Summary
The active thyroid hormone, triiodothyronine (T3), regulates mitochondrial uncoupling protein activity and related thermogenesis in peripheral tissues. An enzyme that catalyzes active thyroid hormone production, type 2 deiodinase (DII), and mitochondrial uncoupling protein 2 (UCP2) are also present in the hypothalamic arcuate nucleus, where their interaction and physiological significance have not been explored. Here, we report that DII-producing glial cells are in direct apposition to neurons co-expressing neuropeptide Y (NPY), agouti related protein (AgRP) and UCP2. Fasting increased DII activity and local thyroid hormone production in the arcuate nucleus in parallel with increased GDP-regulated UCP2-dependent mitochondrial uncoupling. Fasting-induced T3-mediated UCP2-activation underlied mitochondrial proliferation in NPY/AgRP neurons, an event that was critical for increased excitability of these orexigenic neurons and consequent rebound feeding following food deprivation. These results reveal a physiological role for a thyroid hormone-regulated mitochondrial uncoupling in hypothalamic neuronal networks.
Introduction
Thyroid hormones play major roles during development as well as in adulthood. In adults, an important contribution of the thyroid gland is to regulate metabolism at both the cellular and whole organism levels. Various downstream transcriptional events triggered by the active thyroid hormone, triiodothyronine (T3), have been thought to alter cell- and tissue metabolism (Basset et al., 2003 for review). In addition, non-transcriptional effects of T3 have also been identified (Basset et al., 2003; Harvey and Williams, 2002; Weitzel et al., 2003 for review). With regard to whole animal physiology, a key role for T3 was established in the regulation of brown fat-associated non-shivering thermogenesis (Silva, 1995 for review). The molecular underpinning of thermogenesis in brown fat is the activation of mitochondrial uncoupling protein 1 (UCP1), which dissipates energy in the form of heat (Ricquier, 2005; Nedergaard et al., 2005 for review). UCP1 activation is associated with increased proton conductance of the inner mitochondrial membrane and mitochondrial proliferation (Ricquier, 2005; Nedergaard et al., 2005). The activation of UCP1 is under the control of the sympathetic nervous system and fatty acids (Sell et al., 2004), and it is regulated by T3 (Reitman et al., 1999; Lanni et al., 2003). Based on structure homology, other UCPs with varying tissue distribution were also discovered, including UCP2 and UCP3, but their physiological role remains ill-defined and tissue specific (Krauss et al., 2005). In contrast to UCP1, UCP2 and UCP3 are conditional uncouplers yielding less robust changes in proton permeability of the inner mitochondrial membrane (for review see Andrews et al., 2005 and Brand and Esteves, 2005), and their function is not dependent on sympathetic activation but can be altered by T3 (Horrum et al., 1990; 1995; Kalderon et al., 1995). The hypothalamic arcuate nucleus, which is considered to be the key brain site that responds to changes in peripheral tissue metabolism (see Schwartz et al., 2000 for review), expresses high levels of UCP2 (Horvath et al., 1999) as well as thyroid hormone receptors (Lechan et al., 1993) and also has the capacity for local production of T3 (Coppola et al., 2005a,b). To date, it is unclear, however, whether local thyroid hormone production affects arcuate nucleus UCP activity, and if so, whether such an interaction has any physiological relevance in the regulation of energy metabolism.
Results
DII-producing glia are associated with orexigenic neurons
Local T3 production is catalyzed by type II deiodinase (DII) expressed in arcuate nucleus glial cells (Tu et al., 1997; Diano et al., 1998a,b; 2003a), and both DII activity and local T3 production is increased during fasting (Coppola et al., 2005a,b). UCP2, on the other hand, is expressed exclusively in neurons of the arcuate nucleus (Horvath et al., 1999), including cells that produce neuropeptide Y (NPY) and agouti-related protein (AgRP) (Horvath et al., 1999). These orexigenic cells of the brain are mandatory for feeding regulation (Gropp et al., 2005; Luquet et al., 2005) and their electrical activity is enhanced during fasting (Takahashi and Cone, 2005) when local T3 production is increased (Coppola et al., 2005b). Thus, we first analyzed whether a relationship exists between arcuate nucleus DII-expressing glial cells and NPY/AgRP neurons. Immunostaining for DII in GFP-NPY transgenic mice revealed a close association between DII-producing glial processes (red fluorescence) and NPY/AgRP neurons (green fluorescence; Fig.1A). Electron microscopic examination confirmed that DII immunopositive tanycytes (arrows pointing to the immunoreactivity of the endoplasmic reticulum; Fig.1 panels B, C, D) made contact with GFP-NPY immunoreactive cells (arrowheads). Thus, it would appear possible that the orexigenic NPY/AgRP neurons are exposed to locally formed T3.
Fig. 1.

DII-producing glia are associated with orexigenic neurons Panel A: Immunostaining for DII (red fluorescence) in GFP-NPY transgenic mice revealed a close association between DII-producing glial processes and NPY/AgRP neurons (green fluorescence). Panel B shows an electron micrograph of tanycytes immunostained with DII (arrowheads) in contact with a GFP-NPY immunoreactive cell. Panel C is a high power magnification micrograph showing a tanycyte (T) immunopositive for DII (arrows point to immunostaining in the endoplasmic reticulum) in contact (arrowheads) with an axon hillock (AH) of a GFP-NPY cell in the arcuate nucleus of the hypothalamus. Panel D shows another tanycyte immunostained for DII (arrow points to the immunoreactivity of the endoplasmic reticulum) in contact (arrowhead) with the same GFP-NPY cell of the arcuate nucleus. Bar scale in panel A represents 10 μm. Bar scale in panel B represents 1 μm. Bar scale in panel C (for D) represents 500 nm.
UCP2 in NPY/AgRP neurons
We previously showed that UCP2 immunoreactivity is expressed in the arcuate NPY neurons (Horvath et al., 1999). To confirm that UCP2 mRNA is present in these same cells, in situ hybridization for UCP2 was performed in NPY/GFP mice. UCP2 mRNA was expressed in various brain regions as previously reported (Richard et al., 1998; Horvath et al., 1999). Intense labeling was found in the hypothalamus where several nuclei showed a strong hybridization signal including the preoptic area, as well as the suprachiasmatic, paraventricular, and ventromedial nuclei. The intensity of the signal was markedly greater in the arcuate nucleus. When combined with immunocytochemistry for GFP/NPY, we found that GFP/NPY labeled neurons (light brown diaminobenzidine reaction) co-expressed UCP2 mRNA (silver grain representing UCP2 mRNA; Fig. 2A-B).
Fig. 2.

UCP2 in NPY/AgRP neurons Panel A-B: Representative light micrographs showing the results of combined in situ hybridization for UCP2 (silver grain representing UCP2 mRNA) and immunocytochemistry for GFP in NPY/GFP mice (light brown diaminobenzidine reaction product). Bar scale represents 10 μm.
