Abstract
Oestrogen treatment down-regulates B lymphopoiesis in the bone marrow of mice. Meanwhile it up-regulates immunoglobulin production. To understand better the oestrogen action on bone marrow male mice lacking oestrogen receptor α (ERα; ERKO mice), lacking ERβ (BERKO mice), lacking both receptors (DERKO mice) or wild-type (wt) littermates were castrated and treated for 2·5 weeks with 30 μg/kg 17β-oestradiol (E2) or vehicle oil as controls. The B lymphopoiesis in the bone marrow was examined by flow cytometry and mature B-cell function was studied using an ELISPOT assay enumerating the B cells in bone marrow and spleen that were actively producing immunoglobulins. In wt mice the frequency of B-lymphopoietic (B220+) cells in the bone marrow decreased from 15% to 5% upon E2 treatment. In ERKO and BERKO mice significant reduction was seen but not of the same magnitude. In DERKO mice no reduction of B lymphopoiesis was seen. In addition, our results show that E2 mediated reduction of different steps in B lymphopoiesis require only ERα or both receptors. In wt and BERKO mice E2 treatment resulted in significantly increased levels of B cells actively producing immunoglobulin, while in ERKO and DERKO mice no such change was seen. Similar results were found in both bone marrow and spleen. In conclusion our results clearly show that both ERα and ERβ are required for complete down-regulation of B lymphopoiesis while only ERα is needed to up-regulate immunoglobulin production in both bone marrow and spleen.
Introduction
Oestrogen is known to exert multiple effects on the immune system. Oestrogen deficiency after oophorectomy up-regulates both B1 and T lymphopoiesis2 while oestrogen treatment decreases lymphopoietic cell number in the bone marrow1 and in the thymus.2 Down-regulation of both B and T lymphopoiesis involves a cell phenotype shift towards lower frequencies of immature lymphocytes.1,2 In animal studies it was demonstrated that oestrogen modulates immune reactivity by decreasing granulocyte-3 and T-cell-4 mediated inflammation, natural killer (NK) cell-mediated cytotoxicity5 and enhancing immunoglobulin production.6
Oestrogen exerts its effects through two related hormone-binding nuclear receptors, named oestrogen receptor α (ERα) and ERβ. They act as transcription factors on different gene promoters and have different tissue distributions. ER knock-out mice, lacking ERα (ERKO mice), ERβ (BERKO mice), or both ERs (DERKO mice) have been used in the study of receptor specificity of oestrogenic effects in different organ systems (reviewed in ref. 7). Recent studies in ER-targeted mice have revealed that male mice negative for ERα have smaller spleens and thymi and lower frequency of mature single positive T cells in the thymus than ERα-positive mice. Oestrogen treatment of oophorectomized BERKO mice induced thymic atrophy to a similar extent as in wild-type (wt) littermates but no T-cell phenotype shift.8 When castrated ERKO males were treated with oestrogen they displayed only a minor thymic atrophy and they failed to shift towards more mature T cells.9 Taken together these data suggest that both ERα and ERβ are needed to achieve full thymic atrophy.
Oestradiol is naturally found in males as a result of extra-gonadal aromatization of testosterone. It is known that oestrogen is of great importance in males, both for bone growth and maintenance10 and for reproduction and behaviour.11 Interestingly it has recently been shown that the up-regulation of B lymphopoiesis in castrated male rats correlates with serum oestrogen rather than testosterone levels.12 Following these data the aim of the present study was to elucidate the receptor specificity for oestrogen-induced effects on B lymphopoiesis and immunoglobulin production in male mice. For this purpose male mice of all four ER genotypes were castrated and treated with oestradiol or vehicle as controls.
Materials and methods
Mice
Generation of DERKO mice: male double heterozygous (ERα+/− ERβ+/−) mice were mated with female double heterozygous (ERα+/− ERβ+/−) mice on a mixed C57BL/6J/129 background resulting in wt (ERα+ ERβ+), ERKO (ERα− ERβ+), BERKO (ERα+ ERβ−) and DERKO (ERα− ERβ−) offspring. Genotyping of tail DNA was performed with polymerase chain reaction as previously described.8,13 Mice were housed four to six animals in each cage under standard conditions of temperature and light and were fed standard laboratory chow ad libitum.
