Abstract
It is known that β-lactam antibiotics can conjugate to lysine and histidine residues on proteins via the carbonyl group of the opened β-lactam ring. However, it is not known which proteins these drugs target and there is little work addressing whether conjugation is preferential for some proteins over others or if conjugation has functional consequences for the protein. We have previously shown that the β-lactam antibiotic benzylpenicillin (BP) conjugates to IFN-γ and reduces its activity. This interaction demonstrates selectivity, as BP does not bind to IL-4. Here, we extend our study to include other Th1 and Th2 cell-associated cytokines and two cytokines associated with inflammatory responses. We demonstrate by Western blotting that BP also conjugates to IL-1β, IL-2, IL-5, IL-13 and TNF-α but not to IL-10. Densitometric analysis of leading cytokine bands on blots revealed that IFN-γ always gave more intense BP-positive bands than any other cytokine analysed. Cytokines pre-incubated with BP at 37°C in a protein-containing, serum-free medium were assayed for their biological activity. By in vitro bioassay, BP inhibited the ability of IFN-γ but not IL-1β or TNF-α to induce CD54 expression on epithelial cells. In addition, BP did not affect IL-4 or IL-13 inhibition of mast cell proliferation. When the pre-incubation temperature was reduced to 4°C, BP did not conjugate to IFN-γ or modulate its activity. BP retained its inhibitory effect on IFN-γ activity when 20% FCS was added to the pre-incubation medium. In conclusion, BP conjugates to some cytokines but not others and this does not appear to be related to primary protein structure. Furthermore, of the cytokines studied, conjugation only to IFN-γ is accompanied by inhibition of activity. This phenomenon is temperature dependent and occurs in the presence of serum. These findings provide further evidence for differential, direct drug–cytokine interactions. Such interactions may have therapeutic implications in terms of targeting cytokines to regulate their activity.
Keywords: benzylpenicillin, conjugation, allergy, β-lactam, cytokine
Introduction
We have shown previously that the β-lactam antibiotic benzylpenicillin (BP) conjugates to IFN-γ and reduces its activity in a number of in vitro assays [1]. We also showed that BP does not bind to IL-4. Although generally nontoxic, β-lactams are one of the classes of drug most frequently associated with IgE-mediated allergy [2–5]. Our data led us to hypothesize that selective impairment of IFN-γ activity by β-lactams during the early phase of an immune response may favour the generation of Th2 over Th1 responses, thus leading to IgE production and allergy. Here, we extend our studies to question whether BP conjugates to and affects the activity of other cytokines, using conditions previously optimized for BP interactions with IFN-γ. We selected several cytokines to include Th1 (IL-2 and IFN-γ) and Th2 (IL-5, IL-10, IL-13 and IL-4) lymphocyte-associated cytokines and two cytokines that promote inflammation, TNF-α and IL-1β.
Western blotting revealed that BP bound in varying degrees to IFN-γ, IL-1β, IL-2, IL-5, IL-13 and TNF-α but did not bind to IL-4 or IL-10. Of interest, bands for BP conjugated to human IFN-γ were considerably more intense than those for murine IFN-γ, demonstrating interspecies heterogeneity. In bioassays for IFN-γ, IL-1β, TNF-α, IL-4 and IL-13 activity, BP affected only IFN-γ activity, showing that conjugation is not always associated with impairment of biological activity. Furthermore, the inhibitory effect of BP on IFN-γ activity does not occur when the drug and cytokine are incubated at 4, rather than 37°C, but does occur in the presence of 20% FCS.
Methods
Cells and cytokines
A549 human lung epithelial cells (ECACC, Salisbury, UK) were cultured in DMEM containing 5% FCS. The human mast cell line HMC-1 was a generous gift from J.H. Butterfield [6] and was maintained by subculturing 1 : 8 weekly in IMDM + 5% FCS. Carrier-free recombinant human IFN-γ, IL-2, IL-5, IL-10, IL-4, IL-13, TNF-α, IL-1β and murine IFN-γ were purchased from Peprotech (London, UK).