Hypothalamic DII associated T3 levels are elevated during fasting
We have shown that during food deprivation, DII mRNA and activity levels are increased in the hypothalamus (Diano et al., 1998a; Coppola et al., 2005). Moreover, we recently demonstrated that the elevation in DII activity also induces increased tissue levels of T3 in rats (Coppola et al., 2005b). To confirm that this also occurs in mice, we performed T3 measurements in the hypothalamic tissues of fasted and fed mice. As reported earlier (Coppola et al., 2005b), hypothalamic T3 levels were significantly higher in fasted mice (4.77±1.045 pg/mg wet tissue) compared to ad libitum fed animals (2.29±0.21 pg/mg wet tissue; Fig.3A).
Fig. 3.

Fasting and T3 induce hypothalamic uncoupling protein 2 activity and mRNA levels and an increase in mitochondrial density Panel A: Graph showing hypothalamic T3 levels in wild type fed and 24 hour fasted animals. Values are expressed as mean±SE. Panel B: Fatty acid-induced uncoupling in fasted, fed, T3-treated and saline treated mice. Exposure to the free fatty acid palmitate induces increased mitochondrial uncoupling in fasted mice compared to their fed controls (left panel) and T3-treated compared to saline-treated mice (right panel). The increase in uncoupling activity is due specifically to the elevation in UCP2 since UCP2KO mice showed no alteration in uncoupling activity following either fasting (left panel) or T3 treatment (right panel). Data are expressed as the percentage of increase above oligomycin-induced state 4 respiration (±SE).
Panel C: Increase in hypothalamic UCP2 mRNA in fasted compared to fed controls and T3-treated compared to saline-treated mice (graph on the left). On the other hand, UCP2 mRNA was downregulated under hypothyroid conditions (graph on the right). The graphs show the expression levels of UCP2 mRNA(±SE) relative to the expression levels in fed or saline treated mice. D: Representative electron micrographs of GFP-NPY neurons in the hypothalamic arcuate nucleus of a fed and fasted mouse (upper micrographs), and T3-treated and saline-treated mouse (lower micrographs). Red points indicate some of the mitochondria which appear to be more numerous in fasted and T3-treated NPY/AgRP neurons compared to the values of their respective controls. Bar scale represents 1 μm. Unbiased stereology and statistical analyses confirmed that in fasted and T3-treated arcuate nuclei, the mitochondrial number in NPY/AgRP neurons (±SE) was significantly higher compared to the values of their respective controls. * P<0.05 compared to fed animals, saline-treated mice or hypothyroid controls.
Fasting or T3 administration induce hypothalamic uncoupling activity
We analyzed whether uncoupling activity of the arcuate nucleus is induced by fasting or T3 administration by measuring the uncoupling activity in isolated hypothalamic mitochondria of fasted, fed, T3-treated and saline-treated animals. Serum free T3 levels in T3 treated mice were significantly higher (27.14±2.5 pg/ml) than the saline controls (1.24±0.09 pg/ml). The ratio of hypothalamic FFA-induced uncoupling and state 4 respiration (baseline uncoupling) was significantly elevated in T3-treated (6.9±0.28 vs. 4.3±0.14 of saline-treated animals) and fasted wild type animals (5.96±0.16 vs. 4.16±0.19 of fed animals) (Fig.3B).
Increased hypothalamic mitochondrial uncoupling activity during fasting is UCP2-dependent and nucleotide-regulated
To analyze whether the increase in hypothalamic mitochondrial uncoupling activity was UCP2-dependent, GDP regulated, we analyzed the effect of fasting or T3 administration in hypothalamic uncoupling activity UCP2KO animals. Neither of these interventions altered mitochondrial uncoupling in UCP2 KO animals (Fig. 3B). To test whether changes in expression levels of the adenine nucleotide translocator (ANT), a mitochondrial protein that is regulated by thyroid hormones in the periphery (Dummler et al., 1996) and was shown to alter mitochondrial uncoupling (Brustovetsky et al., 1990; Skulachev, 1991, 1998; Brustovetsky and Klingenberg, 1994), may also be associated with increased hypothalamic uncoupling, we measured ANT mRNA levels by Real Time PCR after fasting or T3 treatment. Fasting did not alter ANT mRNA levels (0.83±0.03) compared to fed controls (0.79±0.06). On the other hand, T3-treatment induced a slight, but significant elevation of ANT mRNA levels (0.92±0.03) compared to saline controls (0.82±0.03). However, because T3 administration did not induce elevated fatty acid-driven mitochondrial uncoupling in UCP2 KO mice, it is reasonable to suggest that the role of ANT in fasting-induced hypothalamic mitochondrial uncoupling is negligible.
To analyze whether UCP2-dependent mitochondrial uncoupling during fasting is nucleotide-regulated, we measured uncoupling activity either in the presence or absence of GDP: when GDP was added to the respiration buffer, the fasting-induced increase in the ratio of palmitate-induced uncoupling and baseline uncoupling (5.96±0.16 vs. 4.16±0.19 of fed animals) was abolished (GDP-treated fasted: 4.14±0.31; GDP-treated fed: 4.31±0.002; P=0.64).
T3 regulates hypothalamic UCP2 expression
To test whether a relationship exists between hypothalamic T3 levels and UCP2 transcription, we measured UCP2 mRNA levels in the hypothalamus of fasted, T3-treated and propyl-thiouracil (PTU)-plus iopanoic acid (IOP)-treated mice. Fasting (1.44±0.2 versus 0.9±0.07 of fed mice) and T3 treatment (1.40±0.11 versus 0.7±0.2 of saline mice) induced a significant increase in hypothalamic UCP2 mRNA levels (Fig. 3C). Under hypothyroid conditions, in which the production of thyroid hormones as well as the activity of all the deiodinases were inhibited (by PTU or IOP plus PTU, respectively), hypothalamic UCP2 mRNA levels were significantly lower (0.45±0.15) than those of euthyroid control mice (1.06±0.1; Fig.3C).
Increased mitochondrial uncoupling is associated with elevated mitochondrial density in NPY/AgRP neurons
Increased mitochondrial uncoupling induces mitochondrial proliferation both in brown fat (Klingenspor, 2003 for review) and brain (Diano et al., 2003b). Thus, we tested whether increased mitochondrial density is also associated with increased UCP2 activity in the arcuate nucleus during fasting or T3 administration by stereological assessment of mitochondrial number in NPY/AgRP neurons of fasted and fed NPY/GFP mice and saline and T3-treated mice. In NPY/AgRP neurons, fasting induced approximately a 200% rise in mitochondrial number (Fig.3D). Similarly, in the NPY/AgRP neurons of T3-treated mice, the number of mitochondria was 160% higher than their saline-treated controls (Fig.3D).