Castration
All mice were castrated at the age of 7 months and then left to rest for 2 weeks prior to the start of treatment. Testes were removed after a single scrotal incision. The operation procedures were carried out under Ketalar/Dormitor anaesthesia.
Hormones and treatment
The mice were treated with subcutaneous (s.c.) injections of 17β-oestradiol benzoate (E2; Sigma, St Louis, MO) (30 μg/kg). The hormone was dissolved in olive oil (Apoteksbolaget, Göteborg, Sweden) and injected s.c. in a final volume of 100 μl 5 days/week for 2·5 weeks. This treatment resulted in serum oestradiol levels of about 60 pg/ml.14 Controls were given vehicle, 100 μl olive oil/mouse 5 days/week. In a recently published study we have shown that normal intact female mice display serum oestradiol levels of approximately 12 pg/ml, oophorectomized mice 6 pg/ml and female mice treated with E2 about 60 pg/ml.15
Cellular parameters
Tissue collection and single cell preparation. The mice were killed by cervical dislocation. Spleens were autopsied and single cell suspensions were obtained after tissue was mashed and passed through a nylon wool sieve. The cells were centrifuged at 515 g for 5 min and pelleted spleen cells were resuspended in Tris-buffered 0·83% NH4Cl solution pH 7·29 to lyse erythrocytes. Bone marrow cells were harvested from the cavity of the right femur using a syringe with 2 ml of phosphate-buffered saline (PBS). Cells were kept in complete Iscove's medium/PBS (50/50) until use. After being washed in PBS the total number of leucocytes was calculated, using an automated cell counter (Sysmex, Kobe, Japan) The cells were subjected to fluorescence-activated cell sorter (FACS) analysis and an ELISPOT assay determining number of immunoglobulin-producing cells.
Flow cytometry for analysis of cell phenotypes. Isolated splenocytes were stained with fluorescein isothiocyanate (FITC) -labelled antibodies to CD45R/B220 (clone RA3-6B2) (Becton Dickinson, Franklin Lakes, NJ) and phycoerythrin (PE)-conjugated antibodies to CD3ε-PE (clone 145-2C11) (B-D). Bone marrow cells were labelled with anti-CD45R/B220-FITC and anti-μPE (cat no. 1021-09; Southern Biotechnology, Birmingham, AL). All cells were analysed in a FACSCalibur (Becton Dickinson). Bone marrow cells were expressed as percentage of all nucleated cells.
ELISPOT. The ELISPOT technique16 was used for enumeration of immunoglobulin M- (IgM), IgG- and IgA-secreting bone marrow and spleen cells. Briefly, 96-well nitrocellulose plates (Millipore, Bedford, MA) were coated with 100 μl PBS containing 5 μg/ml of affinity purified F(ab′)2 fragments of goat anti-mouse IgM, IgG and IgA (Cappel, Oragon Teknika, Turnhout, Belgium). After overnight incubation at 4° and blocking with 5% fetal calf serum, 50 μl of Iscove's culture medium containing 106 or 105 freshly isolated spleen or bone marrow cells/ml was added to each well. The plates were incubated for 3·5 hr at 37° in 5% CO2 and 95% humidity. After being rinsed each well was incubated stepwise with 100 μl of alkaline-phosphatase-conjugated goat anti-mouse IgM, IgG, or IgA (Southern Biotechnology Inc) diluted in 1 : 750 PBS and finally with the substrate 5-bromo-4-chloro-3-indolyl phosphate (Sigma, St Louis, MO). After 1 hr the reaction was stopped by washing in tap water and each well was examined for the appearance of dark blue spots. The samples were run in triplicates and the numbers of immunoglobulin-secreting cells were expressed as the frequency of spot-forming cells (SFC) per 103 B220+ cells.
Serological parameters
Oestradiol radioimmunoassay (RIA). E2 was measured using an RIA detecting E2 (DiaSorin, Saluggia, Italy) with a sensitivity below 5 pg/ml at 95% confidence limits.
Statistic analysis. Two-way analysis of variance (anova) was used to find a statistical difference with respect to the expression of ERα and ERβ. To find differences with respect to hormone treatment the non-parametric Mann–Whitney test was used for each genotype separately. A P value <0·05 was considered statistically significant. Data are expressed as mean ± SEM.
