Abstract
While the synaptic properties of Golgi cell-mediated inhibition of granule cells are well studied, less is known of the afferent inputs to Golgi cells so their role in information processing remains unclear. We investigated the responses of cerebellar cortical Golgi cells and Purkinje cells in Crus I and II of the posterior lobe cerebellar hemisphere to activation of peripheral afferents in vivo, using anaesthetized rats. Recordings were made from 70 Golgi cells and 76 Purkinje cells. Purkinje cells were identified by the presence of climbing fibre responses. Golgi cells were identified by both spontaneous firing pattern and response properties, and identification was confirmed using juxtacellular labelling of single neurones (n = 16). Purkinje cells in Crus II showed continuous firing at relatively high rates (25–60 Hz) and stimulation of peripheral afferents rarely evoked substantial responses. The most common response was a modest, long-latency, long-lasting increase in simple spike output. By comparison, the most common response evoked in Golgi cells by the same stimuli was a long-latency, long-lasting depression of firing, found in ∼70% of the Golgi cells tested. The onsets of Golgi cell depressions had shorter latencies than the Purkinje cell excitations. Brief, short-latency excitations and reductions in firing were also evoked in some Golgi cells, and rarely in Purkinje cells, but in most cases long-lasting depressions were the only significant change in spike firing. Golgi cell responses could be evoked using air puff or tactile stimuli and under four different anaesthetic regimens. Long-lasting responses in both neurone types could be evoked from wide receptive fields, in many cases including distal afferents from all four limbs, as well as from trigeminal afferents. These Golgi cell responses are not consistent with the conventional feedback inhibition or ‘gain control’ models of Golgi cell function. They suggest instead that cerebellar cortical activity can be powerfully modulated by the general level of peripheral afferent activation from much of the body. On this basis, Golgi cells may act as a context-specific gate on transmission through the mossy fibre–granule cell pathway.
The precise geometric organization of the cerebellar cortical circuitry has allowed connectivity between the individual neurones to be well studied (Eccles et al. 1966; Ito, 1984). Most electrophysiological analyses of cerebellar processing focus on Purkinje cell activity (Llinas & Sasaki, 1989; Keating & Thach, 1995; Welsh et al. 1995; Lang et al. 1999). While Purkinje cells are the output cells of the cerebellar cortex, these studies usually assume that Purkinje cell activity is a ‘simple read-out’ of the underlying activity in the granular layer. Golgi cells are an important element of the granular layer, being activated by mossy fibres both directly and via parallel fibres (Palay & Chan-Palay, 1974). Golgi cells inhibit local granule cells, so modulate excitatory transmission through mossy fibre–parallel fibre pathways. At the synaptic level, Golgi cell inhibition of granule cells has both fast synaptic and slower extrasynaptic ‘spillover’ components (e.g. Rossi & Hamann, 1998; Mitchell & Silver, 2000; Watanabe & Nakanishi, 2003). The circumstances in which Golgi cell firing is modulated in vivo are poorly understood, therefore their contribution to granule cell and Purkinje cell firing remains elusive. Based on connectivity, and assuming that Golgi cells sample inputs from parallel fibres that also excite local Purkinje cells, it was proposed that Golgi cells mediate feedback inhibition to exert ‘gain control’ over mossy fibre–granule cell transmission. In this scheme, if more granule cells become active, they excite Golgi cells and as a result produce greater inhibition of granule cells, which modulates overall excitation (Marr, 1969; Albus, 1971; Ito, 1984; see Maex & De Schutter, 1998). This feedback inhibition will be accompanied by feedforward inhibition driven by direct mossy fibre connections to the Golgi cells (Precht & Llinas, 1969). Several lines of evidence suggest that Golgi cells are not well suited for gain control, and modelling studies (Maex & De Schutter, 1998; De Schutter et al. 2000) have suggested an alternative role for Golgi cells; that is, to synchronize granule cell activity through closed loops formed by the granule cell–Golgi cell pathway, so that granule cell spiking is precisely timed by Golgi cell inhibition. Such patterns of Golgi cell–granule cell firing should be represented in the simple spike output of Purkinje cells.
Modulated activity of putative Golgi cells in vivo has been described during vestibulo-occular reflex adaptation (Miles et al. 1980), locomotion in cats (Edgley & Lidierth, 1987) and limb movement in monkeys (Van Kan & Gibson, 1993). De Schutter and coworkers have investigated the response properties of putative Golgi cells in Crus I and II of the rat cerebellar hemispheres (Vos et al. 1999, 2000; Volny-Luraghi et al. 2002). However, only recently have data been reported from Golgi cells identified definitively using juxtacellular labelling (Simpson et al. 2005).
Here we have employed juxtacellular labelling to characterize Golgi cells. We show that in addition to brief excitatory responses from focal, mainly trigeminal afferents (Vos et al. 1999), Golgi cell firing is frequently depressed over several hundred milliseconds after peripheral afferent stimulation, while the same stimuli frequently evoke modest increases in Purkinje cell firing over a similar time course. These responses can be evoked with or without preceding excitations, are of long duration and can be evoked by stimuli from much of the body suggesting a highly convergent sensory input to the cerebellar cortex. These responses have not been described before and imply a functional role for Golgi cells that is very different from current models.
Methods
Experiments were performed on 55 adult rats (Hooded and Wistar) weighing 350–500 g. Most experiments were performed under general anaesthesia with urethane (n = 45 animals, initial dose 1000 mg kg−1i.p.), although additional experiments were performed using ketamine and xylazine (n = 5 animals, 100 mg kg−1 and 10 mg kg−1, i.p. initially, supplemented with additional single doses of 25 mg kg−1 and 2.5 mg kg−1), pentobarbitone (n = 3 animals, 40 mg kg−1i.p. initially, maintained if necessary with additional doses of 5 mg) and or chloralose as the anaesthetic (n = 1 animal, 50 mg kg−1i.p. initially, maintained if necessary with additional doses of 10 mg) to verify that the responses that we describe were not anaesthesia-specific. All procedures were approved by the local ethical review panel of the University of Cambridge and the UK Home Office (Animals (Scientific Procedures) Act) regulations. Depth of anaesthesia was maintained so as to eliminate limb withdrawal reflexes to noxious stimuli.
Surgery
The femoral vein was cannulated to allow fluid delivery and anaesthetic maintenance. During some experiments, 0.9% saline solution (1 ml h−1) was delivered and a single dose of prednisolone (20 mg kg−1i.v.) was administered to alleviate cerebral oedema. The trachea was cannulated. The animals were fixed in a stereotaxic frame. Core body temperature was maintained at 37–38°C with a servo-controlled heating blanket. The muscles and soft tissue overlying the posterior cerebellum were removed and a small craniotomy was performed in order to expose the cortex. The surrounding soft tissues were formed into a pool and filled with warm paraffin oil (37–38°C) to prevent drying of the exposed cerebellar cortex; oil levels were maintained as necessary during the experiment.
Stimulation
In these experiments, the aim was to determine the distribution of inputs to Golgi cells and Purkinje cells from widely different areas of the body. In most experiments afferents were activated non-selectively by electrical stimulation through pairs of stimulating percutaneous pins (insect pins or modified 25-gauge hypodermic needles) inserted bilaterally across the skin of the mystacial whisker pads (ipsi.Vib and co.Vib) to activate afferents in the trigeminal (infraorbital) nerve and in the main forelimb footpads (ipsi.FL and co.FL) and hindlimb footpads (ipsi.HL and co.HL) to activate distal limb afferents. Stimuli were delivered at intensities sufficient to evoke small local muscle twitches of the digits or vibrissal pads, indicating direct activation of local motoneurone axons or local withdrawal reflexes. Stimulus intensities expressed relative to this motoneurone activation threshold were used during the experiment (0.5–2 times the movement threshold). In no cases was withdrawal movement evoked at more proximal limb joints. In some experiments, field potentials were recorded from the contralateral sensorimotor cortex or from the dorsum of the spinal cord to verify that the initial stimulus intensities we used were 4–10 times the threshold for detectable cortical field potentials or spinal volleys.
More physiological stimuli were also used in some experiments. Tactile stimuli were delivered by prodding or moving the skin or applying gentle pressure to the skin surface with a probe. Air puff stimuli were generated using a pressurized vessel and a solenoid valve to produce puffs with a duration of between 20 and 50 ms, with muzzle pressures of 35–350 kPa. These were delivered to the vibrissae on each side of the body and to the glabrous skin of the feet using a thin tube (muzzle diameter, 2 mm), positioned 3–5 cm away from the skin surface. The glabrous skin of the feet was indented by the air puffs, but the limb did not move.