The lack of UCP2 blunts NPY/AgRP neuronal responses to fasting.
c-fos activation
To analyze whether the lack of UCP2 could affect the activation of arcuate neurons, we first examined c-fos mRNA in the arcuate nucleus of UCP2KO and wild type controls after 24h fasting. We found that c-fos mRNA induction by fasting was blunted in UCP2KO animals compared to wild type littermates (Fig. 4A). In accordance with the mRNA study, immunocytochemistry for c-fos in the arcuate nucleus showed that UCP2KO mice exhibited just 41% of c-fos staining compared to their wild type controls (Fig. 4B). Next, we performed double immunocytochemistry for NPY/GFP and c-fos and observed that in wild types, 71% of the NPY/GFP cells were c-fos positive, while in the UCP2KO mice, only 27% of this cell population expressed c-fos (Fig.4B). It is worthy to note that no difference in the number of NPY neurons in the wild type controls and UCP2KO animals was found (data not shown).
Fig. 4.
Lack of UCP2 decreases c-fos expression in NPY/AgRP neurons and prevents the elevation of NPY mRNA levels during fasting. Panel A shows representative blackfield micrographs of c- fos mRNA expression in the arcuate nucleus of wild type (WT) and UCP2KO mice following 24 hours of fasting. The graph shows the significant decrease in c-fos mRNA (±SE) in the UCP2KO mice compared to their wild type controls. *P<0.05. Bar scale represents 100 μm. Arc= Arcuate Nucleus; 3v=third ventricle. Panel B: Low and high power magnification of c-fos and GFP staining in the arcuate nucleus of GFP/NPY mice (WT) and UCP2KO/GFP-NPY mice (UCP2KO) following 24 hours of fasting. Note the difference in the amount of c-fos staining in the wild type compared to the UCP2KO mouse. Arrows show double labeled cells for GFP and c-fos, while arrowheads indicate GFP immunopositive neurons that are negative for c-fos staining. Graphs show the percentage of c-fos immunopositive nuclei (±SE) in the arcuate nucleus of UCP2KO mice compared to that of wild type controls and the percentage of cells double stained for c-fos and GFP in GFP/NPY mice and UCP2KO-GFP/NPY mice compared to the total number of GFP/NPY neurons within the arcuate nucleus. It is worthy to note that no difference in the number of NPY neurons in the wild type controls and UCP2KO animals was found (data not shown). Bar scales in low and high power magnification micrographs represent 50 μm and 10μm, respectively. 3v=third ventricle; Arc=Arcuate Nucleus; ME= Median Eminence. *P<0.001 compared to wild type controls. Panel C: Graph showing the expression levels of NPY mRNA relative to the expression levels in fed wild type and fed UCP2KO mice (±SE). Following fasting, wild type animals exhibited an increase in NPY mRNA levels, while fasted UCP2KO mice did not show this elevation.
NPY and POMC gene expression in fed and fasted wild type and UCP2KO mice.
To assess whether the lack of UCP2 would impair the induction of NPY mRNA during fasting, we performed real time PCR on hypothalamic tissues from wild type and UCP2KO mice in fed and fasted conditions. In wild type animals fasting significantly increased NPY mRNA levels (2.51±0.17; Fig 4C) compared to animals fed ad libitum (1.03±0.26; Fig. 4C). On the other hand, in UCP2KO mice, fasting did not induce the increase in NPY mRNA (1.24±0.3; Fig. 4C) seen in wild type mice (1.18±0.16; Fig.4C). Note, however, that no difference in NPY mRNA levels was found in fed wild type and UCP2KO animals.
We then analyzed POMC mRNA and found that in contrast to NPY, POMC mRNA levels in UCP2KO animals showed the same changes than the wild type controls. In wild type mice, fasting induced a decrease in POMC mRNA (0.62±0.12) compared to fed controls (1.004±0.05). Similarly, fasted UCP2KO animals showed a significant reduction of POMC mRNA (0.66±0.03) compared to fed UCP2KO mice (1.011±0.06).
Mitochondrial density
Coinciding with the lack of UCP2 activation by fasting, the mitochondrial number within the NPY neurons did not change after a 24h fast in UCP2 KO animals (0.19±0.02 μm2 in fed UCP2KO and 0.16±0.02 μm2 in fasted UCP2KO; P=0.2). When we compared the mitochondrial number of fasted UCP2KOs with their fasted wild type littermates, UCP2KO mice exhibited only 30% of the number of mitochondria seen in the wild type controls (Fig.5A).
Fig. 5.
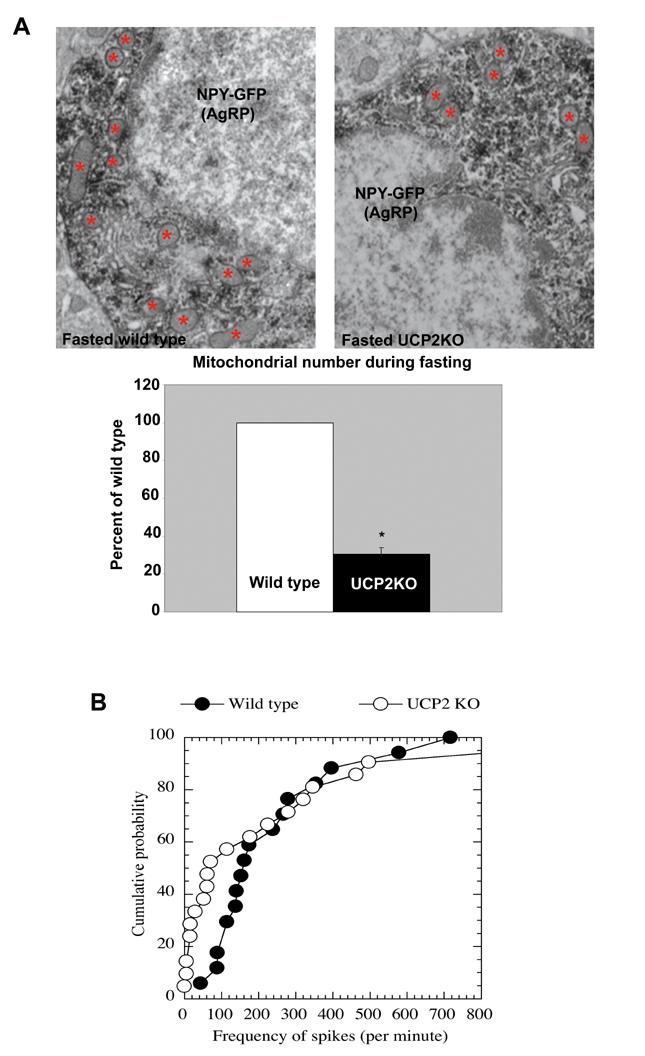
Lack of UCP2 prevents fasting-induced increase in mitochondrial density and alters electrical properties. Panel A: Representative electron micrographs of GFP-NPY neurons in the hypothalamic arcuate nucleus of 24 hour-fasted wild type and UCP2KO mice. Red points indicate some of the mitochondria which appear to be less numerous in fasted UCP2KO NPY/AgRP neurons compared to the values of the controls. Bar scale represents 1 μm. Unbiased stereology and statistical analyses confirmed this observation. * P<0.05 compared to wild type mice. Panel B: Cumulative probability curves for frequency of spikes recorded from arcuate NPY/GFP neurons of fasted wild type and fasted UCP2KO mice shows that frequency of spike firing is significantly lower in UCP2 KO mice than in wild type controls. The median frequency of spikes of NPY/GFP neurons were 157 per minute for wild type and 71 per minute for UCP2KO mice. The distribution of the frequency of spikes from these two groups are significantly different as indicated by the Kolmgorov-Smirnov test (P<0.05).