Results
Effects on B lymphopoiesis
Male mice of all four ER genotypes were castrated and treated for 2·5 weeks with 30 μg E2/kg or olive oil as controls. Femur bone marrow cellularity was determined using an automated cell counter and, as expected, the cell yield decreased upon E2 treatment in wt mice. In mice lacking one or both ERs no reduction was seen and a two-way anova revealed that both ERα and ERβ are needed to get reduction of bone marrow cellularity upon E2 treatment (Table 1).
Table 1.
Bone marrow cellularity after E2 and B lymphocyte number
| Total bone marrow cellularity (106 cells/femur) | B220+ cells (106 cells/femur) | ||||
|---|---|---|---|---|---|
| Controls | 30 μg E2/kg | Controls | 30 μg E2/kg | ||
| Wild-type mice | |||||
| ERα+ ERβ+ | 33·9 ± 3·9 | 13·5 ± 3·0* | 5·3 ± 0·5 | 0·6 ± 0·2* | |
| BERKO mice | |||||
| ERα+ ERβ− | 32·6 ± 4·0 | 22·5 ± 1·8 | 4·3 ± 0·7 | 1·6 ± 0·2** | |
| ERKO mice | |||||
| ERα− ERβ+ | 27·1 ± 3·5 | 24·4 ± 1·0 | 4·1 ± 0·3 | 2·5 ± 0·3* | |
| DERKO mice | |||||
| ERα− ERβ− | 32·6 ± 2·4 | 26·7 ± 3·3 | 5·1 ± 0·4 | 4·3 ± 0·7 | |
| anova ERα | † | ††† | |||
| anova ERβ | ‡ | ‡‡ | |||
Male mice of all four ER genotypes were castrated and treated for 2·5 weeks with subcutaneous doses of E2 or olive oil as controls. Bone marrow cells were harvested from the cavity of the right femur and counted using an automated cell counter. Mean ±SEM.
P < 0·05.
P < 0·01 (Mann–Whitney: controls versus E2).
P < 0·05.
P < 0·001 (anova: ERα+ versus ERα−).
P < 0·05.
P < 0·01 (anova: ERβ+ versus ERβ−).
Cell phenotypes in the B lymphopoiesis in bone marrow were analysed by use of flow cytometry. As expected, in wt mice the frequency of B lymphopoietic cells (B220+) in bone marrow decreased dramatically from more than 15% to less than 5% upon E2 treatment. A decrease of smaller magnitude, although still significant, was found in both BERKO and ERKO mice, whereas no decrease at all was seen in DERKO mice (Fig. 1). A two-way anova revealed that both ERα and ERβ are required for complete E2-induced reduction of B lymphopoiesis. The total number of B220+ cells in femur is shown in Table 1. The effects of exposure to E2 on mice with different ER expressions on total number of B220+ cells were similar to the frequency of B220+ cells.
Figure 1.
Both ERα and ERβ are needed to achieve a full inhibitory effect on B lymphopoiesis after oestrogen treatment. The figure shows the percentage of B220+ cells in bone marrow of castrated mice of all ER genotypes treated 5 days/week for 2·5 weeks with 30 μg E2/kg or olive oil as controls. A two-way anova reveals that both ERα and ERβ contribute to achieve full inhibitory effect. ERα: P < 0·001, ERβ: P < 0·001. Bars represent mean ± SEM. *P < 0·05, **P < 0·01 versus controls (non-parametric Mann–Whitney U-test).
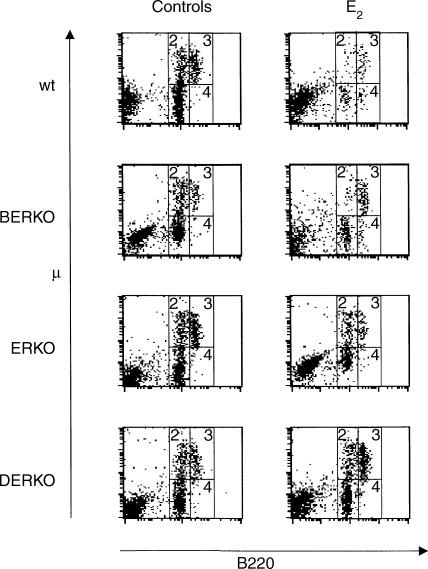
Three different stages in the B lymphopoiesis were identified as follows: (1) pro-B cells (B220low μ−), (2) pre-B cells (B220low μ+), (3) B cells (B220high μ+) (Fig. 2). These stages constitute the late part of B lymphopoiesis from just before rearrangement of the immunoglobulin genes to newly formed mature B cells. Representative FACS plots are shown in Fig. 2 and means and SEMs are given in Table 2.