Recording
Single-unit recordings were made from neurones located in lobules Crus Ic and IIa/b. Electrode penetrations were made at angles perpendicular to the folial surface (typically 40–50 deg from vertical) with penetrations spanning a mediolateral axis extending approximately 4 mm lateral to the paravermal vein. The depth from the folial surface rarely exceeded 3 mm.
In seven experiments, recordings were made using a system comprising 16 independently moveable microelectrodes (platinum–tungsten coated with quartz glass; shaft diameter, 80 μm; impedance, 2–3 MΩ) arranged in a 4 × 4 array (Eckhorn & Thomas, 1993), using the control system described by Baker et al. (1999). The spacing between individual electrodes was set to 305 μm. In other experiments, single Parylene-C-insulated stainless steel electrodes (2 MΩ, MPI Inc.) were used. Signals from the microelectrodes were amplified (gain, × 10000), filtered (band-pass, 0.3–10 kHz) and digitized at 25 kHz. In experiments where juxtacellular labelling was performed (n = 9), micropipettes pulled from filament glass were broken to give tip impedances of 10–25 MΩ when filled with 2% (w/v) biocytin in 0.5 m NaCl, as described by Pinault (1996).
Analysis
Single-unit spikes were discriminated using a custom-written, spike-shape analysis and cluster-cutting package (GetSpike; Dr S. N. Baker, Department of Anatomy, University of Newcastle upon Tyne). Peri-stimulus–time histograms (PSTHs) and interspike interval histograms (ISIHs) were constructed from the time series. Instantaneous frequencies were calculated for each interspike interval as the reciprocal of the interspike interval; mean instantaneous frequency as the arithmetic mean of all values.
All PSTHs were quantified using a custom-written program (Matlab; The MathWorks Inc.) incorporating a cumulative sum (CUSUM) derivative analysis (Davey et al. 1986), which facilitates the detection of persistent changes in the histogram. A period of 200 ms immediately preceding the stimulus was used as a control spontaneous activity measure and to establish the CUSUM baseline. Responses were measured relative to this background measure. For each response, cursors were positioned marking response onset and termination based on the CUSUM. The bin values within the response were compared to those of the control period using unpaired Student's t tests, and only those that were significantly different (P < 0.05) were considered to be responses. To standardize the measures of long-lasting changes in cell firing, a time window between 100 and 300 ms after the stimulus presentation was used; a response was accepted when the spike counts for these bins were significantly different from the bin counts in the 200 ms before the stimulus (unpaired Student's t test, P < 0.05). Bin size was usually 5, 10 or 20 ms and at least 50 sweeps (usually > 100) were used to construct each PSTH. A standard package was used for other statistical analyses (SigmaStat, Jandel Scientific).
Juxtacellular labelling
To label single Golgi cells and Purkinje cells, we used the juxtacellular labelling technique described by Pinault (1996), which has been applied to cerebellar interneurones recently (Simpson et al. 2005). Juxtacellular labelling was achieved using glass electrodes (filled with 2% neurobiotin in 0.5 m NaCl) by passing square-wave current pulses (0.5–3 nA; duration, 200 or 300 ms; duty cycle, 400 or 600 ms) through the electrode tip to entrain the spike discharge and electroporate the recorded cell. After allowing at least 30 min for transport of tracer, the surface of the craniotomy was marked using pontamine blue, and animals were killed by transcardiac perfusion with heparinized 0.1 m phosphate-buffered saline solution (PBS, pH 7.3) followed by 4% paraformaldehyde in the same buffer. After fixation for 24 hours in the same solution, 75 μm parasagittal sections were cut using a vibrotome. Neurobiotin-labelled neurones were visualized using standard diaminobenzidine (DAB) peroxidase histochemistry (Vector ABC Elite kit), weakly counterstained with thionine, dehydrated and cleared. Sections and labelled neurones were examined using conventional bright-field and dark-field microscopy.
Results
Identification of Golgi cells and Purkinje cells and their firing patterns
The data include recordings from a total of 146 cerebellar cortical neurones. Of these, 76 were identified as Purkinje cells, based on the presence of both simple and complex spikes and the pauses in simple spike activity that followed complex spikes. In these experiments, Purkinje cells discharged simple spikes continuously at mean rates of 23–60 Hz. The remainder of the sample of neurones (n = 70), were classified as Golgi cells according to several criteria. In previous studies, putative Golgi cells were identified from their characteristic spontaneous firing pattern and their location in the granule cell layer (Edgley & Lidierth, 1987; Atkins et al. 1997; Vos et al. 1999). Qualitatively, the spontaneous spike trains of these neurones have a distinctive rhythm of continuous, relatively low-frequency spontaneous activity. This differs from the pattern of spontaneous firing of Purkinje cells, which includes complex spikes, post complex spike pauses in simple spike firing, and characteristic interspike interval distributions that include many short (< 20 ms) intervals. Quantitative criteria for identification of Golgi cells were also applied, such as resting firing rates of 8–30 Hz, with characteristic ISIHs that have broad peaks and a scarcity of interspike intervals of less than 30 ms (Eccles et al. 1966; Schulman & Bloom, 1981; Edgley & Lidierth, 1987; Van Kan & Gibson, 1993). In these studies, we also considered the units to be Golgi cells because the discharges originated from large neurones (based on the size of the extracellular spikes and the observation that single units can be recorded over 100–150 μm of electrode travel; Schulman & Bloom, 1981) and because microlesions at recording sites identify them as originating from the granule cell layer (e.g. Edgley & Lidierth, 1987; Vos et al. 1999). In recent studies, neurones with these characteristic firing patterns have been definitively identified as Golgi cells using juxtacellular labelling (Simpson et al. 2005).
Here we describe the spontaneous firing and response patterns of a large sample of neurones that conform to the properties described above. In addition, in 16 cases we filled the recorded neurones with neurobiotin using the juxtacellular labelling technique (Pinault, 1996). Morphological analysis of these neurones confirmed that they were Golgi cells (Ramón y Cajal, 1911, Palay & Chan-Palay, 1974). As examples, data from two such neurones are illustrated in Fig. 1, which shows two neurobiotin-filled Golgi cells (Fig. 1A and E). The typical Golgi cell morphology shown in Fig. 1A and E includes dendrites that extend up into the molecular layer as well as ramifying in the granule cell layer and an axonal arborization, often very extensive and tortuous, in the granule cell layer. These stained cells all had relatively large somata (maximal diameters, 20 ± 1.2 μm (mean + sem; range, 16–26 μm and minimal diameters, 14 ± 0.7 μm; range, 11–16 μm) located in the granule cell layer. These are similar to the ranges quoted by Palay & Chan-Palay (1974) for Golgi cells (10–24 μm).
Figure 1. Characterization of Golgi cells.
A and E, photomicrographs of two typical Golgi cells, juxtacellularly filled with neurobiotin and viewed in darkfield. The Purkinje cell layer is marked by the dashed line. Scale bars, 100 μm. Within the granular layer, the axonal arbour of neurone ramifies extensively, giving rise to a profuse spray of synaptic boutons. B–D, show data from the neurone illustrated in A. B, a 1-s trace of the spontaneous spike train. C, an interspike interval histogram (bin size, 2 ms) of spontaneous activity. D, a peri-stimulus time histogram (bin size, 40 ms) showing a long-lasting depression in firing following a somatosensory stimulus at t = 0 ms. Similar data for the neurone illustrated in E are shown in panels F–H. ML, molecular layer; GL, granular layer; WM, white matter.
Example spontaneous spike trains for the two labelled Golgi cells shown in Fig. 1A and E are shown in Fig. 1B and F, with ISIHs constructed from the spontaneous spike trains of these neurones shown in Fig. 1C and G. Example responses of these neurones (PSTHs) following somatosensory stimulation (Fig. 1D and H) show that each neurone responded with a long-lasting depression of firing lasting many hundreds of milliseconds. All of the juxtacellularly labelled Golgi cells showed similar spontaneous firing patterns and the long-lasting depression response, which will be described below for these and a large group of neurones that were not juxtacellularly labelled.