SDS-PAGE and Western blotting
Cytokines were incubated at 10 µg/ml, as previously optimized for visualization for Western blot and amido black staining [1], with or without BP at a final concentration of 5 mg/ml in PBS at 37°C, unless otherwise stated. After overnight incubation, 5× loading buffer (50% glycerol (v/v), 10% SDS (w/v), 100 µm DTT in 50 mm TRIS-HCl) was added 1 : 5 to each sample and 30 µl then loaded onto SDS-10% PAGE vertical slab gels (Hoefer Mighty Small apparatus Amersham, Bucks, UK), each gel including molecular weight markers. Gels were run in duplicate (30 mA/gel for 2 h) and proteins transferred electrophoretically by semidry blotter (Biometra, Berks, UK) to nitrocellulose membranes (Hybond ECL, Amersham). To detect BP conjugation, one blot was incubated in 1 : 5000 rabbit anti-BP antibody followed by 1 : 25 000 peroxidase-labelled goat antirabbit IgG and developed in ECL™ reagent (Amersham) as previously decribed [7]. IFN-γ was included as an internal reference in all experiments and other cytokines were related to IFN-γ as a ratio of band intensity, following densitometric analysis using the ‘Image’ programme (National Institutes of Health, USA). Ratios of test cytokine to IFN-γ band intensity were calculated for leading bands (verified by molecular weight) and for the total intensity of all bands in the lane. For analysis of BP effects on cytokine multimer formation, blots were incubated with polyclonal antibody to IFN-γ, TNF-α or IL-2 (Peprotech), at 0·2 or 0·4 µg/ml and visualized in the same way. The second blot from each experiment was stained for total protein in 0·1% amido black to confirm protein transfer.
Drug-cytokine incubations prior to functional assays
Cytokines (100 ng/ml) were incubated at 37°C, unless otherwise stated, for 4 days, with or without BP at a final concentration of 2 mg/ml. The medium used wasRPMI-1640 containing either 2% TCH™ serum replacement (RT2: ICN, Thame, UK) to give a final protein concentration of 0·65%, or various concentrations of FCS. Solutions of BP (Sigma, Poole, UK) were made up fresh for each experiment in RT2 or serum containing RPMI-1640 and filter-sterilized. As controls, cytokines were incubated alone for 4 days and BP was added immediately before bioassay, as previously described [1].
Assay for IL-4 and IL-13 activity
IL-4 and IL-13 each inhibit the proliferation of the human mast cell line HMC-1 [8]. HMC-1 cells were seeded into 96-well plates with RT2, BP, IL-4 or IL-13 preparations to give concentrations of 2, 10 or 50 ng/ml, in replicates of four. After 4–6 days incubation at 37°C, 5% CO2, 3H-thymidine (0·5 µCi/well) was added for the final 4 h of culture, the cells harvested and incorporation of radioactivity measured (PerkinElmer Life Sciences, Cambridge, UK).
Assay for IL-1β, TNF-α and IFN-γ activity
IL-1β, TNF-α and IFN-γ each induce expression of CD54 on A549 human lung epithelial cells [9]. A549 cells were grown to confluence in 96-well plates before the appropriate medium, BP or cytokine preparations were added in triplicate. After overnight incubation, CD54 expression was measured by ELISA. Medium was removed and cells incubated for 30 min at room temperature in Cellfix (Becton Dickinson, Oxford, UK). Plates were then washed with PBS + 0·05% Tween 20 (Sigma) (PBS-T) before addition of mouse IgG1 anti-CD54 monoclonal antibody (Pharmingen, Oxford, UK, 1/10 000) or isotype control (at the same final concentration) in PBS plus 2% goat serum plus 2% FCS (PBS-D) (100 µl/well). After 1 h at room temperature, plates were washed in PBS-T and HRP-labelled F(ab)2 goat antimouse-IgG (Caltag, Silverstone, UK) 1/4000 or EnVision™+/HRP (DAKO, Ely, UK) 1/50 in PBS-D (100 µl/well) added for 1 h at room temperature. After further washing, 2–2′-Azinobis(3-ethylbenzthiazoline-sulphonic Acid) (ABTS: Sigma) or 3,3′,5,5′-tetramethylbenzidine (TMB: Sigma) (100 µl) was added to each well. The TMB reaction was stopped with 100 µl 1 m H2SO4 and absorbance read at 450 nm with a reference of 630 nm on an automatic plate reader (MRX; Dynatech Instruments, Torrance, CA, USA). For ABTS absorbance was read at 405 nm.