Figure 2.
E2 treatment changes the phenotype pattern of B220+ cells in bone marrow. The figure shows representative FACS plots from bone marrow of castrated mice of all ER genotypes treated 5 days/week for 2·5 weeks with 30 μg E2/kg or olive oil as controls. Means and SEM for the B lymphopoietic fractions 1–3 are shown in Table 2 and means and SEM for the activated immunoglobulin-switched B cells (fraction 4) in Table 3.
Table 2.
Different stages of the B lymphopoiesis after E2 treatment
| Pro-B cells (B220low μ−) | Pre-B cells (B220low μ+) | B cells (B220high μ+) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls | 30 μg E2/kg | % change | Controls | 30 μg E2/kg | % change | Controls | 30 μg E2/kg | % change | |
| WT ERα+ ERβ+ | 8·49 ± 0·52 | 2·32 ± 0·81* | −73 | 3·31 ± 0·10 | 0·97 ± 0·39* | −70 | 3·40 ± 0·19 | 0·66 ± 0·19* | −81 |
| BERKO ERα+ ERβ− | 6·75 ± 1·43 | 3·70 ± 0·19 | −45 | 3·96 ± 0·54 | 1·05 ± 0·15** | −75 | 1·84 ± 0·20 | 1·54 ± 0·30 | −17 |
| ERKO ERα− ERβ+ | 9·10 ± 0·68 | 5·00 ± 0·77* | −45 | 3·21 ± 0·26 | 2·31 ± 0·26 | −28 | 2·78 ± 0·21 | 2·17 ± 0·60 | −12 |
| DERKO ERα− ERβ− | 9·47 ± 0·66 | 8·57 ± 0·69 | −10 | 2·96 ± 0·17 | 2·77 ± 0·18 | −7 | 2·63 ± 0·41 | 3·56 ± 0·23 | +38 |
| anova ERα | ††† | ††† | ††† | ||||||
| anova ERβ | ‡‡ | ns | ‡‡ | ||||||
Male mice of all four ER genotypes were castrated and treated for 2·5 weeks with subcutaneous doses of E2 or olive oil as controls. The frequency of B lymphopoietic cells in bone marrow was obtained by use of flow cytometry. All groups consist of four to six mice. Representative FACS plots are shown in Fig. 2. Mean ±SEM.
P = 0·05.
P < 0·01 (Mann–Whitney: controls versus E2).
P < 0·001 (anova: ERα+ versus ERα−).
P < 0·01 (anova: ERβ+ versus ERβ−).
ns, not significant.
In wt mice all these three B lymphopoietic populations were dramatically decreased after E2 treatment. In mice lacking ERβ the frequencies of pro- and pre-B cells decreased but not the newly formed B cells, while in mice lacking ERα only the pro-B cells decreased upon E2 treatment. Mice lacking both ERα and ERβ displayed no reduction. A two-way anova revealed that to reduce the pro-B cells completely both ERα and ERβ are required. ERKO and BERKO mice were thus displaying significant reduction of pro-B cells but not of the same magnitude as seen in wt mice. To get reduction of pre-B cells upon E2 treatment only ERα was required while both receptors were needed to get any significant reduction of the newly formed B cells.
Effects on immunoglobulin switched mature B cells
The immunoglobulin switched mature B-cell population (B220highµ−) was identified in bone marrow by FACS (Fig. 2, fraction 4). The frequency of these cells increased significantly upon E2 treatment in wt and BERKO mice but not in ERKO and DERKO mice. A two-way anova revealed that ERα but not ERβ was required for oestrogen-mediated stimulation of immunoglobulin-switched B cells (Table 3).
Table 3.