Electrophysiology
To determine whether the electrical activity of NPY neurons is also affected in UCP2KO mice during fasting, we analyzed the firing rate of GFP/NPY neurons. In order to keep intracellular content intact to eliminate the rundown of action potentials during the entire experiment, extracellular recording of spikes was performed in fasted wild type and fasted UCP2 KO mice. We found that during fasting in both wild type and UCP2KO mice, the firing rate of the arcuate NPY neurons was highly heterogeneous. After fasting, the firing rate was 44 to 717 spikes per minute (median frequency= 157 per minute, n=16) in wild type mice and 0 (no spikes) to 1029 spikes per minute (median frequency= 71 per minute, n=21) in UCP2KO mice. Thus, we generated cumulative probability curves for frequencies of spikes recorded from wild type and UCP2KO mice, as shown in Fig 5B. The cumulative probability curve for UCP2KO mice shifted to the left, compared to that of wild type mice. The distribution of the frequencies of spikes from these two groups was significantly different as indicated by the Kolmgorov-Smirnov test (P<0.05). In summary, our data show that the frequency of spike firing is significantly lower in UCP2 KO mice than in wild types after fasting, which is reflected by relatively more NPY neurons firing at a low frequency.
Diminished rebound feeding in animals lacking either UCP2 or DII.
It has been shown that NPY/AgRP neurons are mandatory for feeding to occur (Gropp et al., 2005; Luquet et al., 2005). Thus, to test whether UCP2-dependent cellular mechanisms in NPY neurons are important for appropriate feeding responses, we analyzed the rebound feeding of UCP2KO and wild type animals after 24 hour fasting. The initial feeding response of UCP2 KO animals to a 24 h fast was diminished (0.52±0.13g; 0.35±0.03g after the first hour of food) compared to the values of fasted wild type littermates (0.86±0.09 g; 0.54±0.05 g after the second hour of food; Fig.6A). Note, however, that the normal feeding behavior of these animals is not affected nor do they show visible signs of gross metabolic alterations (Arsenijevic et al., 2000; Zhang et al., 2001). These observations indicate that UCP2 may be necessary for activation of the arcuate nucleus orexigenic NPY/AgRP neurons to evoke an appropriate response to fasting.
Fig. 6.

Diminished rebound feeding in animals lacking either UCP2 or DII. Panel A shows graphs of the percentage of food intake (±SE) during the first and second hours after 24hrs of fasting in UCP2KO mice in relation to wild type values. *P<0.05 compared to wild type controls. Panel B shows graphs of the percentage of food intake (±SE) after 24 hours of fasting in DIIKO mice and DIIKO mice injected with T3, compared to their wild type controls between 0-30 minutes and 30-60 minutes after re-feeding. *P<0.05 compared to wild type controls; # P<0.05 compared to DIIKO. Panel C shows graphs of the hypothalamic T3 levels and NPY mRNA levels (±SE) measured in fed and fasted wild type and DIIKO mice. * P<0.05 compared to wild type animals.
Because we showed that DII-produced T3 plays a role in UCP2 activation during fasting, we analyzed the acute responses of DII knockout animals (DIIKO; Schneider et al., 2001) to a 24h fasting regimen. Fifteen male DIIKO and 6 male wild type animals were fasted. Six hours before food was replaced, 7 DIIKO mice were injected i.p. with T3. Food was replaced after 24 h at which time the body weight and core body temperature were recorded and food intake was measured in a 30 minute period following re-feeding. Food intake at the end of the 24 h fast was significantly lower in DIIKO animals compared to wild type littermates in the first 30 minutes after re-feeding (0.38±0.05 g in DIIKO; 0.65±0.02 in the wild type; Fig.6B). When DIIKO mice were treated with T3, food intake was significantly increased compared to DIIKO saline-treated mice (0.57±0.05) in both the first and second 30 minute periods after re-feeding (0.39±0.05g in DIIKO T3-treated; 0.18±0.05 g in DIIKO Saline-treated; 0.24±0.02 g in the wild type after the second hour of food; Fig.6B).
The lack of DII prevents the increase in hypothalamic T3 and NPY mRNA levels during fasting
Finally, we analyzed whether the reduction in food intake after fasting seen in DIIKO mice was associated with hypothalamic T3 and NPY mRNA levels.
First, the serum levels of free T4 and free T3 in wild types and DIIKOs were analyzed. As previously reported, fasting decreased serum T4 and T3 levels (3.3±0.06 ng/dl and 0.35±0.021 pg/ml, respectively) compared to fed ad libitum conditions (6.8±1.1 ng/dl for T4 and 0.51±0.020 pg/ml for T3). Similarly, fasted DIIKO animals showed a significant reduction in serum T4 and T3 levels (4.9±0.6 ng/dl and 0.33±0.023 pg/ml, respectively) compared to fed DIIKO mice (12.6±1.2 ng/dl for T4 and 0.46±0.026 pg/ml for T3). When we examined hypothalamic T3 levels in fasted DIIKO mice, no changes were observed compared to fed DIIKO animals (2.39±0.30 pg/mg wet tissue versus 2.11±0.50 pg/mg wet tissue, respectively; Fig. 6C). Note that no difference in hypothalamic T3 levels was observed between fed wild type and fed DIIKO mice. In addition, supporting the feeding data, hypothalamic NPY mRNA levels in fasted DIIKO mice (1.37±0.23; Fig 6C) did not differ compared to the levels of fed DIIKOs (1.05±0.18; Fig. 6C). In contrast to NPY, POMC mRNA levels in fasted DIIKO were significantly decreased (0.72±0.05) compared to fed DIIKO controls (1.004±0.04). This reduction was comparable to that observed in wild type animals (1.003±0.03 in fed WTs and 0.63±0.09 in fasted WTs).
Discussion
Our results present evidence of a physiological role for UCP2 in hypothalamic neuronal functions. The observed increase in the activity of hypothalamic mitochondrial uncoupling protein 2 is thyroid hormone-regulated and nucleotide (GDP)-dependent. This T3-regulated mitochondrial uncoupling by UCP2 was found to be critical for the appropriate activation of arcuate nucleus NPY/AgRP neurons during fasting, thereby altering rebound feeding in mice. Because we found that mitochondrial proliferation within NPY/AgRP neurons is inherent to the response to negative energy balance and is UCP2-dependent, we suggest that neuronal UCP2 in the hypothalamus plays an important role in the adaptation of these neurons to changing metabolic demand. These observations provide experimental evidence for the hypothesis that neuronal UCP2 plays a physiological role in the regulation of neuronal functions (Horvath et al., 1999; Horvath et al., 2002a; Horvath et al., 2002b; Diano et al., 2003; Horvath et al., 2003; Andrews et al., 2005b; Andrews et al., 2006).