Results
Benzylpenicillin conjugates to IFN-γ, IL-1β, IL-2, IL-5, IL-13 and TNF-α but not IL-4 or IL-10
Western blot analysis revealed that BP conjugated to seven out of the nine cytokines analysed, as shown in Fig. 1. Blots were aligned with amido black tranfers that contained molecular weight markers and the molecular weight of the bands determined. Using this method, it was evident that the leading bands visualized on Westerns with anti-BP antibodies corresponded to the molecular weight of a single molecule of each cytokine. The trailing bands represent multiples of this molecular weight. Multimers were observed on Western blots of all positive cytokines except for TNF-α (Fig. 1). Each cytokine was analysed in this way three times and, using densitometric analysis, ratios to IFN-γ determined for primary bands (monomers for all except IL-5, which is an interdigitating dimer, 10) and for total intensity of all bands, as described in Materials and Methods. Considering the primary bands, none of the cytokines gave a ratio of signal intensity of 1 or more, when compared to IFN-γ (Table 1). The mean ratios for IL-5, IL-13 and TNF-α were similar to each other, IL-2 being lower, whilst IL-1β was higher. BP bound minimally to IL-4 and not at all to IL-10 under these conditions and bound much less to murine compared to human IFN-γ. For every cytokine tested, protein transfer was confirmed by amido black staining of parallel blots. Ratios were also calculated for the total binding to all the bands in each lane. Here, the cytokines fell into three distinct groups: IL-5, which was equivalent to IFN-γ; IL-1β, IL-2, TNF-α and IL-13, which were similar to each other but less than IFN-γ and IL-5; and murine IFN-γ, IL-4 and IL-10, had the lowest ratios (Table 1). Comparison of these ratios of Western blot band intensities with the lysine and histidine content of the cytokines does not reveal any relationship (Table 1). For example, TNF-α, which contains only 6 lysine and 3 histidine residues, is conjugated relatively strongly by BP, whereas IL-10, with 13 lysines and 3 histidines is negative.
Fig. 1.
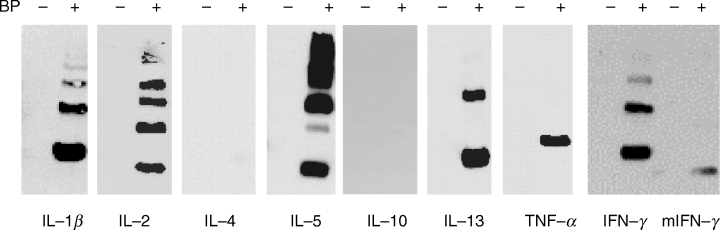
BP conjugation to cytokines. Cytokines, each at 10 µg/ml in PBS, were incubated with or without 5 mg/ml BP overnight at 37°C. Samples were analysed by SDS-PAGE and Western blotted with rabbit polyclonal anti-BP antibody. In each case, one blot representative of three is shown. m, murine.
Table 1.
Conjugation of BP to cytokines relative to IFN-γ
| Amino acid residues per molecule | Relative band intensity | ||||
|---|---|---|---|---|---|
| Cytokine | MW | Lysine | Histidine | Leading bandMean ± SD Lysine | TotalMean ± SD Histidine |
| IFN-γ | 16·7 | 20 | 2 | 1 | 1 |
| IL-1β | 17 | 15 | 1 | 0·82 ± 0·06 | 0·66 ± 0·15 |
| IL-2 | 15·4 | 11 | 3 | 0·21 ± 0·59 | 0·59 ± 0·33 |
| IL-4 | 14·9 | 12 | 5 | 0·05 ± 0·05 | 0·06 ± 0·05 |
| IL-5* | 25 | 16 | 6 | 0·68 ± 0·11 | 1·03 ± 0·14 |
| IL-10 | 18·6 | 13 | 3 | 0 | 0 |
| IL-13 | 12·5 | 7 | 3 | 0·67 ± 0·16 | 0·51 ± 0·14 |
| TNF-α | 17·4 | 6 | 3 | 0·66 ± 0·14 | 0·46 ± 0·09 |
| mIFN-γ | 15·6 | 10 | 3 | 0·24 ± 0·27 | 0·16 ± 0·19 |
Cytokines were incubated at 10 µg/ml with or without 5 mg/ml of BP in PBS. After overnight incubation at 37°C, samples were analysed by SDS-PAGE and Western blotted with rabbit polyclonal anti-BP antibody. IFN-γ was included as an internal reference in all experiments and other cytokines were related to IFN-γ as a ratio of band intensity, following densitometric analysis. Ratios of test cytokine to IFN-γ were calculated for monomer bands (determined from molecular weight) and for the total intensity of all bands in each lane. Results represent the mean ± standard deviation of three experiments. MW, molecular weight in kD. m, murine.
dimer, see text and [ 10].