The frequency of immunoglobulin-switched mature B cells (B220high μ−) in bone marrow after E2 treatment
| % B220high μ− bone marrow cells | ||
|---|---|---|
| Controls | 30 μg E2/kg | |
| Wild-type mice | ||
| ERα+ ERβ+ | 3·2 ± 0·4 | 18·6 ± 3·7* |
| BERKO mice | ||
| ERα+ ERβ− | 2·9 ± 0·6 | 13·1 ± 1·2** |
| ERKO mice | ||
| ERα− ERβ+ | 3·4 ± 0·5 | 6·7 ± 0·9 |
| DERKO mice | ||
| ERα− ERβ− | 4·2 ± 0·3 | 5·0 ± 0·6 |
| anova ERα | ††† | |
| anova ERβ | ns | |
Male mice of all four ER genotypes were castrated and treated for 2·5 weeks with subcutaneous doses of E2 or olive oil as controls. Frequency of B220+ cells of B220high μ− phenotype was obtained by use of flow cytometry. All groups consisted of four to six mice. Mean ±SEM.
P < 0·05.
P < 0·01 (Mann–Whitney: controls versus E2).
P < 0·001 (anova: ERα+ versus ERα−).
ns, not significant.
Effects on immunoglobulin secretion in bone marrow and spleen
Cells from bone marrow and spleen of castrated male mice treated with E2 or control oil were subjected to an ELISPOT assay enumerating cells producing immunoglobulins M, G and A. The frequency of B lymphocytes from spleen and bone marrow actively producing immunoglobulins was increased in wt mice after E2 exposure. A similar effect was found in BERKO mice, but in ERKO and DERKO mice no such increase was found (Fig. 3a,b). A two-way anova confirmed that ERα is needed and that ERβ is not involved in oestrogen-mediated increase of immunoglobulin production.
Figure 3.
ERα but not ERβ is required to achieve an increased frequency of B cells actively producing immunoglobulins. The figure shows the frequency of B220+ cells actively producing immunoglobulin in bone marrow(a)and spleen (b)of castrated mice of all ER genotypes treated 5 days/week for 2·5 weeks with 30 μg E2/kg or olive oil as controls. Data are shown as immunoglobulin SFC/103 B220+ cells. A two-way anova reveals that ERα, but not ERβ, is required to achieve full effect. ERα: P < 0·001 (a); P < 0·05 (b); ERβ: not significant. Bars represent mean ± SEM for the summation of IgM, IgG and IgA. *P < 0·05, **P < 0·01 versus controls (non-parametric Mann–Whitney U-test).
There were no clear-cut changes in number of lymphocytes in spleen.
Discussion
Castration of both male and female mice increases bone marrow cellularity and stimulates B lymphopoiesis, as demonstrated by increased frequency of B220+ cells in bone marrow.17,18 It was recently shown that in intact aged male rats the frequency of B lymphopoietic cells correlated to serum oestradiol rather than serum testosterone levels.12 Replacement of oestrogen in physiological or pharmacological doses decreases bone marrow cellularity and suppresses B lymphopoiesis19 in female as well as male mice. The erythroid and myeloid precursors are not altered after oestrogen exposure.18 Smithson et al.20 have shown that bone marrow stroma cells from female ERKO mice are not fully oestrogen responsive, suggesting a role for ERβ. We now show that both ERα and ERβ contribute to the E2-induced inhibition of B lymphopoiesis in male mice and that in the absence of both receptors no oestrogen-induced effects were found. The bone marrow cellularity is difficult to quantify and variances are often high. Nevertheless a two-way anova revealed that both ERα and ERβ are needed to achieve a decrease in bone marrow cellularity after E2 exposure.
Newly formed B cells and B-cell precursors are depressed in oestrogen-treated mice.21,22 We now point out that to achieve oestrogenic effect on the pre-B cells ERα but not ERβ is required and to achieve any effect on the mature B cells both receptors are required (Table 2). However, to achieve the full oestrogenic effect on the pro-B cells both receptors are needed. On the other hand, lack of any of the receptors is enough to partly decrease the pro-B cell compartment, a hint that oestrogen plays a part earlier in B lymphopoiesis, before the expression of B220. Lack of both ERs results in no change in the pro-B cell compartment upon E2 treatment (Table 2). Recently Kincade and colleagues have shown that cells referred to as early pro-B cells or common lymphoid progenitors (lineage marker negative, B220−, TdT+ and c-kitlo) are sensitive to oestrogen treatment. These cells are found up-streams in the lymphopoietic lineage and have the potential to differentiate into both B and T lymphocytes.23,24
Oestrogen is known to increase immunoglobulin production. Long-term treatment with low doses of E2 results in increased serum levels of immunoglobulin.6 After short-term treatment no significant changes are found on systemic immunoglobulin levels, but the frequency of B cells actively producing immunoglobulin increases.15 In the present study we show that the frequency of B cells in bone marrow which have switched from IgM increases after E2 treatment and this effect is ERα dependent (Table 3). At the same time the frequency of bone marrow B cells actively producing immunoglobulin was enhanced after E2 treatment of ERα+ mice (Fig. 3a). Together these data suggest that to affect the mature B cells, represented by the immunoglobulin-switched B cells and the immunoglobulin-producing cells, only ERα is required. The mandatory role of ERα for E2 mediated stimulation of mature switched B cells was further established because B220+ cells in the spleen actively producing immunoglobulin displayed the same pattern as in the bone marrow (Fig. 3b).