As the previously used criteria for distinguishing Golgi cells and Purkinje cells were primarily qualitative, we compared some basic descriptors of the ISIH distributions from their spontaneous firing patterns to seek more definitive descriptors, using a sample of 30 Purkinje cells and 45 Golgi cells for which at least 1500 spikes were recorded. Raw data from an example Purkinje cell is shown in Fig. 2A. This shows a portion of the spike train composed of simple spikes and a complex spike (marked with an asterisk). The ISIH for this neurone is plotted in Fig. 2B and C (black bins) on which the ISIHs of the two example Golgi cells illustrated in Fig. 1 are superimposed for comparison (grey bins). Considerable overlap exists between the ISIH distributions, and basic measures of firing, such as mean spike frequency, could not be used to separate the populations (Fig. 2D: Golgi cells had mean firing frequencies in the range 2–25 Hz (cf. 8–30 Hz reported by Schulman & Bloom, 1981) whereas Purkinje cells had higher mean firing frequencies 23–75 Hz, but the populations overlap). An alternative measure we used was to take the mean of the instantaneous frequencies (the average of the instantaneous frequency of each interval in the spike train; see Edgley & Lidierth, 1987) This gave non-overlapping populations (P < 0.001; unpaired Student's t test); all Golgi cells had mean instantaneous frequencies below 28 Hz, and all Purkinje cells above 32 Hz (Fig. 2E). Similarly, the median and modal interspike interval distributions were also non-overlapping (P < 0.001; unpaired Students t test). All Golgi cell ISIHs had median intervals of 45 ms or greater, whereas Purkinje cells had median intervals of 35 ms or less (Fig. 2F). All Golgi cell ISIHs had modal intervals of 40 ms or greater, whereas Purkinje cells had modal intervals of 30 ms or less (Fig. 2G). The neurobiotin-labelled Golgi cells fell within the population of non-labelled Golgi cells, which also failed to overlap with the Purkinje cells on the three measures described above, and all of these neurones had long-lasting depression responses (described below), suggesting that the non-labelled neurones were also Golgi cells.
Figure 2. Spontaneous activity of Purkinje and Golgi cells.
A, shows raw data from a juxtacellularly labelled Purkinje cell, showing 1 s of spontaneous discharge of simple spikes and a complex spike (*), alongside 20 superimposed examples of simple spikes and complex spikes. B and C, show the interspike interval histogram (ISIH; bin size, 2 ms) for the Purkinje cell shown in A (black bins) superimposed on the ISIHs from the two juxtacellularly labelled Golgi cells illustrated in Fig. 1 (grey bins). D–G, shows four measures of spontaneous firing of Purkinje and Golgi cells, taken from a sample of 30 Purkinje cells and 45 Golgi cells: mean spike frequency, mean instantaneous frequency, median and modal interspike interval, respectively. Purkinje cells are shown as black bins, juxtacellularly labelled Golgi cells as grey bins and non-labelled Golgi cells as unshaded bins. Mean spike frequency (D) proved not to be useful in separating the firing patterns of the two neurone types, whereas any of the three other parameters provided separation (P < 0.001 Student's unpaired t test).
Response patterns of Golgi cells
Three distinct patterns of response to stimulation of peripheral afferents were seen in Golgi cells, each with characteristic latency and duration. In order of their frequency of occurrence, these were (i) a long-lasting depression of discharge, (ii) a brief, short-latency depression and (iii) a brief, short-latency excitation. Our observations on response patterns were drawn from a larger population of neurones, all of which conformed to the properties of the juxtacellularly labelled Golgi cells described above.
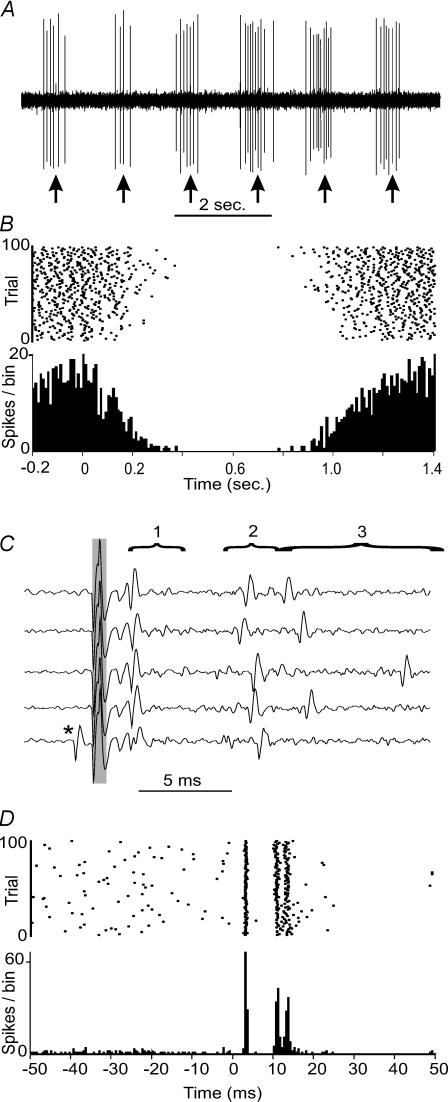
By far the most commonly encountered responses in Golgi cells were long-lasting (usually several hundred milliseconds) depressions of ongoing spike activity. These responses were present in all of the juxtacellularly labelled Golgi cells. A response of this type is illustrated in the unitary recording in Fig. 3A and the PSTH and raster in Fig. 3B, in this case evoked by stimulation of afferents from the distal ipsilateral hindlimb. Shorter duration excitations and/or depressions were also seen, but were less frequent. Figure 3C and D illustrates short-latency excitations evoked by stimulation of ipsilateral trigeminal afferents in the same neurone that responded to stimulation of the ipsilateral hindlimb with a long-lasting depression (in Fig. 3A and B). Figure 3C shows five traces of raw data (stimulus artefact marked by grey bar) in which the individual spikes can be seen. In the four upper traces an initial spike was discharged at short latency (2–4 ms after the stimulus, marked as period 1) whereas in the lowermost trace a spontaneous spike occurred just before the stimulus (marked with an asterisk) and there was no short-latency evoked spike, although a small field potential at the same latency was still evoked. Spikes also occurred within two additional time windows, 7–11 ms and 12–20 ms after the stimulus (periods 2 and 3). The corresponding raster and PSTH for this response (Fig. 3D) shows the timing of these spikes clearly. These responses (Fig. 3C and D) are very similar to those described in Golgi cells by Vos et al. (1999, 2000) that were also evoked by activation of trigeminal afferents (by prod stimuli). In addition, a brief silent period followed the excitatory responses of the Golgi cells, was also described by Vos et al. (1999). The inset panels of Fig. 6A–D show four further examples of short latency excitations evoked by stimulation of trigeminal afferents in Golgi cells, each recorded under a different anaesthetic.
Figure 3. Response patterns of Golgi cells.
A, shows a 10-s extract of spike activity from a Golgi cell during stimulation of ipsilateral hindlimb afferents (stimuli indicated by the arrows). Following each stimulus the firing was strongly and consistently depressed, which is clear in the PSTH (bin size, 10 ms) and raster plots shown in B. C and D, show the responses of the same Golgi cell to stimulation of ipsilateral trigeminal afferents. The five traces in C show that following the stimulus (artefact marked by grey bar), the Golgi cell was excited at short latency, in this case in three precisely timed periods (marked 1–3). In the lowermost trace, a spontaneous spike occurred just before the stimulus (*) and in this trial a spike was not evoked in the first period of increased excitability following the stimulus, although the underlying small field potential at similar latency was evoked. D, shows the corresponding PSTH (100 stimuli; stimulus at t = 0; bin size, 0.5 ms) and raster plots for these responses. Three peaks in the PSTH correspond to the three periods of excitation following the stimulus.
