Abstract
PspA and PsaA are Streptococcus pneumoniae surface proteins and potential pneumococcal vaccine antigens. The aim of this study was to characterize the transplacental transfer of antibodies to PspA and to PsaA. Paired mother and cord blood sera were obtained at delivery from 28 women. Concentrations of antibodies against PspA, PsaA, tetanus toxoid (vaccine-induced antibodies) and P6-outer membrane protein (OMP) of nontypeable Haemophilus influenzae were determined by ELISA. Antibodies to PspA of the IgG, IgG1 and IgG2 antibodies were also determined. The geometric mean percentage (GM%) of the paired infant:mother antibody were calculated. Results: The GM% of the infant:mother antibody concentrations against PspA, PsaA and P6-OMP antibodies were 64·7% (3·3 µg/ml in infants vs. 5·1 µg/ml in mothers), 50·4% (6·8 µg/ml vs. 13·5 µg/ml) and 66·7% (5·6 µg/ml vs. 8·4 µg/ml), respectively; the GM% of antibodies against tetanus toxoid was 104·5% (4·6 µg/ml vs. 4·4 µg/ml). Transplacental transfer of IgG1 was more efficient than that of IgG2 (approximately 120%vs. 65%). A transplacental transfer of antibodies to PspA and to PsaA exist. Moreover, these data suggest an active placental transfer of IgG1 antibodies to PspA since the concentration of these antibodies were consistently higher in cord sera than in the mother's sera.
Keywords: PspA, PsaA, materno-fetal transfer, antibody, clinical vaccinology
INTRODUCTION
Respiratory tract infections and meningitis due to Streptococcus pneumoniae are among the most frequent life-threatening infectious diseases of infants, particularly in developing countries [1]. Pneumococcal disease can now be prevented in infants by vaccination with protein–polysaccharide conjugates [2]. However, these new vaccines are costly, cannot be administered before two months of age, and do not cover all pathogenic pneumococcal capsular serotypes. Vaccines containing one or more pneumococcal protein antigens would likely be highly immunogenic in children because of their ability to induce a T cell dependent immune response. Although the efficacy of pneumococcal protein antigens is still unproven in humans, studies in animals are encouraging and a phase 1 study has been conducted in healthy adults [3,4]. Using noncapsular antigens as vaccines for pneumococcal infection is also appealing to prevent otitis media because conjugate vaccines are not as effective for otitis as for invasive disease.
Among the pneumococcal proteins identified as potential vaccine antigens, the pneumococcal surface protein A (PspA) and the pneumococcal surface adhesin A (PsaA) are promising candidates. PspA inhibits complement deposition on pneumococci [5], is essential for full virulence of pneumococci [6], and is present on all clinically important pneumococcal strains [7]. Immunization with a recombinant PspA derived from strain Rx1 has been shown to be safe and to induce a broadly cross-reactive immune response in humans [3]. Human antibodies to PspA elicited by recombinant PspA can protect mice challenged with highly virulent S. pneumoniae [4]. The expression of PsaA appears to be important for the adherence of pneumococci to host mucosal tissue [8].
Materno-fetal transfer of antibodies to pneumococcal capsular polysaccharide (PPS) seems not to occur after a 23-valent PPS vaccination of the mothers during the third trimester of pregnancy in developing countries [9,10] but results are more favourable to this approach in a US trial [11]. Unlike PPS vaccines, a surface-protein vaccine is expected to induce IgG1 antibodies that would cross the placenta. These antibodies could provide natural passive immunity to neonates. However, before undertaking the development of a pneumococcal surface protein-based vaccine, it is important to characterize the antibodies against the protein antigens that are induced by natural infection or exposure to S. pneumoniae. Several investigators have already reported that the antibody levels to PspA are maximal during childhood [12–14]. In the present study, we have asked the following question: does the mother transfer antibodies against these proteins to their infant?