Since Western blots for BP conjugation revealed bands corresponding to multimers of cytokine molecules (Fig. 1), we determined whether BP induced the formation of these multimers, using polyclonal anticytokine antibody in place of anti-BP antibodies. IFN-γ, TNF-α and IL-2 each gave the same band pattern on Westerns blots whether or not they had been incubated with BP (Fig. 2). Therefore, BP did not affect the aggregation of these cytokines. Of interest, although TNF-α could be visualized as multimers on SDS-PAGE using an anti-TNF-α polyclonal antibody (Fig. 2), only the monomeric form of this cytokine was positive for BP (Fig. 1).
Fig. 2.

BP has no effect on cytokine oligomerization. IFN-γ, IL-2 and TNF-α, each at 10 µg/ml in PBS, were incubated with or without 5 mg/ml BP overnight at 37°C. Samples were analysed by SDS-PAGE and Western blotted with the appropriate polyclonal anti-cytokine antibody.
BP does not affect IL-4 or IL-13 activity
IL-4 and IL-13 were each incubated in RT2 at 37°C at 100 ng/ml for 4 days with or without BP (2 mg/ml), conditions previously optimized for experiments with IFN-γ[1]. The cytokines were then added to HMC-1 cells and their effect on proliferation measured. Both IL-4 and IL-13 caused a concentration-dependent inhibitory effect on HMC-1 proliferation which was significant at 10 and 50 ng/ml (Fig. 3a and b, respectively). Pre-treatment of either of these two cytokines with BP did not affect their activity (Fig. 3a,b). Likewise, addition of BP to these cytokines immediately before bioassay had no effect on their activity. In addition, BP added to the bioassays at the same final concentration as in these samples had no effect (data not shown).
Fig. 3.
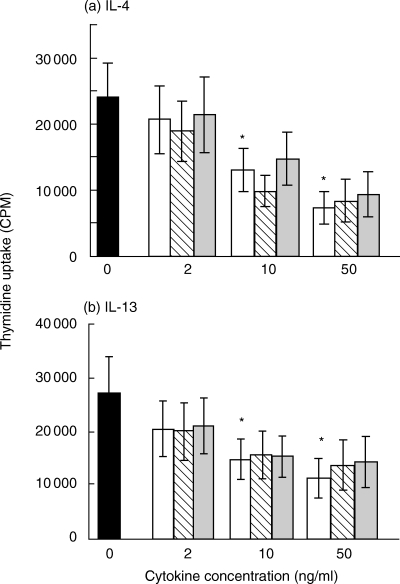
Effect of BP on the ability of(a)IL-4 or(b)IL-13 to inhibit HMC-1 cell proliferation. Each cytokine (100 ng/ml) was incubated in RT2 with or without BP (2 mg/ml) for 4 days at 37°C, then each preparation was assayed at 2, 10 or 50 ng/ml. Proliferation was measured as uptake of 3H-thymidine after cells had been incubated for 4–6 days with the IL-4 or IL-13 preparations. Results represent mean ± SEM of 4 experiments, each performed in replicates of at least 4. *P < 0·05, by paired Student's t-test, for comparison of cells cultured with or without cytokine. ▪ No cytokine, □ Cytokine incubated 4 days alone,  Cytokine incubated 4 days + 2 mg/ml BP,
Cytokine incubated 4 days + 2 mg/ml BP,  Cytokine incubated 4 days alone, 2mg/ml BP added immediately before assay.
Cytokine incubated 4 days alone, 2mg/ml BP added immediately before assay.
BP affects IFN-γ but not TNF-α or IL-1β activity
TNF-α, IL-1β and IFN-γ were each incubated in RT2 at 37°C at 100 ng/ml for 4 days with or without BP (2 mg/ml), conditions previously optimized for experiments with IFN-γ[1]. TNF-α, IL-1β and IFN-γ each induced CD54 expression on A549 cells, IL-1β being the most potent (Fig. 4a-c). Pre-treatment of either IL-1β or TNF-α with BP, or addition of BP immediately before assay, had no effect their activity (Fig. 4a and b, respectively). In contrast, IFN-γ pre-incubated with BP had a significantly reduced activity compared to IFN-γ incubated alone or to IFN-γ to which BP was added immediately before assay (Fig. 4c). BP-treated IFN-γ preparations assayed at 2 and 10 ng/ml had a reduced activity of 71 and 38%, respectively, compared to untreated IFN-γ. BP alone did not affect CD54 expression (data not shown).