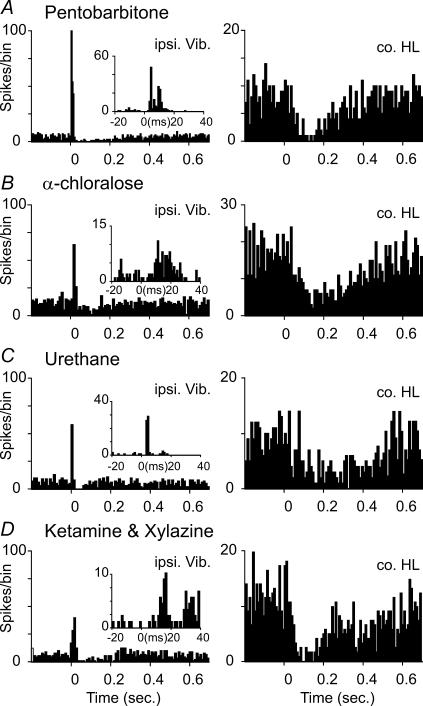
Figure 6. Similar Golgi cell responses are seen under different types of general anaesthesia.
A–D, show responses of four different Golgi cells recorded under pentobarbitone, α-chloralose, urethane and ketamine/xylazine anaesthesia, respectively. The PSTHs on the left show short-latency excitations of these neurones from stimulation of ipsilateral trigeminal afferents (ipsi.Vib), with insets showing the time course of the short-latency excitations on a shorter time scale. The PSTHs to the right show that each of these Golgi cells also responded to stimulation of contralateral hindlimb afferents (co.HL) with long-lasting depressions and no preceding excitation. All PSTHs based on 100 stimulus presentations, the stimulus was at t = 0 ms, and the bin size was 10 ms in the main PSTHs and 1 ms in the insets.
The three response types (short-latency excitations, short-lasting depressions and long-lasting depressions) were seen in individual Golgi cells in different combinations, as well as in the same neurone by stimulation of afferents from different parts of the body. Figure 4A–D illustrates a Golgi cell in which a short-lasting depression was evoked by stimulation of both ipsi.Vib and ipsi.FL, but stimulation of the ipsi.FL also evoked a long-lasting depression of firing. A different Golgi cell is illustrated in Fig. 4E and F which responded to stimulation of both co.FL and co.Vib with a long-lasting depression, but stimulation of the latter also evoked an additional short-latency excitation. These results indicate that the three response types are independent of one another (in individual Golgi cells) and thus different neural pathways are likely to underlie each response type. Individual Golgi cells could respond with one, any two or all three of these response types.
Figure 4. Different components of Golgi cell responses to somatosensory stimulation.
A and C, responses of a Golgi cell to stimulation of the ipsilateral trigeminal afferents (ipsi.Vib) and ipsilateral forelimb afferents (ipsi.FL). The horizontal grey line indicates the background firing level, based on firing during the 200 ms before the stimulus. B and D, show the initial parts of the same PSTHs, together with rasters for 300 sweeps, on an expanded time scale. Both stimuli evoked a short-lasting depression of firing; the ipsi.FL stimulus induced an additional longer-latency, longer-lasting depression. E and F, show responses of a different Golgi cell in which similar long-lasting depressions of activity were evoked by stimulation of the contralateral forelimb pads (co.FL) and contralateral trigeminal afferents (co.Vib). The co.Vib stimulus also evoked a brief short-latency excitation. PSTHs based on 300 (A–D) or 100 (E–F) presentations of a single stimulus onset at t = 0 ms, and the bin size was 5 ms.
Distribution of peripheral inputs to Golgi cells
Figure 5A, C and E shows the frequencies with which these types of response were evoked in a subgroup of 51 Golgi cells in which inputs from all four limbs and trigeminal afferents from both sides were tested; the onset latencies and durations of the responses are shown in Fig. 5B, D and F.
Figure 5. Frequencies, onsets and latencies of responses of Golgi cells.
The histograms on the left (A, C and E) show the frequencies of occurrence of each of the response types from a population of 51 Golgi cells tested with six different inputs. A, short latency excitations were most common from the ipsi.Vib and ipsi.FL stimuli. C, shows that short-latency inhibitions were seen with similar frequencies from all of the inputs. E, shows that long-latency inhibitions were most commonly evoked from the limbs than from the vibrissae (*χ2, P < 0.001). The charts in B, D and F show the onset latencies and durations of these response types, from each of the stimulus sites tested. Note the different scaling of the axes. B, shows that the short-latency excitations evoked from the vibrissae had the shortest latencies. D, shows that short-latency depressions evoked from all sources had similar latencies and durations. F, shows that long-latency depressions evoked from the limbs had longer durations than those evoked from the vibrissae, and the difference between the duration of responses from the ipsilateral vibrissae and the limbs was significant (*one-way ANOVA on ranks, P < 0.01).
Short-lasting excitations were evoked in 12/51 neurones (24%), most frequently from trigeminal afferents (Fig. 5A, but by stimulation of limb afferents in some neurones; examples are shown in Figs 7 and 10). Note that within the sample of 51 Golgi cells tested for responses from each of the six standard inputs, none responded with short-lasting excitations from either of the hindlimbs, although hindlimb-evoked, short-lasting excitations were seen in other Golgi cells, thus their latencies and durations (Fig. 5B) are drawn from outside of the sample of 51 Golgi cells in which all standard inputs were tested. The durations of short-lasting excitations were brief (Fig. 5B), usually less than 20 ms.
Figure 7. Long-lasting depressions are elicited from wide areas of the periphery in individual Golgi cells.
The PSTHs show the responses of a single Golgi cell recorded in Crus IIb following stimulation of six different skin areas (trigeminal, Vib; forelimb pads, FL; hindlimb pads, HL). Short-latency excitations are apparent from ipsi.Vib, co.Vib, ipsi.FL and co.FL, especially ipsilaterally. Stimulation of afferents from all of the limbs evoked a long-lasting depression as did the ipsi.Vib. The PSTHs were based on 100 stimulus presentations; stimuli were delivered at t = 0 ms; bin size, 10 ms.
Figure 10. Onsets and frequencies of Purkinje cell simple spike excitation responses and comparison with Golgi cell long-lasting depression responses.
A, shows the occurrence of simple spike excitation responses from each of the skin areas tested. In particular, simple spike excitation responses were significantly less likely to be evoked from the ipsilateral vibrissal skin when compared to the other skin areas (indicated by *). Otherwise, simple spike excitation responses were equally likely to be evoked from the other skin areas. B, shows the onset latencies and durations of Purkinje cell simple spike excitation responses from each of the skin areas tested. No significant differences between the onset latencies of vibrissal and limb evoked simple spike excitation responses were found although, both of the hindlimb onset latencies were significantly later than both of the forelimb onset latencies (indicated by *). No significant differences in duration of simple spike excitation responses from any of the skin areas tested were found. C, shows comparative data relating to the onset latencies and durations of the Golgi cell long-lasting depression (LLD) responses (open bars) and the Purkinje cell simple spike excitation responses (SSE; grey bars). When compared to Purkinje cell simple spike excitation onset latencies, the Golgi cell responses had significantly earlier onset latencies with the exception of the contralateral forelimb. In terms of durations, Golgi cell responses were significantly shorter than Purkinje cell responses with the exception of those responses from the contralateral forelimb. (all significant differences indicated by *). Error bars represent s.e.m.
Short-lasting depressions were seen in 21/51 neurones (41%). They had similar onset latencies to the short-lasting excitations, and had durations of 30–50 ms (Fig. 5D). In contrast to the short-lasting excitations, the short-lasting depressions were evoked with approximately equal frequency (in 10–15% of neurones) from all of the sources of afferents tested (Fig. 5C).
In total, 35/51 (69%) of Golgi cells showed a significant long-lasting depression following stimulation of peripheral inputs. The mean onset latencies of long-lasting depressions were between 40 and 60 ms, and their durations were much greater, usually several hundred milliseconds (see Fig. 5F). Given the duration of these responses, they represent a substantial change in the spike output of the Golgi cell over a prolonged period. To our knowledge, these responses have not been described before. Brief (duration, 100–200 ms) depressions of firing rate follow short-latency excitations (Vos et al. 1999; and e.g. Fig. 6 responses to ipsi.Vib), but in most cases the depressions in firing that we encountered (both short lasting and long lasting) were not preceded by excitations (see Figs 3A and B, and 6A–D for co.HL responses and Fig. 7 for ip.HL and co.HL responses), had longer onset latencies, and were longer lasting.