We measured antibodies to PspA and PsaA in the mothers’ sera and the levels of antibody the mothers had transferred transplacentally into the cord blood of their infants. The geometric mean percentages (GM%) of the paired infant:mother antibody concentrations were determined for antibodies to PspA and PsaA as well as for two additional protein antigens from other nonpneumococcal bacteria. The nonpneumococcal responses selected for comparison were vaccine-induced antibodies to tetanus toxoid and natural infection induced immunity to the P6 outer membrane protein (OMP) of nontypeable Haemophilus influenzae infection. This study also includes comparisons of the GM% of IgG, IgG1 and IgG2 antibody to PspA in paired sera from mothers and infants.
METHODS
All the women were living in or near Lyon (France), had uncomplicated pregnancies of at least 37 weeks duration, and had given birth to healthy infants. The appropriate Ethics Committee approved the study before any subjects were enrolled, and the study was conducted in accordance with the Declaration of Helsinki. All subjects gave written informed consent before entering the study.
Twenty-eight paired sera were obtained at delivery from mothers and from their infant's cord blood. Sera were stored at − 80°C and shipped frozen to Birmingham (AL, USA) for assays. Sera were analysed for their content of antibody reactive with the various test antigens using an enzyme-linked immunosorbent assays (ELISA). Briefly, ELISA plates (NUNC, Weisbaden, Germany) were coated overnight at 4°C in phosphate buffered saline (PBS) at pH = 7·2 with different antigens: 1 µg/ml Rx1-strain recombinant PspA (Aventis Pasteur, Swiftwater, PA, USA), 1 µg/ml PsaA (Aventis Pasteur, Toronto, Canada), 1 µg/ml tetanus toxoid (Wyeth Laboratories Inc., Marietta, PA, USA), and 1 µg/ml OMP P6 from nontypeable H. influenzae (provided by TF Murphy [15]). All assays included a control plate coated with a bovine serum albumin (BSA) to verify the specificity of the assays for the coating antigen. The low levels of reactivity with the BSA plates were subtracted from the values on the antigen coated plates. Plates were washed with PBS containing 0·05% Tween 20 (ELISA wash buffer). The plates were blocked with PBS containing 1% BSA for one hour at room temperature followed by incubation with the subject's sera overnight at 4°C, then washed with ELISA wash buffer. The ELISA plates were then incubated with biotin-conjugated goat anti-human immunoglobulin (Ig) serum or mouse monoclonal antibodies specific for human IgG, IgG1, or IgG2 (Southern Biotechnology Associates, Birmingham, AL, USA) for two hours at room temperature, washed and then incubated with streptavidin-alkaline phosphatase (Southern Biotechnology Associates) for one hour at room temperature. After a final wash, the plates were developed with p-nitrophenyl phosphate (Sigma, St. Louis, MO, USA) absorbance was read at 405 nm. All antibodies against PspA and PsaA were standardized to pooled human antibodies (HSP5) with a known concentration [3]. Antibody concentrations to tetanus toxoid and P6-OMP were calculated by assuming that the relationship between absorbance and antibody concentration for antibody to these antigens was the same as for antibody to PspA in the HSP5 pool.
When calculating geometric mean concentrations (GMC) of the different antibodies, sera with no detectable antibody were assigned a value 10% below the detection limit. Antibody concentrations were log10 transformed to obtain a normal distribution and the results were reported as the antilogarithm of the GMC log10 with the range of values (maximum–minimum). Student's paired t-test was used to compare the mother and infant antibody concentrations. When the log-transformed data were not normally distributed, Wilcoxon's nonparametric test was used. For each antibody specificity, the geometric mean percentage of antibody in each infant's cord blood as compared to its mother's serum was calculated. Comparisons of the GM% of antibody in cord blood to mother's serum at delivery were made by Spearman rank correlation. Data sets were analysed with SPSS software (version 10·0, SPSS Inc. Chicago, IL, USA) and P < 0·05 was considered to be statistically significant.
RESULTS
Ig antibody to PspA, PsaA, P6-OMP and tetanus toxoid were present in the sera from all mothers and their infants (Table 1). For each antigen the correlation between antibody concentrations in the cord blood and maternal serum were statistically significant at P < 10−4. The percentage of antibody in the cord blood were significantly lower than that in the maternal sera for PspA, PsaA and P6-OMP, but not for tetanus toxoid.