Fig. 4.

Effect of BP on the ability of(a)IL-1β,(b)TNF-α or(c)IFN-γ to induce CD54 expression on A549 cells. Each cytokine (100 ng/ml) was incubated in RT2 with (○) or without (•) BP (2 mg/ml) for 4 days at 37°C, then each preparation was assayed at 0·02, 0·2, 2 or 10 ng/ml. Confluent layers of A549 cells in 96-well plates were incubated overnight with cytokine preparations. After fixing, CD54 expression was measured by ELISA. ▴ Cytokine incubated 4 days alone, 2 mg/ml BP added immediately before assay. Results represent mean ± SEM of 3 (IL-1β and TNF-α) or 4 (IFN-γ) experiments, each performed in triplictate. *P < 0·05, by paired Student's t-test, for comparison of untreated to BP-treated IFN-γ.
Effect of temperature on BP conjugation to IFN-γ
BP conjugated to IFN-γ at 37°C but not at 4°C, as revealed by Western blotting (Fig. 5)
Fig. 5.
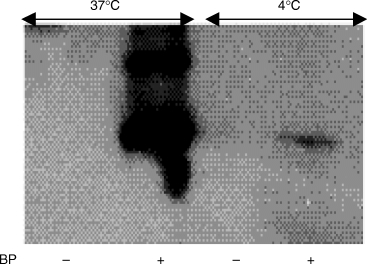
Effect of temperature on BP conjugation to IFN-γ. IFN-γ, at 10 µg/ml in PBS, was incubated with or without 5 mg/ml BP overnight at 4 or 37°C. Samples were analysed by SDS-PAGE and Western blotted with rabbit polyclonal anti-BP antibody.
Influence of temperature and serum on the inhibitory action of BP on IFN-γ activity
When BP and IFN-γ were pre-incubated together at 37°C, BP inhibited IFN-γ activity. However, if the pre-incubation temperature was reduced to room temperature or 4°C, BP did not influence IFN-γ activity (Fig. 6). The inhibitory action of BP on IFN-γ activity was retained when pre-incubation was performed in medium containing 20% or 2% FCS (Fig. 6).
Fig. 6.

Effect of temperature and serum on BP reduction of IFN-γ activity. IFN-γ was incubated, at 100 ng/ml, at different temperatures in RT2, or in varying concentrations of FCS for 4 days with or without 2 mg/ml BP. Confluent layers of A549 cells in 96-well plates were incubated overnight with each cytokine preparation at a final concentration of 2 ng/ml. After fixing, CD54 expression was measured by ELISA. Results represent mean ± SEM of 4 experiments, each performed in triplictate.*P < 0·01 by paired Student's t-test, for comparison of untreated to BP-treated IFN-γ.□ medium alone,  IFN-γ, ▪ IFN-γ+ BP.
IFN-γ, ▪ IFN-γ+ BP.
Discussion
Here we demonstrate that the β-lactam antibiotic BP conjugates differentially to cytokines. Band intensity for primary bands on Western blots was greatest for IFN-γ, followed by IL1-β, IL-5, IL-13 and TNF-α, less for IL-2 and murine IFN-γ, very weak for IL-4 and no bands could be detected for IL-10. TNF-α resolved as multimers on PAGE but BP was only visualized as binding to the monomeric form, suggesting that only this form of TNF-α contains sites accessible for BP conjugation. If this is the case, it is not surprising that BP does not modulate TNF-α activity in our in vitro assays, as the active form of this cytokine is a trimer [11]. In contrast, the other cytokines to which BP bound displayed positive monomeric and oligomeric bands.