Lack of dependence of long-lasting depressions of Golgi cell firing on type of anaesthesia
One concern when dealing with responses in the cerebellar cortex is that responses may differ under different anaesthetic regimens. In order to ensure that the appearance of these responses was not dependent on the urethane anaesthesia that we used in most experiments, other experiments were performed under different anaesthetic regimens; ketamine and xylazine (n = 5 animals, n = 12 cells), pentobarbitone (n = 3 animals, n = 14 cells) and α-chloralose (n = 1 animal, n = 6 cells). We were able to observe long-lasting depressions (as well as the short-latency responses) under all of these anaesthetic regimens and in all cases the long-duration depressions of firing were the most frequent responses. Figure 6 illustrates the responses of four different Golgi cells, one recorded under each anaesthetic regimen. In each neurone, a short-latency excitation was also elicited by stimulation of the ipsi.Vib (see inset panels for PSTHs with an expanded time scale), and stimulation of the co.HL evoked a long-lasting depression of firing. These findings suggest that the neural pathways/mechanism underlying each Golgi cell response type function similarly under each of the anaesthetic regimens, and that there are common patterns of response in this part of the cerebellum.
Widespread convergence of peripheral afferent information onto Golgi cells
A striking feature of the long-lasting depressions of Golgi cell firing was that they could be evoked in individual cells from widespread areas including both sides of the face and the limbs on both sides of the body. An example of such bilateral convergence of afferent information reaching a single Golgi cell is shown in Fig. 7, with long-lasting depressions from the trigeminal afferents and vibrissal skin and from each of the limbs. In some cases, bilateral convergence from different limbs was also observed in Golgi cells with short-lasting depressions (data not shown).
Among the 51 Golgi cells in which inputs from all of the limbs and from trigeminal afferents bilaterally were tested, long-lasting depressions were evoked from all six skin areas in 6% of neurones, from three to five areas in each of 21% of neurones, from two areas in 6% of neurones and from only one skin area in 25%. By comparison, short-lasting excitations were evoked from three or more sources in only two neurones (4%), from two sources in two neurones (4%), but most frequently (eight neurones, 16%) from a single source, most commonly ipsi.Vib or ipsi.FL (Fig. 5A). Short-lasting depressions followed a similar pattern with responses evoked from three or more sources in five neurones (10%), from two sources in four neurones (8%) and most frequently from a single skin area (12 neurones, 24%). Short-lasting depressions were evoked with similar frequencies from each of the stimulation sites (10–15%, Fig. 5C).
It is surprising that for a region of the cerebellum in which short-lasting excitations were most frequent from trigeminal afferents, long-lasting depressions were evoked more frequently from limb afferents than from trigeminal afferents. Figure 5E shows the frequencies with which long-lasting depressions were evoked from the different stimulation sites. The frequency of occurrence of these responses following stimulation of ipsi.Vib or co.Vib was significantly lower than following stimulation of the limbs (χ2, P < 0.001; asterisks in Fig. 5E). The depressions evoked by different stimuli also had different durations, as shown in the bar plots in Fig. 5F. Responses evoked by stimulation of ipsilateral trigeminal afferents (ipsi.Vib) were briefer than those evoked by stimulation of any of the limb footpads (P < 0.05, one-way ANOVA on ranks, Dunn's method for multiple comparisons), although they were not significantly different from those responses evoked from co.Vib.
Golgi cell responses are readily evoked using air puff stimuli
As the principal aim of these experiments was to assess the presence of pathways from different regions of the body, we used electrical stimuli to activate peripheral afferents. The long-lasting depressions in Golgi cells were evoked from many peripheral sites and were therefore likely to be evoked from a pathway with high spatial convergence, these pathways themselves can also have input from several different sensory modalities (Bloedel & Courvile, 1981). Nevertheless, electrical stimulation generates non-physiological nerve volleys (Kolb et al. 1997). Mechanical stimulation using a paintbrush or rod produced these responses in a small number of neurones tested (n = 6), suggesting that relatively low-threshold mechanoreceptors contribute to the responses. We therefore tested whether the long-lasting depressions could be evoked by more physiological stimulation using brief air puffs directed against the glabrous skin of the limb pads. These stimuli, usually delivered to the hindlimb, generated small indentations of the glabrous skin and moved the hairs surrounding it. They readily evoked long-lasting depressions of firing. When directed to the vibrissae, short-lasting excitations were readily evoked from Golgi cells that responded to electrical stimulation of trigeminal afferents. A total of 10 neurones were tested in this way. Figure 8 shows responses of an example Golgi cell following air puff stimuli to the vibrissae on either side of the face (which evoked short-lasting excitations followed by depressions, Fig. 8A and B) and to each of the hindlimbs (which evoked robust long-lasting depressions Fig. 8Ca and Da), although these were less prominent than the responses to electrical stimulation (shown for comparison in Fig. 8Cb and Db).
Figure 8. Short-latency excitations and long-lasting depressions are readily evoked using air puff stimuli.
A and B, show PSTHs showing responses of a single Golgi cell following brief air puff stimuli that deflected the vibrissae on either side of the face. In both cases, short-lasting excitations responses were evoked and each was followed by a long-lasting depression. In Ca and Da, long-lasting depressions were evoked in the same neurone following air puff stimuli delivered to either of the hindlimb footpads, and for comparison Cb and Db show the responses evoked by electrical stimulation of hindlimb afferents. PSTHs were based on 100 stimulus presentations, stimulus delivered at t = 0 ms; bin size, 20 ms (A–B) or 40 ms (C–D).
Response patterns of Purkinje cells
Purkinje cell responses were less pronounced than Golgi cell responses. Of most importance, none showed long-lasting depressions of firing like those shown by Golgi cells. The majority (91%) of Purkinje cells showed changes in simple spike output that were classified into two types, a brief short-latency excitation (in 9% of cells), and a longer-latency, longer-lasting increase in simple spike output (in 82% of cells), with the remaining 9% of Purkinje cells showing no obvious changes in their simple spike activity. Climbing fibre responses occurred spontaneously at low rate, but in this region of the cerebellum, none of the Purkinje cells responded detectably at short latency with complex spike responses to the stimuli we used.
The PSTHs and CUSUMs illustrated in Fig. 9 show examples of the two different types of Purkinje cell simple spike responses. Figure 9A shows a short-lasting excitation, followed by a longer-lasting period of excitation separated by a brief period of reduced firing, evoked by stimulation of trigeminal afferents. Similar responses in Purkinje cells in Crus II have been reported (Bower & Woolston, 1983; Brown & Bower, 2001) in response to vibrissal stimulation. The early component of this type of response has also been described by Armstrong & Drew (1980) following electrical stimulation of trigeminal afferents. These responses were seen in 4/46 Purkinje cells.
Figure 9. Response patterns of Purkinje cells.
A and B, show PSTHs and CUSUMs of typical simple spike responses of Purkinje cells following stimulation of trigeminal afferents (A, ipsi. Vib) or limb afferents (B, ipsi. HL). Note the long duration, but subtle increase in simple spike firing following the limb stimulus, shown more clearly by the CUSUM trend line. C, the CUSUM trend lines show the responses of a single Purkinje cell recorded in Crus IIa following stimulation of six different skin areas, as in Fig. 8. Each of the CUSUM trend lines indicated with an asterisk indicates a significant increase in simple spike occurrence (P < 0.05, see Methods). PSTHs based on 200 stimulus presentations; stimuli were delivered at t = 0 ms; bin size 5 ms (A) and 20 ms (B).
Longer-latency, longer-lasting accelerations of Purkinje cell firing were seen in 82% of cells indicated by an upward sloping CUSUM trend line. These responses were seldom obvious when listening to the spike train: note the y-axis scale of the PSTH in Fig. 9B. These Purkinje cell responses represented increases in simple spike firing of 2–12% relative to the baseline period of firing before stimulus onset (−200 to 0 ms). These relatively small increases in simple spike firing imposed on the continuous firing of Purkinje cells tend to be masked, but are nonetheless consistent changes in simple spike output.
Long-lasting simple spike responses could be evoked from any of the six standard skin areas tested and their frequency of occurrence is plotted in Fig. 10A. Like the long-lasting depressions of Golgi cell firing, stimulation of ipsilateral trigeminal afferents evoked significantly fewer responses than limb inputs (P < 0.024, χ2 test, asterisk in Fig. 10A). The summary data of Fig. 10B show the onset latencies and durations of the long-lasting simple spike responses. In terms of onset latencies, no statistical differences were found between trigeminal afferent- and limb-evoked responses. However, responses evoked by hindlimb stimulation had later onset latencies when compared to responses evoked from either of the forelimbs (P < 0.05, Wilcoxon rank sum test and unpaired Student's t test, marked by asterisk in Fig. 10B). No statistically significant differences were found between the response durations of Purkinje cells from any of the skin areas tested. These findings suggest that Purkinje cell simple spike excitations have similar durations irrespective of skin areas from which the sensory afferents arise.