Table 1.
Mother and infant specific immunoglobulins (Ig) concentrations against PspA, PsaA, P6-OMP and tetanus toxoid antigens
| GMC† (µg/ml) (range) | Antibody in cord blood (%) vs. mother serum | |||||
|---|---|---|---|---|---|---|
| n | Mother at delivery | Infant cord blood | GM%‡ | (95% CI§) | Spearman’s coefficient (Rs)¶ | |
| Ig to PspA | 28 | 5·1 (1·34–26·3) | 3·3 (0·8–22·2) | 64·7%* | (56·9–94·4) | 0·825 |
| Ig to PsaA | 28 | 13·5 (0·03–241·0) | 6·8 (0·0015–241·0) | 50·4%** | (42·9–96·5) | 0·849 |
| Ig to P6-OMP | 26 | 8·4 (1·5–38·6) | 5·6 (0·34–19·1) | 66·7%*** | (47·8–123·5) | 0·675 |
| Ig to tetanus toxoid | 25 | 4·4 (0·21–32·1) | 4·6 (0·21–41·2) | 104·5% | (89·2–158·0) | 0·832 |
GMC: Geometric Mean Concentration, in µg/ml.
GM%: Geometric Mean percentage.
95% CI: 95% Confidence Intervals.
All Spearman rank correlation tests were statistically significant with P < 10−4 Comparison of the mother and infant antibody concentrations
P = 0·0004 (Student's paired t-test)
P < 10−3 (paired Wilcoxon's non parametric test)
P < 10−4 (Student's paired t-test).
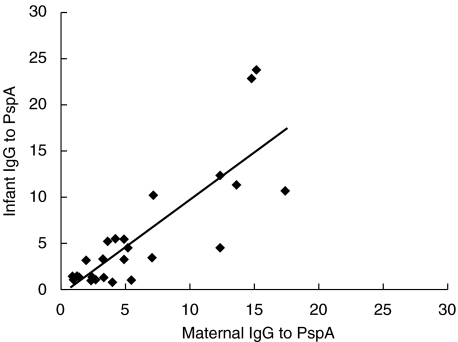
Antibodies to PspA were characterized according to total IgG, IgG1, and IgG2 (Table 2). As expected, most of the antibodies to PspA were of IgG isotype in cord blood sera (Ig = 3·3 µg/ml vs. IgG = 3·1 µg/ml) whereas IgG to PspA represents three quarters of the total Ig from the mothers’ sera. Moreover for each of these assays the correlation between antibody concentrations in cord blood and maternal serum were statistically significant at P < 10−4. Figure 1 shows that the higher the mother IgG to PspA concentration the higher the infant's IgG to PspA concentration. The percentage of IgG1 in the cord blood (as compared to the maternal serum) was higher than that for IgG2 (Table 2). Consistent with this finding, the percentage values for IgG antibody to PspA were intermediate to those for IgG1 and IgG2.
Table 2.
Mother and infant serum concentrations of IgG, IgG1 and IgG2 antibodies to PspA
| GMC† (µg/ml) (range) | Antibody in cord blood (%) vs. mother serum | |||||
|---|---|---|---|---|---|---|
| n | Mother at delivery | Infant cord blood | GM%‡ | (95% CI§) | Spearman's coefficient (Rs)¶ | |
| IgG to PspA | 27 | 3·8 (0·8–17·4) | 3·1 (0·8–23·9) | 81·6%* | (76·8–114·8) | 0·711 |
| IgG1 to PspA | 23 | 1·35 (0·2–8·8) | 1·6 (0·2–6·6) | 118·5% | (98·5–181·8) | 0·727 |
| IgG2 to PspA | 19 | 0·9 (0·12–6·6) | 0·6 (0·045–5·7) | 66·7%** | (54·2–99·5) | 0·906 |
GMC: Geometric Mean Concentration.
GM%: Geometric Mean percentage.
95%CI: 95% Confidence Intervals.