Until now the only human protein to which BP conjugation has been well characterized is serum albumin, in which lysine residues are the sites of binding [12] but other protein targets for BP are poorly defined. The major interaction between BP and proteins is via the β-lactam ring, to form penicilloyl determinants. The amino acids that could be involved in this situation are lysine and histidine [12–14]. Comparison of the primary amino acid sequence of these cytokines shows that the extent of BP binding is not determined purely by the quantity of lysine and histidine residues in the polypeptide. Further analysis of the structural features of these cytokines reveals that many of them (IL-2, IL-4, IL-5, IL-10, IL-13 and IFN-γ) share a common four-helical bundle topology [15–17], whilst IL-1β and TNF-α belong to the β-trefoil and β-jellyroll families, respectively [15, 18, 19]. IL-1β, IL-2, IL-4 and IL-13 exist as monomers [15, 17, 18], IFN-γ, IL-5 and IL-10 as dimers [15, 16, 20] whilst TNF-α exists predominantly as a trimer [11,19]. However, there is no correlation between the structural groupings of these proteins and their susceptibility to conjugation by BP. Of interest, the cytokine with a structure most similar to IFN-γ is IL-10, to which BP does not conjugate at all. Therefore, the chemical environment of individual lysine and histidine residues within each protein and the relative availability of these residues within the 3D structure of the molecule must determine whether or not BP will conjugate to them.
When five of these cytokines were assayed to see if BP modulates their in vitro biological activity, only IFN-γ was affected. Predictably, the activity of IL-4, to which BP does not conjugate, was unaffected. However, the activity of three cytokines to which BP does bind (IL-1β, TNF-α and IL-13) also remained unaltered. This may be a consequence of BP binding to functionally unimportant sites within these cytokine molecules. For IFN-γ the regions of the molecule important for receptor (residues 1–42, 108–124 and 130–132) and glycosaminoglycan (GAG: residues 125–131) binding are known [20–22]. These regions all contain histidine and/or lysine groups. In the case of TNF-α, corresponding regions have not yet been defined. Regions of IL1-β and IL-13 that bind to their receptors have been identified [23–25]. These are thought to be multiple single amino acid residues and peptide sequences in IL-1β[23,24], whereas, for IL-13, the areas predicted to be important in receptor binding are helices A, C and D [25]. Again, there are lysine and histidine residues within these regions. Therefore, there appears to be something unique in the interaction of BP with IFN-γ that results in the decreased activity of this cytokine.
The temperature at which BP is pre-incubated with IFN-γ had a marked effect on its ability to conjugate to and inhibit the activity of the cytokine. This supports the notion that the modulation of IFN-γ activity by BP is due to a chemical interaction. In the presence of 20% FCS during the pre-incubation of BP with IFN-γ, the drug mediated a significant reduction the biological activity of the cytokine, supporting our hypothesis that BP may regulate IFN-γ activity under physiological conditions.
BP interaction with amino acid residues within a cytokine could have several possible outcomes. First of all, it could disrupt the secondary or tertiary structure of the molecule, causing functional modulation. Secondly, BP could bind to residues within the receptor-binding region of the molecule, affecting receptor engagement and therefore biological activity. Thirdly, conjugation to residues within GAG-binding regions of a cytokine could disrupt GAG-binding and have an effect not only on activity but also on biodistribution and half-life [26]. Our biological assays performed to date do not allow us to distinguish between any effect that BP may be having on receptor versus GAG-binding, as we have not determined the possible contribution of GAGs in these assays. However, it is known that many cytokines, including most of those in our study, do conjugate to GAGs and that this has important functional consequences [26, 27, 28, 29, 30, 31].
If BP conjugates to cytokines in vivo, there could be several outcomes. One of these is the favouring of Th2 over Th1 lymphocyte responses due to the differential effects we have shown on IFN-γ over Th2 cytokine (IL-4 and IL-13) in vitro activity. It is possible that other haptens behave in a similar fashion, conjugating to and disrupting the activity of cytokines in a differential manner. Cytokines structurally modified by BP or other haptens may also form neoantigens, with various immunological consequences. In addition to our work showing that the commonly used drug BP can reduce IFN-γ activity, others have shown that a reactive metabolite of acetaminophen can covalently bind to and inhibit the function of MIF [32]. Thus, drug modulation of cytokine activity is not restricted to β-lactams and IFN-γ.
In conclusion, we have shown that BP differentially conjugates to cytokines and preferentially disrupts the activity of IFN-γ. These findings have implications for understanding possible mechanisms by which BP and other haptens cause allergy or disrupt immune regulation. They also have therapeutic implications, as they provide a rationale for the targeting and selective modulation of cytokine bioactivity.