The wide receptive fields from which responses were evoked in Purkinje cells is reminiscent of the large, bilateral receptive fields from which long-lasting depressions were evoked in Golgi cells. Furthermore, individual Purkinje cells could show long-lasting excitatory responses from more than one skin area, with 50% (12/24) of neurones tested with all six standard skin areas being responsive to two or more skin areas. Convergence between limb and trigeminal input was also observed in 6/24 neurones (25%), with 11 (46%) neurones having bilateral receptive fields (limbs and/or trigeminal).
Comparison of Golgi and Purkinje cell responses
In direct comparison (Fig. 10C) long-lasting depressions evoked from a given location in Golgi cells had shorter onset latencies than the Purkinje cell excitations (P≤ 0.01, Wilcoxon rank sum test or unpaired Student's t test, as appropriate), with the exception of responses from the contralateral forelimb (Fig. 10C). The Purkinje cell responses also had significantly greater durations than those of Golgi cells, assessed from the CUSUMs as described in the Methods (Fig. 10B, P ≤ 0.03 Wilcoxon rank sum test or unpaired Student's t test, as appropriate). Taken together, these observations suggest that Golgi cell long-lasting depressions begin approximately 100 ms before the excitatory Purkinje cell responses, and that these outlast the Golgi cell responses by 200–400 ms.
Discussion
Characterization of Golgi cells and Purkinje cells
To our knowledge this is the first study in which the somatosensory responses of definitively identified Golgi cells have been described. Morphologically, the neurones we recovered using juxtacellular labelling show the classic features of Golgi cells (e.g. Ramón y Cajal, 1911; Eccles et al. 1966). All of the labelled Golgi cells had similar patterns of spontaneous activity and similar long-lasting depressions of activity following peripheral afferent stimulation. As all of the 16 labelled neurones were unambiguously Golgi cells, we infer that the other non-biotin-filled neurones, which had similar spontaneous and response patterns, were also Golgi cells. All of our Golgi cells showed characteristics previously attributed to juxtacellularly labelled Golgi cells (Simpson et al. 2005) and to putative Golgi cells: particularly firing rates of 8–30 Hz (very few interspike intervals < 30 ms) and a distinctive ISIH shape (Eccles et al. 1966; Schulman & Bloom, 1981; Edgley & Lidierth, 1987; Van Kan & Gibson, 1993; Atkins et al. 1997; Vos et al. 1999). Patterns of spontaneous discharge and response were qualitatively similar using four different anaesthetics; notably the spontaneous firing pattern is qualitatively similar to that described in vitro (e.g. Dieudonné, 1998; Carta et al. 2004).
Purkinje cells were easily identified by their complex spike discharges and in a small number of cases (n = 3) we juxtacellularly labelled Purkinje cells. Their spontaneous firing patterns were easily distinguished from Golgi cell firing patterns in our experiments by mean instantaneous frequency and median or modal interspike interval (Fig. 2) but not by mean rate.
Patterns of Golgi cell responses
Previous studies of putative Golgi cells using tactile stimuli described brief excitations, frequently followed by ‘silent periods’ of 100–200 ms (Vos et al. 1999, 2000; Van Kan & Gibson, 1993). Very similar responses were also evoked in this study by electrical stimuli (cf. Fig. 3C and D with Fig. 3 of Vos et al. 1999), although they were less frequent than the long-lasting depressions of firing. The generation of both long- and short-lasting depressions in the same neurones shows that more than one pathway can influence Golgi cell activity. Furthermore, since air puff stimuli (as well as electrical stimuli) generated these responses, tactile mechanoreceptors can effectively activate the ascending pathway (Fig. 8).
In agreement with Vos et al. (1999), excitatory responses in Golgi cells were most frequently evoked from trigeminal afferents. In contrast, stimulation of forelimb or hindlimb afferents also evoked these responses, albeit in a small proportion of neurones. It is surprising that long-lasting depressions were more frequently evoked than excitations and were significantly more frequent following limb afferent stimulation than following trigeminal afferent stimulation. Short-latency excitations of mossy fibres and Purkinje cells in Crus I and II have been reported to be evoked principally by trigeminal inputs (e.g. Shambes et al. 1978; Bower & Woolston, 1983), like the excitations of Golgi cells (Vos et al. 1999; and Fig. 5).
Mechanism of Golgi cell long-lasting depressions
Well-established inhibitory pathways to Golgi cells are few. Purkinje cell collaterals are unlikely to have initiated the long-lasting depressions, as local Purkinje cells were not excited at the appropriate latencies, either through parallel or climbing fibre pathways (see Figs 9 and 10C). Golgi cells possess glycinergic and GABAergic synaptic currents (Dieudonné, 1995; Dieudonné & Dumoulin, 2000; Donmoulin et al. 2001), so local basket or stellate cells may play a role. The recently described inhibitory Lugaro cell may also contribute to the depression (Dieudonné & Dumoulin, 2000). There is little information on afferent connections to Lugaro cells, which are considered to be sparse (Dieudonné & Dumoulin, 2000), although there is evidence for climbing fibre input to them (Ekerot & Jorntell, 2003). One possibility is that our stimuli activated serotoninergic systems and as a result activated Lugaro cells to produce depressions of Golgi cell firing. Our finding that depressions were readily evoked with air puff stimuli suggests that this is unlikely. Lugaro cells are also reported to inhibit not only Golgi cells but also basket and stellate cells (Laine & Axelrad, 1998), suggesting a broader function promoting flow of mossy fibre–granule cell signals through to Purkinje cells. However, Lugaro cells have also been reported to inhibit Purkinje cells (Dean et al. 2003), which might be consistent with the modest responses of Purkinje cells seen in this study.
An alternative mechanism may involve group 2 metabotropic glutamate receptors (mGluRs), which generate postsynaptic inhibition in Golgi cells (Watanabe & Nakanishi, 2003). However, most of the depressions (short or long latency) in Golgi cells were not preceded by excitations, which should also be evoked by excitatory glutamate receptors (Watanabe & Nakanishi, 2003). If the depressions were generated through the activation of inhibitory mGluRs, then some mechanism must prevent preceding excitatory responses. This could arise if many parallel fibre–Golgi cell synapses were ‘silent’ with respect to excitation (as proposed for parallel fibre–Purkinje cell synapses by Isope & Barbour, 2002), yet still generate inhibition. However, different populations of Golgi cells may be mGluR2 positive or negative (Neki et al. 1996), so this mechanism may not fully explain the depressions of Golgi cell firing.
Patterns of Purkinje cell response
The Purkinje cell responses found in this study following trigeminal afferent stimulation are very similar to those reported previously in this region by Armstrong & Drew (1980) and Bower & Woolston (1983), although responses evoked from limb afferents were not reported in their studies. The most common response of Purkinje cells in our study was a long-lasting increase in simple spike output. In comparison to the responses in Golgi cells, the magnitudes of these simple spike responses were modest (2–12% increase above background firing rate): they may have been overlooked in previous studies. However, these were consistent responses. The relative subtlety of these responses may be related to the low efficacy of the parallel fibres synapses, especially if some are ‘electrically silent’ (Isope & Barbour, 2002). Robust granule cell activity, as would be predicted during Golgi cell long-lasting depressions, is likely also to drive stellate and basket cells which in turn may dampen large increases in simple spike output.
Convergence of afferent information
The convergence of information observed in Golgi cells and Purkinje cells is in strong contrast to the small focal receptive fields reported previously, but could be mediated by a variety of ascending pathways that terminate as mossy fibres which have broad spatial convergence as well as input convergence from different somatosensory modalities (Bloedel & Courvile, 1981). The spatial convergence could occur at spinal level in ascending tract neurones, supraspinally in brainstem precerebellar nuclei, or within the cerebellum. Golgi cells are excited by both mossy fibres and parallel fibres and the long-lasting depressions could reflect reduced activity in afferents that specifically target Golgi cells, but not Purkinje cells, as proposed for spinoreticular afferents by Arshavsky et al. (1986). Given the wide receptive fields from which these responses could be evoked, several afferent pathways which have spatially convergent inputs that project to the cerebellum as mossy fibres might underlie them (e.g. ‘non-specific’ pathways, see Oscarsson, 1973; Bloedel & Courvile, 1981; Arshavsky et al. 1986). As these responses could be evoked by air puff stimuli, low threshold mechanoreceptors are likely to contribute to this convergence. The best studied non-specific pathway involves the lateral reticular nuclei (LRN), which receive ascending spinal afferent input from all limbs (Ekerot & Oscarsson, 1975) and from trigeminal afferents (Clendenin et al. 1975). Ascending inputs to the LRN arise from spinal pathways that themselves relay convergent information. The spinoreticular systems have therefore been considered to be responsible for controlling general levels of excitability within the cerebellar cortex (see Arshavsky et al. 1986).
