI] [PubMed] [Google Scholar]
- 6.Belz G, Vremec D, Febbraio M, Corcoran L, Shortman K, Carbone FR, Heath WR. CD36 is differentially expressed by CD8+ splenic dendritic cells but is not required for cross-presentation in vivo. J Immunol. 2002;168:6066, 70. doi: 10.4049/jimmunol.168.12.6066. [DOI] [PubMed] [Google Scholar]
- 7.Valdez Y, Mah W, Winslow MM, Xu L, Ling P, Townsend SE. Major histocompatibility complex class II presentation of cell-associated antigen is mediated by CD8alpha+ dendritic cells in vivo. J Exp Med. 2002;195:683–94. doi: 10.1084/jem.20010898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pooley JL, Heath WR, Shortman K. Cutting edge: Intravenous soluble antigen is presented to CD4 T cells by CD8(−) dendritic cells, but cross-presented to CD8 T cells by CD8(+) dendritic cells. J Immunol. 2001;166:5327–30. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 9.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 10.Higgins DE, Shastri N, Portnoy DA. Delivery of protein to the cytosol of macrophages using Escherichia coli K-12. Mol Microbiol. 1999;31:1631–41. doi: 10.1046/j.1365-2958.1999.01272.x. [DOI] [PubMed] [Google Scholar]
- 11.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–86. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 12.Kamath AT, Pooley J, O'Keeffe MA, et al. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol. 2000;165:6762–70. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- 13.Yrlid U, Wick MJ. Antigen presentation capacity and cytokine production by murine splenic dendritic cell subsets upon salmonella encounter. J Immunol. 2002;169:108–16. doi: 10.4049/jimmunol.169.1.108. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich G, Hess J, Gentschev I, Knapp B, Kaufmann SH, Goebel W. From evil to good: a cytolysin in vaccine development. Trends Microbiol. 2001;9:23–8. doi: 10.1016/s0966-842x(00)01893-x. [DOI] [PubMed] [Google Scholar]
- 15.Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–62. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 16.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA. 1993;90:4942–6. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–33. [PubMed] [Google Scholar]
- 18.Reis e Sousa C, Germain RN. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J Exp Med. 1995;182:841–51. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–6. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 20.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–71. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 21.Shortman K, Liu Y. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 22.Iyoda T, Shimoyama S, Liu K, et al. The CD8(+) dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–23. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 24.Bachmann MF, Lutz MB, Layton GT, Harris SJ, Fehr T, Rescigno M, Ricciardi-Castagnoli P. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol. 1996;26:2595–600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 25.Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–8. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 26.Regnault A, Lankar D, Lacabanne V, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–80. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–8. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 28.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]