Acknowledgments
This work was funded by the European Commission Framework IV Biotechnology Programme contract number ERBBIO4CT960246
References
- 1.Brooks BM, Flanagan BF, Thomas AL, Coleman JW. Penicillin conjugates to interferon-γ and reduces its activity: a novel drug–cytokine interaction. Biochem Biophys Res Comm. 2001;288:1175–81. doi: 10.1006/bbrc.2001.5896. [DOI] [PubMed] [Google Scholar]
- 2.De Weck AL. Immunopathological mechanisms and clinical aspects of allergic reactions. In: DeWeck AL, Bundgaard H, editors. Allergic Reactions to Drugs. Berlin: Springer-Verlag; 1983. pp. 75–133. [Google Scholar]
- 3.Anderson J. Allergic reactions to drugs and biological agents. J Am Med Assoc. 1992;268:2845–57. doi: 10.1001/jama.268.20.2844. [DOI] [PubMed] [Google Scholar]
- 4.Blanca M. Allergic reactions to penicillins – a changing world. Allerg. y. 1995;50:777–82. doi: 10.1111/j.1398-9995.1995.tb05048.x. [DOI] [PubMed] [Google Scholar]
- 5.Coleman JW. Keeping up with drug allergy. Clin Exp Allergy. 1996;26:1341–2. [PubMed] [Google Scholar]
- 6.Butterfield JH, Weiler D, deWald G, Gleich GJ. Establishment of an immature mast cell line from a patient with a mast cell leukemia. Leukemia Res. 1988;12:345–55. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 7.Warbrick EV, Thomas AL, Stejskal V, Coleman JW. An analysis of β-lactam-derived antigens on spleen cell and serum proteins by ELISA and Western blotting. Allergy. 1995;50:910–7. doi: 10.1111/j.1398-9995.1995.tb02498.x. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson G, Nilsson K. Effects of interleukin (IL)-13 on immediate-early response gene expression, phenotype and differentiation of human mast cells. Comparison with IL-4. Eur J Immunol. 1995;25:870–3. doi: 10.1002/eji.1830250337. [DOI] [PubMed] [Google Scholar]
- 9.Wellicome SM, Thornhill MH, Pitzalis C, Thomas DS, Lanchbury JSS, Panayi GS, Haskard DOA. Monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumour necrosis factor, IL-1 or lipopolysaccharide. J Immunol. 1990;144:2558–65. [PubMed] [Google Scholar]
- 10.Milburn MV, Hassell AM, Lambert MH, Jordan SR, Proudfoot AE, Graber P, Wells TN. A novel dimer configuration revealed by the crystal structure at 2.4A resolution of human interleukin-5. Nature. 1993;363:172–6. doi: 10.1038/363172a0. [DOI] [PubMed] [Google Scholar]
- 11.Smith RA, Baglioni C. The active form of tumour necrosis factor is a trimer. J Biol Chem. 1987;262:6951–4. [PubMed] [Google Scholar]
- 12.Yvon M, Anglade P, Wal J-M. Identification of the binding sites of benzyl penicilloyl, the allergenic metabolite of penicillin, on the serum albumin molecule. FEBS Letts. 1990;263:237–40. doi: 10.1016/0014-5793(90)81382-x. [DOI] [PubMed] [Google Scholar]
- 13.Bachelor FR, Dewney JM, Gazzard D. Penicillin allergy. the formation of the penicilloyl determinant. Nature. 1965;206:362–4. doi: 10.1038/206362a0. [DOI] [PubMed] [Google Scholar]
- 14.Bundgaard H. Chemincal aspects of penicillin allergy: mechanism of imidazole-catalysed penicilloylation. J Pharm Pharmacol. 1976;28:721–8. doi: 10.1111/j.2042-7158.1976.tb02851.x. [DOI] [PubMed] [Google Scholar]
- 15.Sprang SR, Bazan JF. Cytokine stryctural taxonomy and mechanisms of receptor engagement. Curr Opin Struct Biol. 1993;3:815–27. [Google Scholar]
- 16.Walter MR, Nagaabhushan TL. Crystal structure of interleukin 10 reveals an interferon-γ-like fold. Biochemistry. 1995;34:12118–25. doi: 10.1021/bi00038a004. [DOI] [PubMed] [Google Scholar]
- 17.Eisenmesser EZ, Horita DA, Altiri AS, Byrd RA. Solution structure of interleukin-13 and insights into receptor engagement. J Mol Biol. 2001;310:231–41. doi: 10.1006/jmbi.2001.4765. [DOI] [PubMed] [Google Scholar]