Ekerot & Jorntell (2001) reported that parallel fibre receptive fields of cat C3 zone Purkinje cells (and Golgi cells) were related to specific climbing fibre receptive fields. In their study, the parallel fibre- and climbing fibre-specific receptive fields were small, with distinct borders restricted to one forelimb. Our data differ from this pattern in general, but crucially were recorded from regions of the cerebellum without the specific focal somatosensory input to climbing fibres; the somatosensory stimuli we used did not generate any substantial climbing fibre activation in Purkinje cells. In contrast, Eccles et al. (1971) reported convergent cutaneous and muscle inputs from both forelimbs and hindlimbs reaching Purkinje cells in the anterior lobe vermis. Although not easily comparable, the convergence of inputs from each of the limbs reported by Eccles et al. (1971) suggests similarities to our own observations of convergent inputs to Purkinje cells. Overall, this indicates that some regions of the cerebellum can be influenced from a broad receptive field.
Implications of the different responses of Golgi and Purkinje cells for cerebellar cortical function
Golgi cells have long been viewed as forming a negative feedback loop, controlling activity in the granule cells that supply them with parallel fibres (gain control; Eccles et al. 1966; Marr, 1969; Albus, 1971). This view has been criticised before (Maex & De Schutter, 1998; De Schutter et al. 2000). Edgley & Lidierth (1987) compared the firing patterns of Purkinje and putative Golgi cells located close to each other in the granular layer and found that the two types of cells showed a wide variety of different firing patterns. The findings reported here add to these arguments and are inconsistent with such gain control: long-lasting depressions were commonly evoked from a highly convergent pathway, rather than from a focal receptive field, and the responses of Purkinje cells and Golgi cells from most of the body were of opposite sign. In response to a stimulus that evokes mossy fibre activity, the most frequent response seen in Golgi cells was a depression of firing, which would not limit granule cell firing as suggested in the original ideas of gain control, but permit more of it. Alternatively, Maex & De Schutter (1998) proposed that Golgi cells function to determine the precise timing of granule cell activity, setting up synchronous oscillations in granule cell populations. Repetitive Golgi cell activity is essential to sustain these oscillations. During long-lasting depressions, some Golgi cells fell silent, substantially limiting their ability to contribute to the proposed oscillations.
Decreased Golgi cell firing over a sustained period will reduce both direct inhibition and the tonic spillover inhibition maintained by GABA released into the glomerulus (e.g. Rossi & Hamann, 1998; Mitchell & Silver, 2000). Granule cells under inhibitory control from the depressed Golgi cells should be more excitable during this period, and this might explain the observed increase in Purkinje cell output. The delay between the onset of the Golgi cell depression and the Purkinje cell excitations (see Fig. 10C) could be a reflection of the time taken for the tonic inhibition of granule cells to subside sufficiently to allow them to drive the Purkinje cells.
When considered at the level of the cerebellar nuclei, given that each nuclear cell receives convergent input from many Purkinje cells (Ito, 1984) the relatively modest increases in simple spike firing generated by peripheral afferent stimulation in individual cells may sum to produce substantial increases in inhibition at the cerebellar nuclei. Indeed, the effects of increased Purkinje cell output may be further enhanced by spillover mechanisms at the Purkinje cell–nuclear cell synapses (Telgkamp et al. 2004). Recent nuclear cell recordings in the rat revealed that many nuclear neurones receive convergent inputs and are responsive to air puff stimulation of trigeminal afferents from either side of the facial skin and from each of the limbs (Rowland & Jaeger, 2005). These nuclear cell responses consisted of a mixture of short-latency, short-lasting excitations and inhibitions and in particular long-latency, long-lasting excitations occurring over similar time scales as the Purkinje cell responses reported here.
Our data demonstrate that excitatory transmission to Purkinje cells is enhanced during the long-lasting depressions of Golgi cell firing. Golgi cells may therefore act as associative filters, normally stifling the firing of weakly excited granule cells generated by mossy fibre inputs. Under situations of activation of many afferents from many parts of the periphery are activated, as generated by our stimuli, Golgi cells would be depressed, permitting increased transmission of information through granule cells. This would be contingent upon events from the whole of the body. Recent in vitro demonstrations that increased activity in the mossy fibre–parallel fibre–Purkinje cell pathway may generate long-term potentiation (see e.g. Lev-Ram et al. 2003) suggest that the Golgi cell filter may be of considerable consequence for cerebellar cortical plasticity.
Acknowledgments
This work was supported by the Medical Research Council of Great Britain and the Isaac Newton Trust. Multiple electrode recordings were made possible with the generous assistance of Dr Stuart N. Baker, Demetris Soteropolous, Shelley Rhodes (University of Newcastle upon Tyne) and Daniel Z. Wetmore (Stanford University).
References
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- Armstrong DM, Drew T. Responses in the posterior lobe of the rat cerebellum to electrical stimulation of cutaneous afferents to the snout. J Physiol. 1980;309:357–374. doi: 10.1113/jphysiol.1980.sp013513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovsky GN. Cerebellum and Rhythmical Movements. Studies in Brain Function. Vol. 13. Berlin: Springer; 1986. [Google Scholar]
- Atkins MJ, Van Alphen AM, Simpson JI. Characteristics of putative Golgi cells in the rabbit cerebellar flocculus. Abstr Soc Neurosci. 1997;23:1287. [Google Scholar]
- Baker SN, Philbin N, Spinks R, Pinches EM, Wolpert DM, MacManus DG, Pauluis Q, Lemon RN. Multiple single unit recording in the cortex of monkeys using independently moveable microelectrodes. J Neurosci Methods. 1999;94:5–17. doi: 10.1016/s0165-0270(99)00121-1. [DOI] [PubMed] [Google Scholar]
- Bloedel JR, Courvile J. Cerebellar afferent systems. In: Brookhart J, Mountcastle V, Brookes V, Geiger S, editors. Handbook of Physiology, The Nervous System, Part 2, Motor Control. Bethesda, MD: American Physiological Society; 1981. pp. 735–830. [Google Scholar]
- Bower JM, Woolston DC. Congruence of spatial organization of tactile projections to granule cell and Purkinje cell layers of cerebellar hemispheres of the albino rat: vertical organization of cerebellar cortex. J Neurophysiol. 1983;49:745–766. doi: 10.1152/jn.1983.49.3.745. [DOI] [PubMed] [Google Scholar]
- Brown IE, Bower JM. Congruence of mossy fiber and climbing fiber tactile projections in the lateral hemispheres of the rat cerebellum. J Comp Neurol. 2001;429:59–70. doi: 10.1002/1096-9861(20000101)429:1<59::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clendenin M, Ekerot CF, Oscarsson O. The lateral reticular nucleus in the cat. IV. Activation from dorsal funiculus and trigeminal afferents. Exp Brain Res. 1975;24:131–144. doi: 10.1007/BF00234059. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Ellaway PH, Stein RB. Statistical limits for detecting change in the cumulative sum derivative of the peristimulus time histogram. J Neurosci Methods. 1986;17:153–166. doi: 10.1016/0165-0270(86)90068-3. [DOI] [PubMed] [Google Scholar]
- De Schutter E, Vos B, Maex R. The function of cerebellar Golgi cells revisited. Prog Brain Res. 2000;124:81–93. doi: 10.1016/s0079-6123(00)24009-0. [DOI] [PubMed] [Google Scholar]