All Spearman rank correlation tests were statistically significant with P < 10−4.
Comparison of the mother and infant antibody concentrations
P = 0·1 (Student's paired t-test)
P = 0·0004 (Student's paired t-test).
Fig. 1.
Linear regression of infant IgG to PspA (ordinates) in function of maternal IgG to PspA (abcissas). The estimated regression equation is f(x) = − 0·5052 + 1·02362 x and the squared correlation coefficient is r2 = 0·6859.
DISCUSSION
The presence of antibody to PspA in all cord blood, and the strong correlation between antibody concentrations in mothers and their infants, provides irrefutable evidence for maternal transmission of antibodies to these pneumococcal protein antigens. If human antibodies to PspA turn out to be as protective in humans as they are in mice [4], then the results presented here indicate that infants may be able to be protected by prior immunization of their mothers.
The concentrations of antibody to the pneumococcal antigens was consistent with what had been observed in earlier studies. The concentration of antibodies to PspA and PsaA in the maternal blood was similar to that reported for young adults [3]; and the concentration of antibodies to PspA in the cord blood was similar to that observed in human infants [13]. Antibodies to PspA have been shown to rise following human infections with S. pneumoniae, and the concentrations of antibody to PspA in human serum is thought to be the results of natural exposure.
The mean concentrations of the three naturally induced antibodies (PspA, PsaA and P6-OMP) were higher in the mothers’ sera than in the infants’ sera, whereas those of vaccine-induced antibodies (mainly IgG) against tetanus toxoid were equivalent. Our results showing cross-placental transfer of antibodies to tetanus toxoid are consistent with previous reports [9,16].
Chudwin et al. [17] reported that maternal serum naturally contained a significantly higher concentration of type 7F PPS IgG relative to paired infant cord serum. The results of the present study showed that the materno-fetal transfer of antibodies to pneumococcal proteins is higher than the materno-fetal transfer of antibodies to 7F PPS (55% IgG to PPS in cord blood as compared to maternal serum with the 81·6% IgG to PspA in cord blood as compared to maternal serum). Shahid et al. [9] have also demonstrated that when the 23-valent PPS vaccine was used during the third trimester of pregnancy and an infant:mother antibody percentage of 56% was obtained IgG to 6B PPS and a percentage of 59% for IgG to 19F PPS. These investigators also demonstrated that IgG1 to PPS was more efficiently transported through the placenta than IgG2 to PPS. O’Dempsey et al. [10] also observed that by three months of age there was little difference between the antibody to PPS concentrations between infants born to vaccinated and unvaccinated women. Recently, Munoz et al. [11] reported a better transmission rates of antibodies to serotypes 6B, 14, 19F and 23F than previous studies in US pregnant women vaccinated with a 23-valent PPS in the third trimester of gestation.
As has been identified for the other protein antigens, PspA elicited a stronger IgG1 than IgG2 response. We observed that active transfer of IgG1, but not IgG2, antibody to PspA from the mother's serum into the cord blood, was provided by the almost two-fold higher concentrations of the antibody in cord blood that in maternal serum. This finding was consistent with other studies showing active transfer of IgG1 immunoglobulin into the fetus [18]. Our study demonstrates that the mothers naturally transmit antibodies to PspA and to PsaA to their infants. Higher IgG1 antibodies to PspA in cord sera as compared to mother sera indicates the active transfer of this subclass of antibody. If pneumococcal protein based vaccines are found to be efficacious, then the direct correlation of the magnitude of the mother's IgG to PspA and that of the infant suggest that immunization of women of childbearing age with protein pneumococcal vaccine could protect their infants from pneumococcal disease. The advantage of a protein-based pneumococcal vaccine is that proteins are conserved between pneumococcal strains. Clinical trial could be designed to test the potential of immunization of young adult women or infant immunization with a combination of pneumococcal proteins as vaccine approaches to prevent against invasive pneumococcal disease.