We first revealed a direct relationship between DII-containing (putative T3-producing) glial elements and the NPY/AgRP-producing arcuate nucleus neurons. We also found that NPY cells express UCP2, and that the expression level and activity of mitochondrial uncoupling protein 2 are enhanced by fasting or T3 alone, but reduced under hypothyroid conditions. It has been previously demonstrated that T3 increases UCP2 mRNA and activity in peripheral tissues such as white adipose tissue, skeletal muscle and heart (Masaki et al., 1997; Lanni et al., 1997). Our results now show that similar to the periphery, T3 also alters mitochondrial UCP2 activity in the hypothalamus. Mice lacking UCP2 did not exhibit the enhanced uncoupling activity seen following either fasting or T3 treatment. In addition, free fatty acid-induced GDP-sensitive uncoupling was prevented in fasted wild type animals compared to their fed controls, a strong indication that UCP2, rather than other uncouplers, is responsible for the increased uncoupling activity observed in our study.
An increase in mitochondrial number was also observed in the arcuate NPY/AgRP neurons after fasting and T3 treatment, an event that was absent in UCP2 KO animals. This observation is in line with our earlier demonstration of elevated mitochondrial number in hippocampal pyramidal cells of human UCP2/3 overexpressing mice (Diano et al., 2003). Whether this change in mitochondrial number relates to fission and fusion of mitochondria or if it also entails de novo mitochondrial biogenesis, needs to be further explored. Mitochondrial density increases in neuronal sites with increased energy demand in order to support neurotransmission (Shepherd and Harris 1998; Rowland et al. 2000; Li et al., 2004; Schuman and Chan, 2004). Thus, we suggest that the increase in mitochondrial density in the cell bodies of the NPY neurons is important to provide and maintain the high level of activity of these orexigenic neurons in response to food deprivation. Indeed, such an acute activation of the NPY cells is seen during fasting (Takahashi and Cone, 2005). We propose that although individual mitochondria may be less efficient in producing ATP, overall, due to their increased number, cellular ATP levels may actually rise (Diano et al., 2003; Andrews et al., 2005). This, in turn, could play an important role in sustaining the activity of these neurons during negative energy balance. In accordance with this, we observed that during fasting, c-fos mRNA levels in the arcuate nucleus of UCP2KO mice were significantly reduced, and, arcuate NPY neurons of these animals exhibited a median frequency of spikes per minute significantly lower than that of wild type animals. It is interesting to note, however, that NPY neurons in the arcuate nucleus showed a heterogeneity in their electrophysiological response to fasting suggesting that this neuronal population is more diverse in its functions than previously thought.Strengthening our conclusions that there exists an interplay between arcuate T3 and UCP2 in the hypothalamic regulation of energy balance, both DII knockout mice as well as UCP2 KO animals had impaired elevation in NPY mRNA levels and rebound feeding following fasting.
The arcuate nucleus has emerged as a key region in the central regulation of energy metabolism housing the cell bodies of the melanocortin system, the NPY/AgRP neurons and the POMC-producing perikarya. Under satiety conditions, the adipose hormone, leptin, downregulates the activity of the NPY/AgRP neurons and promotes α-MSH neuronal activity (for review see Schwartz et al., 2000; Zigman and Elmquist, 2003; Horvath and Diano, 2004; Stanley et al., 2005). During fasting, leptin levels rapidly decline while circulating corticosteroid and ghrelin levels rise which is associated with increased NPY/AgRP and decreased α-MSH neuronal stimulation (Schwartz et al., 2000; Zigman and Elmquist, 2003). While ghrelin can directly alter the neuronal activity of the melanocortin system (Cowley et al., 2003), elevated corticosteroid levels will trigger glial DII activity (Coppola et al., 2005a) in hypothalamic tanycytes and astrocytes, and, consequently, the local production of T3 (Coppola et al., 2005b). Our present results indicate that this increase in hypothalamic T3 production may have an indirect action on neuronal functions mediated by UCP2, and that this process plays a role in the physiological regulation of feeding.
In summary, we suggest that hypothalamic DII-derived T3 triggers activation of neuronal UCP2 that, in turn, elevates the activity level of NPY/AgRP neurons. Our results indicate that this mechanism is critical in sustaining an increased firing rate in these orexigenic cells so that appetite remains elevated during fasting (Fig.7). Overall, our study provides strong evidence for an interplay between local T3 production and UCP2 during fasting and reveals a central thermogenic-like mechanism (Horvath et al., 1999; Andrews et al., 2005b) in the regulation of food intake (Fig.7).
Fig. 7.

Interplay between arcuate nucleus DII and UCP2 in fuel sensing Under fed (a) conditions, peripheral anorexigenic hormones, such as leptin, control the melanocortin system. During fasting (b), anorexigenic hormones, such as ghrelin, will reverse the tone of the melanocortin system so that NPY/AgRP neuronal activity will dominate. At the same time, elevating corticosteroid levels will trigger DII activity and local T3 production, which will trigger UCP2 production and activity in mitochondria (red) in NPY/AgRP neurons. The elevated activity of NPY/AgRP neurons will suppress POMC tone. We predict that by the time of re-feeding (c), activated UCP2 will have increased mitochondrial number in NPY/AgRP neurons which, in turn, will be a critical factor in sustaining an increased firing rate by these orexigenic cells so that food intake will remain elevated and POMC neuronal firing will stay suppressed subsequent to re-feeding, at a time when neither circulating orexigenic nor anorexigenic signals dominate.
Experimental Procedures
Double immunocytochemistry for DII and GFP.
Adult C57/Bl6 mice carrying GFP in NPY/AgRP neurons (Pinto et al., 2004) were used in this study. Animals were perfused as previously described (Pinto et al., 2004)
For immunofluorescent microscopy, brain sections were immunostained with rabbit anti-DII (1:500) and subsequently with Alexa Fluor 594 donkey anti-rabbit IgG (Molecular Probes, Eugene, OR).
For electron microscopy, the immunolabeling for DII was carried as previously reported (Diano et al., 2003). Sections were further incubated with a mouse anti-GFP antibody (Molecular Probes, Eugene OR; 1:4000) and the immunoreactivity was visualized with DAB. Ribbons of ultrathin sections (Leica Ultramicrotome) were collected and examined using an FEI Biotwin electron microscope.
Combined GFP immunostaining and in situ hybridization for UCP2
C57/Bl6 mice carrying GFP in NPY/AgRP neurons were sacrificed by transaortic perfusion as previously reported (Richard et al., 1998)
Sections were first immunostained with a mouse anti-GFP antibody (Molecular Probes, Eugene OR; 1:4000; immunoreactivity for GFP was visualized by DAB resulting in a brown reaction product) and then processed for in situ hybridization for UCP2 as previously reported (Richard et al., 1998).
Determination of hypothalamic and serum T3 levels
Ten male mice were sacrificed and the hypothalamus was dissected, weight recorded and stored at -80°C. Hypothalamic triiodothyronine (T3) was measured as previously described (Coppola et al, 2005b) T3 was expressed in pg per mg of wet tissue weight.
Serum free T3 levels were measured by RIA kit (Diagnostic Products Corporation, Los Angeles, CA) according to the manufacture’s protocol.