- Dean I, Robertson SJ, Edwards FA. Serotonin drives a novel GABAergic synaptic current recorded in rat cerebellar Purkinje cells: a Lugaro cell to Purkinje cell synapse. J Neurosci. 2003;23:4457–4469. doi: 10.1523/JNEUROSCI.23-11-04457.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonné S. Glycinergic synaptic currents in Golgi cells of the rat cerebellum. Proc Natl Acad Sci U S A. 1995;92:1441–1445. doi: 10.1073/pnas.92.5.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonné S. Submillisecond kinetics and low efficacy of parallel fibre-Golgi cell synaptic currents in the rat cerebellum. J Physiol. 1998;510:845–866. doi: 10.1111/j.1469-7793.1998.845bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonné S, Dumoulin A. Serotonin-driven long-range inhibitory connections in the cerebellar cortex. J Neurosci. 2000;20:1837–1848. doi: 10.1523/JNEUROSCI.20-05-01837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Dieudonné S. IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J Neurosci. 2001;21:6045–6057. doi: 10.1523/JNEUROSCI.21-16-06045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Faber DS, Murphy JT, Sabah NH, Taborikova H. Investigations on integration of mossy fiber inputs to Purkyne cells in the anterior lobe. Exp Brain Res. 1971;13:54–77. [PubMed] [Google Scholar]
- Eccles JC, Llinas RR, Sasaki K. The mossy fibre-granule cell relay of the cerebellum and its inhibitory control by Golgi cells. Exp Brain Res. 1966;1:82–101. doi: 10.1007/BF00235211. [DOI] [PubMed] [Google Scholar]
- Eckhorn R, Thomas U. A new method for the insertion of multiple microprobes into neural and muscular tissue, including fiber electrodes, fine wires, needles and microsensors. J Neurosci Methods. 1993;49:175–179. doi: 10.1016/0165-0270(93)90121-7. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Lidierth M. The discharges of cerebellar Golgi cells during locomotion in the cat. J Physiol. 1987;392:315–332. doi: 10.1113/jphysiol.1987.sp016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF, Jorntell H. Parallel fibre receptive fields of Purkinje cells and interneurons are climbing fibre-specific. Eur J Neurosci. 2001;13:1303–1310. doi: 10.1046/j.0953-816x.2001.01499.x. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Jorntell H. Program No. 274.3 2003 Abstract Viewer/Itinerary Planner. Washington DC: Society for Neuroscience; 2003. Synaptic input to cerebellar Lugaro and Golgi cells studied with whole cell recording in vivo. [Google Scholar]
- Ekerot CF, Oscarsson O. Inhibitory spinal paths to the lateral reticular nucleus. Brain Res. 1975;99:157–161. doi: 10.1016/0006-8993(75)90619-8. [DOI] [PubMed] [Google Scholar]
- Isope P, Barbour B. Properties of unitary granule cell →Purkinje cell synapses in adult rat cerebellar slices. J Neurosci. 2002;22:9668–9678. doi: 10.1523/JNEUROSCI.22-22-09668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. New York: Raven Press; 1984. [Google Scholar]
- Keating JG, Thach WT. Nonclock behavior of inferior olive neurons: interspike interval of Purkinje cell complex spike discharge in the awake behaving monkey is random. J Neurophysiol. 1995;73:1329–1340. doi: 10.1152/jn.1995.73.4.1329. [DOI] [PubMed] [Google Scholar]
- Kolb FP, Arnold G, Lerch R, Straka HJ. Spatial distribution of field potential profiles in the cat cerebellar cortex evoked by peripheral and central inputs. Neuroscience. 1997;81:1155–1181. doi: 10.1016/s0306-4522(97)00255-8. [DOI] [PubMed] [Google Scholar]
- Laine J, Axelrad H. Lugaro cells target basket and stellate cells in the cerebellar cortex. Neuroreport. 1998;9:2399–2403. doi: 10.1097/00001756-199807130-00045. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Sugihara I, Welsh JP, Llinas R. Patterns of spontaneous Purkinje cell complex spike activity in the awake rat. J Neurosci. 1999;19:2728–2739. doi: 10.1523/JNEUROSCI.19-07-02728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SBV, Kleinfeld D, Tsien R. Reversing cerebellar long-term depression. Proc Natl Acad Sci U S A. 2003;100:15989–15993. doi: 10.1073/pnas.2636935100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sasaki K. The functional organization of the olivo-cerebellar system as examined by multiple Purkinje cell recordings. Eur J Neurosci. 1989;1:587–602. doi: 10.1111/j.1460-9568.1989.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Maex R, De Schutter E. Synchronization of Golgi and granule cell firing in a detailed network model of the cerebellar granule cell layer. J Neurophysiol. 1998;80:2521–2537. doi: 10.1152/jn.1998.80.5.2521. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FA, Fuller JH, Braitman DJ, Dow BM. Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J Neurophysiol. 1980;43:1437–1476. doi: 10.1152/jn.1980.43.5.1437. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus. J Neurosci. 2000;20:8651–8658. doi: 10.1523/JNEUROSCI.20-23-08651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neki A, Ohishi H, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Metabotropic glutamate receptors mGluR2 and mGluR5 are expressed in two non-overlapping populations of Golgi cells in the rat cerebellum. Neuroscience. 1996;75:815–826. doi: 10.1016/0306-4522(96)00316-8. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Functional organisation of spinocerebellar paths. In: Iggo A, editor. Handbook of Sensory Physiology, vol. II, Somatosensory System. New York: Springer-Verlag; 1973. pp. 339–380. [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex: Cytology and Organisation. New York: Springer Verlag; 1974. [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Precht W, Llinas R. Functional organisation of the vestibular afferents to the cerebellar cortex of the frog and cat. Exp Brain Res. 1969;9:30–52. doi: 10.1007/BF00235450. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histology of the nervous system of man and the vertebrates. I and II. Paris: Maloine; 1911. [Google Scholar]
- Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity α6 subunit GABAa receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Rowland NC, Jaeger D. Coding of tactile response properties in the rat deep cerebellar nuclei. J Neurophysiol. 2005;94:1236–1251. doi: 10.1152/jn.00285.2005. [DOI] [PubMed] [Google Scholar]
- Schulman JA, Bloom FE. Golgi cells of the cerebellum are inhibited by inferior olive activity. Brain Res. 1981;210:350–355. doi: 10.1016/0006-8993(81)90908-2. [DOI] [PubMed] [Google Scholar]
- Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol. 1978;15(2):94–140. doi: 10.1159/000123774. [DOI] [PubMed] [Google Scholar]
- Simpson JI, Hulscher HC, Sabel-Goedknegt E, Ruigrok TJ. Between in and out: linking morphology and physiology of cerebellar cortical interneurons. Prog Brain Res. 2005;2005:329–340. doi: 10.1016/S0079-6123(04)48026-1. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Padgett DE, Ledoux VA, Woolley CS, Raman IM. Maintenance of high-frequency transmission at Purkinje to cerebellar nuclear synapses by spillover from boutons with multiple release sites. Neuron. 2004;41:113–126. doi: 10.1016/s0896-6273(03)00802-x. [DOI] [PubMed] [Google Scholar]
- Van Kan PL, Gibson AR. Movement-related inputs to intermediate cerebellum of the monkey. J Neurophysiol. 1993;69:74–94. doi: 10.1152/jn.1993.69.1.74. [DOI] [PubMed] [Google Scholar]
- Volny-Luraghi A, Maex R, Vos B, De Schutter E. Peripheral stimuli excite coronal beams of Golgi cells in rat cerebellar cortex. Neuroscience. 2002;113:363–373. doi: 10.1016/s0306-4522(02)00196-3. [DOI] [PubMed] [Google Scholar]
- Vos BP, Volny-Luraghi A, De Schutter E. Cerebellar Golgi cells in the rat: receptive fields and timing of responses to facial stimulation. Eur J Neurosci. 1999;11:2621–2634. doi: 10.1046/j.1460-9568.1999.00678.x. [DOI] [PubMed] [Google Scholar]
- Vos BP, Volny-Luraghi A, Maex R, De Schutter E. Precise spike timing of tactile-evoked cerebellar Golgi cell responses: a reflection of combined mossy fiber and parallel fiber activation. Prog Brain Res. 2000;124:95–106. doi: 10.1016/s0079-6123(00)24010-7. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Nakanishi S. mGluR2 postsynaptically senses granule cell inputs at Golgi cell synapses. Neuron. 2003;39:821–829. doi: 10.1016/s0896-6273(03)00530-0. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Lang EJ, Suglhara I, Llinas R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]