To explain the phenomena seen above one possible mechanism would involve the presence of oestrogen receptors in target cells. Recent findings show that bone marrow stroma cells express both ERα and ERβ20 while mature B cells in the spleen only express ERα.25 One hypothesis is that the oestrogen-induced effects on bone marrow B lymphopoiesis (requires both oestrogen receptors) are mediated by stroma cells expressing both ERα and ERβ, while the effects on immunoglobulin production in mature B cells (requires only ERα) is mediated by direct oestrogenic effects on B cells.
In the present study we have shown that to completely down-regulate the B lymphopoiesis upon E2 treatment both ERα and ERβ are required, while only ERα is needed for mature immunoglobulin-producing B cells to respond to E2. These results might in the future be of importance when choosing among specific oestrogen receptor ligands that can be used to treat osteoporosis in patients with immune complex driven autoimmune diseases such as systemic lupus erythematosus.
Acknowledgments
We thank Mrs Lena Svensson for excellent technical assistance. We would also like to thank the SWEGENE Center for Bio-Imaging (CBI), and Gothenburg university for technical support regarding Image Analysis. This study was supported by grants from the Swedish cancer foundation, the Börje Dahlin foundation, the Göteborg Medical Society, Association against Rheumatism, the King Gustav V's 80 years foundation, the Anna-Greta Crafoord foundation, Reumaforskningsfond Margareta, the Medical Faculty of Göteborg University (LUA), the Swedish Foundation for Strategic Research, the Torsten and Ragnar Söderbergs Foundation, Petrus and Augusta Hedlunds Foundation and the Swedish Medical Research Council.
References
- 1.Medina KL, Strasser A, Kincade PW. Estrogen influences the differentiation, proliferation, and survival of early B-lineage precursors. Blood. 2000;95:2059–67. [PubMed] [Google Scholar]
- 2.Rijhsinghani AG, Thompson K, Bhatia SK, Waldschmidt TJ. Estrogen blocks early T cell development in the thymus. Am J Reprod Immunol. 1996;36:269–77. doi: 10.1111/j.1600-0897.1996.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 3.Josefsson E, Tarkowski A, Carlsten H. Anti-inflammatory properties of estrogen. I. In vivo suppression of leukocyte production in bone marrow and redistribution of peripheral blood neutrophils. Cell Immunol. 1992;142:67–78. doi: 10.1016/0008-8749(92)90269-u. [DOI] [PubMed] [Google Scholar]
- 4.Carlsten H, Verdrengh M, Taube M. Additive effects of suboptimal doses of estrogen and cortisone on the suppression of T lymphocyte dependent inflammatory responses in mice. Inflamm Res. 1996;45:26–30. doi: 10.1007/BF02263501. [DOI] [PubMed] [Google Scholar]
- 5.Hanna N, Schneider M. Enhancement of tumor metastasis and suppression of natural killer cell activity by beta-estradiol treatment. J Immunol. 1983;130:974–80. [PubMed] [Google Scholar]
- 6.Nilsson N, Carlsten H. Estrogen induces suppression of natural killer cell cytotoxicity and augmentation of polyclonal B cell activation. Cell Immunol. 1994;158:131–9. doi: 10.1006/cimm.1994.1262. [DOI] [PubMed] [Google Scholar]
- 7.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 8.Erlandsson MC, Ohlsson C, Gustafsson JA, Carlsten H. Role of oestrogen receptors alpha and beta in immune organ development and in oestrogen-mediated effects on thymus. Immunology. 2001;103:17–25. doi: 10.1046/j.1365-2567.2001.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staples JE, Gasiewicz TA, Fiore NC, Lubahn DB, Korach KS, Silverstone AE. Estrogen receptor alpha is necessary in thymic development and estradiol-induced thymic alterations. J Immunol. 1999;163:4168–74. [PubMed] [Google Scholar]