Brain mitochondria preparation and uncoupling activity measurements
Thirty-two male wild type mice and 32 UCP2KO mice were used in this study and divided into the following groups: 8 mice were single-injected with T3 (5 μg); 8 mice were injected with saline; 8 mice were fasted for 24 hours and 8 mice were fed ad libitum. Mice injected with saline or T3 were sacrificed 6 hours after the injection. Animals were decapitated under deep anesthesia and hypothalami were dissected. Mitochondria were isolated as previously reported (Andrews et al., 2005b) Protein concentrations were determined by a BCA protein assay kit (Pierce, Rockford, IL). Mitochondrial respirations were assessed using a Clark-type oxygen electrode (Hansatech Instruments, Norfolk, UK) at 37°C as previously described (Andrews et al., 2005b). UCP-mediated proton conductance was measured as increased fatty acid-induced respiration (Echtay et al., 2002), which was then compared with state 4 respiration induced by oligomycin, an inhibitor of H+-transporting ATP synthase. All the above determinations were performed both in the presence and absence of exogenous GDP (500 μM) in the respiration buffer.
Real Time-PCR for UCP2 mRNA in fasted, T3-treated and PTU+IOP-treated mice
Thirty male C57/Bl6 were used in this study and divided in the following groups: 5 mice injected ip with T3 (5 μg), 5 controls injected with saline, 5 mice 24 hour-fasted, 5 mice fed controls, 5 hypothyroid and 5 euthyroid mice. Hypothyroidism was induced in mice by a daily ip. injection of 6-n-propylthiouracil (PTU) (10 mg/kg body weight), together with iopanoic acid (IOP) (100 mg/kg body weight) for 14 days. Euthyroid controls received a daily injection of vehicle control for 14 days.T3- and saline treated animals were sacrificed six hours after the injection; the hypothalamus was dissected and processed for RNA extraction.
Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Reverse transcription was performed by the First-Strand cDNA Synthesis kit (Amersham Biosciences, Piscataway, NJ) using 3 μg of total RNA in 15 μl of total volume. Real time PCR analysis of UCP2 mRNA levels was performed by using iQ SYBR Green Supermix (BIORAD) and 0.5 μM of each primer (sense primer: 5′-CTACAAGACCATTGCACGAGAGG-3′ and reverse 5′-AGCTGCTCATAGGTGACAAACAT-3′). Primers against 18S RNA were included as controls. Measurements were performed on iCycler BIORAD.
Quantification of mitochondria in GFP-NPY/AgRP cells of the arcuate nucleus
For this experiment, we used adult C57/Bl6 mice carrying GFP in NPY/AgRP neurons (Pinto et al., 2004) that were divided in 2 groups: 5 mice were fasted for 24 hours and 5 mice were fed ad libitum. Animals were perfused as described above and their brains were processed for GFP immunolabeling for electron microscopic examination. The unbiased stereological method for the assessment of mitochondrial number that was used is based upon our published paper (Pinto et al., 2004). The central feature of this approach is the use of a systematic random sampling method, which meets the statistical requirements necessary to insure an unbiased estimate of the feature of interest. In selecting a sample series, the first section to be analyzed was randomly selected from an initial interval of sections, which then defined the spacing of the remaining sections to be examined. Thus, if we chose to sample every 10th section to measure mitochondrial number, the starting point of the series for each animal is randomly selected among the first 10 sections and then every tenth section was taken from this starting point through the remainder of the series. Having defined a series of sections with equal spacing of a known distance (t), it is then possible to estimate the volume of the structure of interest using the Cavalieri method (Gundersen and Jensen, 1987; Gundersen et al., 1988). Briefly, a point counting grid with a defined area between points a(p) which is corrected for the magnification at which the section is viewed or drawn, was superimposed over the drawing or image of each section in the series. The number of grid points intersecting the structure of interest (*P) is then calculated for each section, and the volume (V) of the region of interest (ROI) is calculated by the formula: V=*P* a(p)*t. The assessment of mitochondrial number was determined using the optical dissector method on electron micrographs. Here, sections to be sampled were selected as above, and smaller grid areas were chosen from within each section in a similar systematic random manner. These areas (typically 100μm2), were then used to count mitochondria in GFP-immunopositive cells. Within each area, an optical section was established between the surfaces of the tissue section, thus creating a three dimensional sampling area of known dimensions. All mitochondrial planes within this area were counted provided they did not cross three of the borders of the sampling boxes considered as exclusionary borders. The counts obtained from these sampling boxes were determined and represent the number of mitochondria per unit volume of the structure of interest. These counts were then corrected for the total volume of the structure obtained with the Cavalieri method, yielding a value for total mitochondrial number for the cell class and structure sampled.
In situ hybridization for c-fos in UCP2KO mice
In situ hybridization was performed on adult UCP2 KO mice and their WT littermates (n=4) fasted for 24 hours. Twenty-five-μm-thick sections were processed for c-fos in situ hybridization as described by Timofeeva E et al., 2003.
Double immunostaining for c-fos and GFP in fasted UCP2KO and wild type mice
Five adult UCP2KO mice carrying GFP in NPY/AgRP neurons and five wild types carrying GFP in NPY/AgRP neurons were single housed and fasted for 24 hours. Animals were then perfused as described above.
The brain sections were incubated with rabbit anti-c-fos (1:20,000 in PB-Triton X-100; Calbiochem, San Diego, CA) overnight at room temperature and then incubated in Alexa Fluor 594 donkey anti-rabbit IgG (1:200; Molecular Probes, Eugene, OR). The number of c-fos immunopositive nuclei as well as the number of cells double immunostained for c-fos and GFP were counted using ScionImage (NIH).
Real Time PCR for NPY mRNA inUCP2KO, WT controls and DIIKO and WT control mice
Forty male mice were used in this study and divided into the following groups: 5 UCP2KO mice 24 hour-fasted, 5 UCP2KO mice fed controls, 5 WT mice 24 hour-fasted, 5 WT mice fed controls, 5 DIIKO mice 24 hour-fasted, 5 DIIKO mice fed controls, 5 WT mice 24 hour-fasted, 5 WT mice fed controls animals. Mice were sacrificed and hypothalami were dissected. Total RNA extraction and reverse transcription were performed as described above. Real time PCR analysis of NPY, POMC and ANT mRNA levels was performed by using iQ SYBR Green Supermix (BIORAD) and 0.5 μM of each primer (NPY sense primer: 5′-GCTAGGTAACAACGAATGGGG; NPY antisense primer: CAC ATG GAA GGG TCT TCA AGC -3′; POMC sense primer: GGC CTT TCC CCT AGA GTT CA; POMC antisense primer: TTG ATG ATG GCG TTC TTG AA; ANT sense primer: GGA CAG ATT CTC TGG GCT TG; ANT antisense primer TGG AAA TGG CTT TAA GAG AAA AC). Primers against 18S RNA were included as controls. Measurements were performed on iCycler BIORAD.