- 18.Priestle JP, Schar H-P, Grutter MG. Crystal structure of the cytokine interleukin-1β. EMBO. 1988;7:339–43. doi: 10.1002/j.1460-2075.1988.tb02818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones EY, Stuart DI, Walker NPC. Structure of tumour necrosis factor. Nature. 1989;338:225–8. doi: 10.1038/338225a0. [DOI] [PubMed] [Google Scholar]
- 20.Ealick SE, Cook WJ, Vijay-Kumar S, Carson M, Nagabhushan TL, Trotta PP, Bugg CE. Three-dimensional structure of recombinant human interferon-γ. Science. 1991;252:698–702. doi: 10.1126/science.1902591. [DOI] [PubMed] [Google Scholar]
- 21.Walter MR, Windsor WT, Nagabhushan TL, Lundell DL, Lunn CA, Zauodn PJ, Narula SK. Crystal structure of a complex between interferon-γ and its soluble high-affinity receptor. Nature. 1995;376:230–5. doi: 10.1038/376230a0. [DOI] [PubMed] [Google Scholar]
- 22.Lortat-Jacob H, Grimaud J-A. Interferon-γ binds to heparan sulphate by a cluster of amino acids located in the C-terminal part of the molecule. FEBS Lett. 1991;280:152–4. doi: 10.1016/0014-5793(91)80225-r. [DOI] [PubMed] [Google Scholar]
- 23.Vigers GPA, Anderson LJ, Caffes P, Brandhuber BJ. Crystal structure of the type-1 interleukin-1 receptor complexed with interleukin-1β. Nature. 1997;386:190–4. doi: 10.1038/386190a0. [DOI] [PubMed] [Google Scholar]
- 24.Casadio R, Frigimelica E, Bossu P, et al. Model of interaction of the IL-1 receptor accessory protein IL-1RAcP with the IL-1β/IL-1R1 complex. FEBS Letts. 2001;499:65–8. doi: 10.1016/s0014-5793(01)02515-7. [DOI] [PubMed] [Google Scholar]
- 25.Zuegg J, Webb DC, Foster PS, Casarotto MG. Structural model of human IL-13 defines the spatial interactions with the IL-13Rα/IL-4Rα receptor. Immunol Cell Biol. 2001;79:332–9. doi: 10.1046/j.1440-1711.2001.01035.x. [DOI] [PubMed] [Google Scholar]
- 26.Lortat-Jacob H, Baltzer F, Grimaud J-A. Heparin decrease the blood clearance of interferon-γ and increases its activity by limiting the processing of its carboxyl-terminal sequence. J Biol Chem. 1996;271:16139–43. doi: 10.1074/jbc.271.27.16139. [DOI] [PubMed] [Google Scholar]
- 27.Ramsden L, Rider CC. Selective and differential binding of interleukin (IL)-1α, IL-1β, IL-2 and IL-6 to glycosaminoglycans. Eur J Immunol. 1992;22:3027–31. doi: 10.1002/eji.1830221139. [DOI] [PubMed] [Google Scholar]
- 28.Lantz M, Thysell H, Nilsson E, Olsson I. On the binding of tumour necrosis factor (TNF) to heparin and the release in vivo of the TNF-binding protein 1 by heparin. J Clin Invest. 1991;88:2026–31. doi: 10.1172/JCI115530. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Lortat-Jacob H, Garrone P, Banchereau J, Grimaud J-A. Human interleukin 4 is a glycosaminglycan-binding protein. Cytokine. 1997;9:101–5. doi: 10.1006/cyto.1996.0142. [DOI] [PubMed] [Google Scholar]
- 30.Lipscombe RJ, Nakhoul A-M, Sanderson CJ, Coombe DR. Interleukin-5 binds to heparin/heparan sulfate. A model for an interaction with extracellular matrix. J Leuk Biol. 1998;63:342–50. doi: 10.1002/jlb.63.3.342. [DOI] [PubMed] [Google Scholar]
- 31.Brooks B, Briggs DM, Eastmond NC, Fernig DG, Coleman JW. Presentation of interferon-g to nitric oxide producing cells: a novel function for mast cells. J Immunol. 2000;164:573–9. doi: 10.4049/jimmunol.164.2.573. [DOI] [PubMed] [Google Scholar]
- 32.Senter PD, Al-Abed Y, Metz CN, et al. Inhibition of macrophage migration inhibitory factor (MIF) tautomerase and biological activities by acetaminophen metabolites. Proc Natl Acad Sci USA. 2002;99:144–9. doi: 10.1073/pnas.011569399. [DOI] [PMC free article] [PubMed] [Google Scholar]