- 10.Vanderschueren D, Boonen S, Bouillon R. Action of androgens versus estrogens in male skeletal homeostasis. Bone. 1998;23:391–4. doi: 10.1016/s8756-3282(98)00131-8. [DOI] [PubMed] [Google Scholar]
- 11.Lombardi G, Zarrilli S, Colao A, Paesano L, Di Somma C, Rossi F, De Rosa M. Estrogens and health in males. Mol Cell Endocrinol. 2001;178:51–5. doi: 10.1016/s0303-7207(01)00420-8. [DOI] [PubMed] [Google Scholar]
- 12.Erben RG, Eberle J, Stangassinger M. B lymphopoiesis is upregulated after orchiectomy and is correlated with estradiol but not testosterone serum levels in aged male rats. Horm Metab Res. 2001;33:491–8. doi: 10.1055/s-2001-16943. [DOI] [PubMed] [Google Scholar]
- 13.Vidal O, Lindberg M, Hollberg K, et al. Estrogen receptor specificity in the regulation of skeletal changes in male mice. Proc Natl Acad Sci USA. 2000;97:5474–9. doi: 10.1073/pnas.97.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindberg MK, Moverare S, Skrtic S, et al. Two different pathways for the maintenance of trabecular bone in adult male mice. J Bone Miner Res. 2002;17:555–62. doi: 10.1359/jbmr.2002.17.4.555. [DOI] [PubMed] [Google Scholar]
- 15.Erlandsson MC, Jonsson CA, Lindberg MK, Ohlsson C, Carlsten H. Raloxifene and estradiol mediated effects on uterus, bone and B lymphopoiesis in mice. J Endocrinol. 2002;175:319–27. doi: 10.1677/joe.0.1750319. [DOI] [PubMed] [Google Scholar]
- 16.Czerkinsky CC, Nilsson LA, Tarkowski A, Ouchterlony O, Jeansson S, Gretzer C. An immunoenzyme procedure for enumerating fibronectin-secreting cells. J Immunoassay. 1984;5:291–302. doi: 10.1080/01971528408063013. [DOI] [PubMed] [Google Scholar]
- 17.Ellis TM, Moser MT, Le PT, Flanigan RC, Kwon ED. Alterations in peripheral B cells and B cell progenitors following androgen ablation in mice. Int Immunol. 2001;13:553–8. doi: 10.1093/intimm/13.4.553. [DOI] [PubMed] [Google Scholar]
- 18.Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, Suda T. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest. 1994;94:1090–7. doi: 10.1172/JCI117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onoe Y, Miyaura C, Ito M, Ohta H, Nozawa S, Suda T. Comparative effects of estrogen and raloxifene on B lymphopoiesis and bone loss induced by sex steroid deficiency in mice. J Bone Miner Res. 2000;15:541–9. doi: 10.1359/jbmr.2000.15.3.541. [DOI] [PubMed] [Google Scholar]
- 20.Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol. 1998;161:27–34. [PubMed] [Google Scholar]
- 21.Kincade PW, Medina KL, Payne KJ, Rossi MI, Tudor KS, Yamashita Y, Kouro T. Early B-lymphocyte precursors and their regulation by sex steroids. Immunol Rev. 2000;175:128–37. [PubMed] [Google Scholar]
- 22.Medina KL, Kincade PW. Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proc Natl Acad Sci USA. 1994;91:5382–6. doi: 10.1073/pnas.91.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouro T, Medina KL, Oritani K, Kincade PW. Characteristics of early murine B-lymphocyte precursors and their direct sensitivity to negative regulators. Blood. 2001;97:2708–15. doi: 10.1182/blood.v97.9.2708. [DOI] [PubMed] [Google Scholar]
- 24.Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718–24. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 25.Benten WP, Stephan C, Wunderlich F. B cells express intracellular but not surface receptors for testosterone and estradiol. Steroids. 2002;67:647–54. doi: 10.1016/s0039-128x(02)00013-2. [DOI] [PubMed] [Google Scholar]