Quantification of mitochondria in the arcuate GFP-NPY/AgRP neurons of UCP2KO and WT controls after 24 hours of food deprivation
Adult UCP2KO mice and WTs carrying GFP in NPY/AgRP neurons were used. Five UCP2KO and 5 WT mice were fasted for 24 hours. Animals were perfused as described above and their brains were processed for GFP immunolabeling for electron microscopic examination as described above. The unbiased stereological method for the assessment of mitochondrial number was used as described above.
Firing rate of fasted NPY/AgRP neurons in UCP2KO and wild type mice.
NPY/GFP animals were sacrificed. Brains were rapidly removed, immersed in cold (4°C) oxygenated bath solution (containing (mM): sucrose 220, KCl 2.5, CaCl2 1, MgCl2 6, NaH2PO4 1.25, NaHCO3 26, and glucose 10, pH 7.3 with NaOH) and trimmed to a large block containing the hypothalamus. Coronal slices (180 μm) were cut through the full extent of the arcuate nucleus and were maintained in a holding chamber with ASCF (bubbled with 5% CO2 and 95% O2) containing (in mM): NaCl 124, KCl 3, CaCl2 2, MgCl2 2, NaH2PO4 1.23, NaHCO3 26, glucose 2.5, pH 7.4 with NaOH. Slices were transferred to a recording chamber after at least 1hr recovery, and constantly perfused with bath solution (33°C) at 2 ml/min. Slices were maintained for 1 hr at 35°C in 95% O2 5% CO2 saturated ACSF prior to recordings. We routinely got six usable slices containing GFP neurons from each mouse. A slice was then placed on the stage of the upright, hybrid fluorescence/infrared microscope with long working distance objectives (Olympus BX51WI), and the presence of fluorescent cells was verified. Fluorescence filter sets appropriate for Sapphire FP, cyan FP and GFP (Chroma Technology Corp) were used to confirm cell fluorescence. The slices were perfused with oxygenated ACSF at 35 °C. Using established criteria, an appropriate healthy, fluorescent cell was visually selected. Extracellular recordings were made with a glass electrode filled with ACSF (resistance=2-5 M Instruments, Inc.) in the hypothalamic slices from mice. The recording electrode was propelled by a motorized micromanipulator to approach an identified NPY-GFP neuron. A loose seal was formed (resistance= 10-20ΩM when the micropipette touched the surface of a neuron. Ten minutes of spontaneous spikes was recorded from each neuron. Cumulative probability curves of frequency of spikes obtained from wild type and UCP2KO mice were plotted by using KaleidaGraph and examined with the Kolmgorov-Smirnov test in software GB-Stat.
Fasting and re-feeding in UCP2KO mice.
Five male UCP2KO and 5 wild type mice were fasted for 24 hours. Food was replaced after 24 h at which time food intake was measured in a 60 minute period following re-feeding.
Fasting and re-feeding in DII KO mice.
Fifteen male DIIKO and 6 wild type mice (Schneider et al., 2001) were fasted for 24 hours. Six hours before food was replaced, 7 DIIKO mice were injected i.p. 7 DIIKO mice and 6 wild type mice were injected with saline. Food was replaced 24 h after fasting at which time food intake was measured in a 30 minute period following re-feeding.
Acknowledgements
To Nicola Diano con l’amore di sempre. We thank Drs. PR Larsen and VA Galton for the DIIKO animals, Erzsebet Borok for her superb technical assistance and Marya Shanabrough for editing the manuscript. This work was supported by NIH grants DK 061619 and DK 070039 to S.D., DK 070723 to X.B.G., DK 074386, DK 060711 and AG 022880 to TLH and by the Juvenile Diabetes Research Foundation to S.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci. 2005a;6:829–40. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- Andrews Z, Horvath B, Barnstable CJ, Elseworth J, Yang L, Beal MF, Roth RH, Matthews RT, Horvath TL. UCP2 is critical for nigral dopamine cell survival in a mouse model of Parkinson’s disease. Journal of Neuroscience. 2005b;25:184–191. doi: 10.1523/JNEUROSCI.4269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Rivera A, Elsworth J, Roth R, Agnati L, Gago B, Abizaid A, Schwartz M, Fuxe K, Horvath TL. Uncoupling protein-2 promotes nigrostriatal dopamine neuronal function. European Journal of Neuroscience. 2006;24(1):32–6. doi: 10.1111/j.1460-9568.2006.04906.x. [DOI] [PubMed] [Google Scholar]
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26(4):435–9. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- Bassett JH, Harvey CB, Williams GR. Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol. Cell Endocrinol. 2003;213:1–11. doi: 10.1016/j.mce.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2(2):85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Brustovetsky NN, Dedukhova VI, Egorova MV, Mokhova EN, Skulachev VP. Inhibitors of the ATP/ADP antiporter suppress stimulation of mitochondrial respiration and H+ permeability by palmitate and anionic detergents. FEBS Lett. 1990;272:187–189. doi: 10.1016/0014-5793(90)80480-7. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Klingenberg M. The reconstituted ADP/ATP carrier can mediate H+ transport by free fatty acids, which is further stimulated by mersalyl. J Biol Chem. 1994;269:27329–36. [PubMed] [Google Scholar]
- Coppola A, Meli R, Diano S. Inverse shift in circulating corticosterone and leptin levels elevates hypothalamic deiodinase type 2 in fasted rats. Endocrinology. 2005a;146:2827–2833. doi: 10.1210/en.2004-1361. [DOI] [PubMed] [Google Scholar]
- Coppola A, Hughes J, Esposito E, Schiavo L, Meli R, Diano S. Suppression of hypothalamic deiodinase type II activity blunts TRH mRNA decline during fasting. FEBS Letters. 2005b;579:4654–4658. doi: 10.1016/j.febslet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Diano S, Naftolin F, Goglia F, Horvath TL. Fasting-induced increase in type II iodothyronine deiodinase activity and messenger ribonucleic acid levels is not reversed by thyroxine in the rat hypothalamus. Endocrinology. 1998a;139:2879–2884. doi: 10.1210/endo.139.6.6062. [DOI] [PubMed] [Google Scholar]
- Diano S, Naftolin F, Goglia F, Horvath TL. Monosynaptic pathway between the arcuate nucleus expressing glial type II iodothyronine 5′-deiodinase mRNA and the median eminence-projective TRH cells of the rat paraventricular nucleus. J. Neuroendocrinol. 1998b;10:731–742. doi: 10.1046/j.1365-2826.1998.00204.x. [DOI] [PubMed] [Google Scholar]
- Diano S, Leonard J, Meli R, Esposito E, Schiavo L. Hypothalamic type II iodothyronine deiodinase: a light and electron microscopic study. Brain Research. 2003a;976:130–134. doi: 10.1016/s0006-8993(03)02692-1. [DOI] [PubMed] [Google Scholar]
- Diano S, Matthews RT, Patrylo P, Yang L, Beal MF, Barnstable CJ, Horvath TL. Uncoupling protein 2 prevents neuronal death including that occurring during seizures: a mechanism for preconditioning. Endocrinology. 2003b;144:5014–5021. doi: 10.1210/en.2003-0667. [DOI] [PubMed] [Google Scholar]