Acknowledgments
We thank Martine Wallon and François Peyron for their help in managing the serum collection (Department of Parasitology, University of Lyon 1, France). We are also indebted to Laura Ferguson, Mary Ewasyshyn and Elaine Wang from Aventis Pasteur for their support throughout this work and to Stéphanie Michard for her help with data analysis. Aventis Pasteur provided the financial support of this work.
REFERENCES
- Mulholland K, Levine O, Nohynek H, Greenwood BM. Evaluation of vaccines for the prevention of pneumonia in children in developing countries. Epidemiol Rev. 1999;21:43–55. doi: 10.1093/oxfordjournals.epirev.a017987. [DOI] [PubMed] [Google Scholar]
- Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19:187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- Nabors GS, Braun PA, Herrmann DJ, et al. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine. 2000;18:1743–54. doi: 10.1016/s0264-410x(99)00530-7. [DOI] [PubMed] [Google Scholar]
- Briles DE, Hollingshead SK, King J, et al. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis. 2000;182:1694–701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- Ren B, Szalai AJ, Thomas O, Hollingshead SK, Briles DE. Both Family 1 and Family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect Immun. 2003;71:75–85. doi: 10.1128/IAI.71.1.75-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel LS, Yother J, Vijayakumar M, McGarry L, Guild WR, Briles DE. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA) J Exp Med. 1987;165:381–94. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain MJ, Waltman WD, Turner JS, Yother J, Talkington DF, McDaniel LS, Gray BM, Briles DE. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–9. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MC, Pilling PA, Epa VC, Berry AM, Ogunniyi AD, Paton JC. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure. 1998;6:1553–61. doi: 10.1016/s0969-2126(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Shahid NS, Steinhoff MC, Hoque SS, Begum T, Thompson C, Siber GR. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet. 1995;346:1252–7. doi: 10.1016/s0140-6736(95)91861-2. [DOI] [PubMed] [Google Scholar]
- O'Dempsey TJ, McArdle T, Ceesay SJ, et al. Immunization with a pneumococcal capsular polysaccharide vaccine during pregnancy. Vaccine. 1996;14:963–70. doi: 10.1016/0264-410x(96)00009-6. [DOI] [PubMed] [Google Scholar]
- Munoz FM, Englund JA, Cheesman CC, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine. 2001;20:826–37. doi: 10.1016/s0264-410x(01)00397-8. [DOI] [PubMed] [Google Scholar]
- Samukawa T, Yamanaka N, Hollingshead SK, Klingman K, Faden H. Immune responses to specific antigens of Streptococcus pneumoniae and Moraxella catarrhalis in the respiratory tract. Infect Immun. 2000;68:1569–73. doi: 10.1128/iai.68.3.1569-1573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapola S, Jantti V, Haikala R, et al. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J Infect Dis. 2000;182:1146–52. doi: 10.1086/315822. [DOI] [PubMed] [Google Scholar]
- Virolainen A, Russell W, Crain MJ, Rapola S, Kayhty H, Briles DE. Human antibodies to pneumococcal surface protein A in health and disease. Pediatr Infect Dis J. 2000;19:134–8. doi: 10.1097/00006454-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Karalus RJ, Murphy TF. Purification and characterization of outer membrane protein P6, a vaccine antigen of non-typeable Haemophilus influenzae. FEMS Immuno Med Microbiol. 1999;26:159–66. doi: 10.1111/j.1574-695X.1999.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. J Infect Dis. 1993;168:647–56. doi: 10.1093/infdis/168.3.647. [DOI] [PubMed] [Google Scholar]
- Chudwin DS, Wara DW, Schiffman G, Artrip SG, Ammann AJ. Maternal-fetal transfer of pneumococcal capsular polysaccharide antibodies. Am J Dis Child. 1985;139:378–80. doi: 10.1001/archpedi.1985.02140060060029. [DOI] [PubMed] [Google Scholar]
- Einhorn MS, Granoff DM, Nahm MH, Quinn A, Shackelford PG. Concentrations of antibodies in paired maternal and infant sera: relationship to IgG subclass. J Pediatr. 1987;111:783–8. doi: 10.1016/s0022-3476(87)80268-8. [DOI] [PubMed] [Google Scholar]