- Dummler K, Muller S, Seitz HJ. Regulation of adenine nucleotide translocase and glycerol 3-phosphate dehydrogenase expression by thyroid hormones in different rat tissues. Biochem J. 1996;317:913–918. doi: 10.1042/bj3170913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol. Behav. 2001;74:703–708. doi: 10.1016/s0031-9384(01)00613-8. [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Bruning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–91. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Harvey CB, Williams GR. Mechanism of thyroid hormone action. Thyroid. 2002;12(6):441–446. doi: 10.1089/105072502760143791. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Warden CH, Hajos M, Lombardi A, Goglia F, Diano S. Brain UCP2: uncoupler neuronal mitochondria predict thermal synapses in homeostatic centers. J Neuroscience. 1999;19:10417–10427. doi: 10.1523/JNEUROSCI.19-23-10417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B, Spies C, Warden C, Diano S, Horvath TL. Uncoupling protein 2 in primary pain and temperature afferents of the spinal cord. Brain Research. 2002;955:260–263. doi: 10.1016/s0006-8993(02)03364-4. [DOI] [PubMed] [Google Scholar]
- Horvath B, Spies C, Horvath Gy, Kox WJ, Miyamoto S, Barry S, Warden CH, Bechmann I, Diano S, Heemskerk J, Horvath TL. Uncoupling protein 2 (UCP2) lowers alcohol sensitivity and pain threshold. Biochem Pharm. 2002;64:369–374. doi: 10.1016/s0006-2952(02)01167-x. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S, Miyamoto S, Barry S, Gatti S, Alberati D, Livak F, Lombardi A, Moreno M, Goglia F, Mor G, Hamilton J, Kachinskas D, Horwitz B, Warden CH. Uncoupling proteins-2 and 3 influence obesity and infIammation in transgenic mice. International Journal of Obesity. 2003;27:433–442. doi: 10.1038/sj.ijo.0802257. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S. The floating blueprint of hypothalamic feeding circuits. Nat. Rev. Neurosci. 2004;5:662–667. doi: 10.1038/nrn1479. [DOI] [PubMed] [Google Scholar]
- Horrum MA, Tobin RB, Ecklund RE. Thyroid hormone effects on the proton permeability of rat liver mitochondria. Mol. Cell Endocrinol. 1990;68:137–41. doi: 10.1016/0303-7207(90)90186-c. [DOI] [PubMed] [Google Scholar]
- Kalderon B, Hermesh O, Bar-Tana J. Mitochondrial permeability transition is induced by in vivo thyroid hormone treatment. Endocrinology. 1995;136:3552–3556. doi: 10.1210/endo.136.8.7628392. [DOI] [PubMed] [Google Scholar]
- Klingenspor M. Cold-induced recruitment of brown adipose tissue thermogenesis. Exp. Physiol. 2003;88(1):141–148. doi: 10.1113/eph8802508. [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 2005;6(3):248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- Lanni A, Moreno M, Lombardi A, Goglia F. Thyroid hormone and uncoupling proteins. FEBS Lett. 2003;543:5–10. doi: 10.1016/s0014-5793(03)00320-x. [DOI] [PubMed] [Google Scholar]
- Lanni A, De Felice M, Lombardi A, Moreno M, Fleury C, Ricquier D, Goglia F. Induction of UCP2 mRNA by thyroid hormones in rat heart. FEBBS Lett. 1997;418:171–174. doi: 10.1016/s0014-5793(97)01375-6. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Qi YP, Berrodin TJ, Davis KD, Schwartz HL, Strait KA, Oppenheimer JH, Lazar MA. Immunocytochemical delineation of thyroid hormone receptor beta 2-like immunoreactivity in the rat central nervous system. Endocrinology. 1993;132:2461–2469. doi: 10.1210/endo.132.6.7684976. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–5. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Masaki T, Yoshimatsu H, Kakuma T, Hidaka S, Kurokawa M, Sakata T. Enhanced expression of uncoupling protein 2 gene in rat white adipose tissue and skeletal muscle following chronic treatment with thyroid hormone. FEBS Lett. 1997;418:323–6. doi: 10.1016/s0014-5793(97)01404-x. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Ricquier D, Kozak LP. Uncoupling proteins: current status and therapeutic prospects. EMBO Rep. 2005;6(10):917–21. doi: 10.1038/sj.embor.7400532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Reitman ML, He Y, Gong DW. Thyroid hormone and other regulators of uncoupling proteins. Int. J. Obes. Relat. Metab. Disord. 1999;23(Suppl 6):S56–59. doi: 10.1038/sj.ijo.0800948. [DOI] [PubMed] [Google Scholar]
- Richard D, Rivest R, Huang Q, Bouillaud F, Sanchis D, Champigny O, Ricquier D. Distribution of the uncoupling protein 2 mRNA in the mouse brain. J. Comp. Neurol. 1998;397(4):549–560. [PubMed] [Google Scholar]
- Ricquier D. Respiration uncoupling and metabolism in the control of energy expenditure. Proc. Nutr. Soc. 2005;64(1):47–52. doi: 10.1079/pns2004408. [DOI] [PubMed] [Google Scholar]
- Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. J. Neurosci. 2000;20:9135–9144. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol. Endocrinol. 2001;15(12):2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- Schuman E, Chan D. Fueling synapses. Cell. 2004;119:738–40. doi: 10.1016/j.cell.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Sell H, Deshaies Y, Richard D. The brown adipocyte: update on its metabolic role. Int. J. Biochem. Cell Biol. 2004;36(11):2098–2104. doi: 10.1016/j.biocel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3 in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J. Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid. 1995;5(6):481–492. doi: 10.1089/thy.1995.5.481. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J. Histotechnol. 1989;12:169–181. [Google Scholar]
- Skulachev VP. Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 1991;294:158–162. doi: 10.1016/0014-5793(91)80658-p. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim. Biophys. Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- Stanley S, Wynne K, McGowan B, Bloom S. Hormonal regulation of food intake. Physiol Rev. 2005;85:1131–58. doi: 10.1152/physrev.00015.2004. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146(3):1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- Timofeeva E, Baraboi ED, Richard D. Contribution of the vagus nerve and lamina terminalis to brain activation induced by refeeding. Eur J Neurosci. 2005;22:1489–501. doi: 10.1111/j.1460-9568.2005.04330.x. [DOI] [PubMed] [Google Scholar]
- Tu HM, Kim S, Salvatore D, Bartha T, Legradi G, Larsen PR, Lechan RM. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology. 1997;138:3359–3368. doi: 10.1210/endo.138.8.5318. [DOI] [PubMed] [Google Scholar]
- Weitzel JM, Iwen KA, Seitz HJ. Regulation of mitochondrial biogenesis by thyroid hormone. Exp. Physiol. 2003;88:121–128. doi: 10.1113/eph8802506. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Elmquist JK. Minireview: From anorexia to obesity--the yin and yang of body weight control. Endocrinology. 2003;144:3749–3756. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]



