Abstract
Optimedin, also known as olfactomedin 3, belongs to a family of olfactomedin domain-containing proteins. It is expressed in neural tissues and Pax6 is involved in the regulation of its promoter. To study possible effects of optimedin on the differentiation of neural cells, we produced stably transfected PC12 cell lines expressing optimedin under a tetracycline-inducible promoter. Cells expressing high levels of optimedin showed higher growth rates and stronger adhesion to the collagen extracellular matrix as compared with control PC12 cells. After stimulation with nerve growth factor (NGF), optimedin-expressing cells demonstrated elevated levels of N-cadherin, β-catenin, α-catenin, and occludin as compared with stimulated, control PC12 cells. Expression of optimedin induced Ca2+-dependent aggregation of NGF-stimulated PC12 cells and this aggregation was blocked by the expression of N-cadherin siRNA. Expression of optimedin also changed the organization of the actin cytoskeleton and inhibited neurite outgrowth in NGF-stimulated PC12 cells. We suggest that expression of optimedin stimulates the formation of adherent and tight junctions on the cell surface and this may play an important role in the differentiation of the brain and retina through the modulation of cytoskeleton organization, cell-cell adhesion and migration.
Keywords: Olfactomedin, Optimedin, PC12, N-cadherin, β-catenin, siRNA
Introduction
Olfactomedin, a secreted glycoprotein with molecular mass about 57 kDa, was originally identified in the bullfrog olfactory neuroepithelium [1]. The C-terminal half of this protein was named the olfactomedin domain. Regions similar to the olfactomedin domain were subsequently found in a variety of proteins from different tissues and species ranging from sea urchin to human [2–9]. Some of the olfactomedin domain-containing proteins are membrane proteins. For example, gliomedin is a type II transmembrane protein containing the olfactomedin domain in the carboxy-terminal extracellular region [10]. Latrophilins are calcium-independent, seven-transmembrane receptors for latrotoxin (CIRL1-CIRL3) with a large N-terminal extracellular part containing the olfactomedin domain [11–15].
A growing body of evidence indicates that olfactomedin domain-containing proteins may play important roles in normal development and diseases. Olfactomedin 1, which is also known as noelin-1 and pancortin, is involved in regulating the production of neural crest cells by the neural tube in chicken [2] and promotes neurogenesis in Xenopus [16]. Xenopus tiarin may participate in the specification of the dorsal neural tube [9]. Gliomedin may mediate Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier [10]. Sea urchin amassin mediates the massive intercellular adhesion of coelomocytes, the immune cells contained in the coelomic cavity [5]. Mutations in the olfactomedin domain may be deleterious for the functions of these proteins. For example, mutations in the olfactomedin domain of the human MYOCILIN gene may lead to juvenile open-angle glaucoma and in some cases to adult onset glaucoma [17–20].
Optimedin, also know as olfactomedin 3, is a secreted glycoprotein which shows about 65% identity to olfactomedin 1 [8]. Olfactomedin 1 and optimedin have overlapping expression patterns in the eyes and brain [8;16;21]. Two major forms of optimedin, optimedin A and optimedin B have been previously identified [8]. These forms of optimedin are encoded by mRNAs transcribed from two different promoters and have differing N-termini. In the present paper we report that expression of optimedin in PC12 cells, which normally do not express optimedin, elevates the levels of N-cadherin, α-catenin and β-catenin. The activation of N-cadherin expression stimulates aggregation of PC12 cells in the presence of NGF and inhibits neurite outgrowth.
Materials and methods
Preparation of PC12 cell line stably transfected with optimedin
Rat optimedin A and B cDNAs in frame with a Flag epitope [8] were cloned into the tetracycline-inducible vector, pTRE2hyg (BD Clontech, San Diego, CA) using Cla I and Mlu I restriction sites. Tet-on PC12 cells (Clontech) were transfected with the optimedin and vector constructs using Nucleofector™ II (Amaxa Biosystems, Gaithersburg, MD) following the instructions provided by the company. Selection of stably transfected cells was conducted in the presence of G418 and hygromycin B. Individual colonies were amplified and stored in liquid nitrogen. Cells were grown in DMEM supplemented with 10% horse serum and 5% of Tet system (Clontech) approved fetal bovine serum (FBS) (normal growth media). For neuronal differentiation, cells were plated on either collagen or poly-L-lysine coated plates and incubated in 1% horse serum in the presence of 50 ng/ml of nerve growth factor (NGF-2.5S, Sigma, MO) and 1 μg/ml of DOX where indicated (differentiation media).
Western blotting experiments
Cells were lysed in RIPA buffer (50 mM Tris-HCl pH 7.5, 1 mM EDTA , 1% NP-40, 0.2% SDS) (Santa Cruz, CA) supplemented with 20 mM DTT and complete EDTA-free protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). About 20 μg of protein from cell lysates were used per lane in SDS-PAGE. After separation and transfer onto nitrocellulose membranes (Invitrogen, CA), proteins of interest were detected using specific antibodies and peroxidase-conjugated secondary antibodies (Amersham, UK). Following primary antibodies have been used: N-cadherin, α-catenin, β-catenin and β-actin antibodies were from Santa Cruz Biotech (Santa Cruz, CA). Monoclonal antibody against Flag was from Sigma (St. Louis, MO) and anti-occludin was from Zymed Lab (Zymed, CA). The SuperSignal chemiluminescent detection system (Pierce) was used according to the manufacturer’s instructions. All experiments were repeated at least twice. Some filters were used again after the exposure. They were stripped from the signals using stripping buffer following the manufacturer’s instruction (Pierce, IL) and treated with another primary antibody as described above. The relative amounts of proteins of interest in control and experimental cells were estimated by densitometric analysis of Western blots using the Microtek ScanMarker 9800XL scanner and the Image Quant Program.
Cell proliferation, cell adhesion and apoptosis assays
Cells were plated onto collagen-coated 12-well plates with a density of 105 cells/well. Cell were grown in a complete media and counted until day 6 in culture. The experiments were repeated three times. Cell adhesion to different extracellular matrix proteins was estimated using CytoMatrix Screening Kit as recommended by the manufacturer (Chemicon, CA). In brief, cells were seeded onto 96-well plates coated with fibronectin, vitronectin, laminin, collagen 1 or collagen IV with a density of 105 cells/well and incubated for 3 h. Cells were washed three times with PBS containing 1.8mM Ca2+ and 0.5mM Mg2+ and then incubated with 0.2% crystal violet dissolved in 10% ethanol at room temperature for 5 minutes. After removing of the staining solution and washing with PBS, cell-bound stain was solubilized in a solution containing 50 mM NaH2PO4, pH 4.5 and 25% ethanol. The cell attachment was proportional to the crystal violet absorbance of the solubilization solution which was measured at 570 nm using a microplate reader (Wallac, Finland). Apoptosis was estimated using ApoAlert Annexin V kit as recommended by the manufacturer (BD Biosciences, CA). In brief, cells were seeded onto collagen-coated 6-well plates with a density of 5x105 cells/well and incubated in a complete media. After 2 days in culture, cells were treated with 50 nM of staurosporine (Sigma, MO) for 3 h. Cell were washed 3 times with PBS containing 1.8 mM Ca2+ and 0.5 mM Mg2+, rinsed with a binding buffer and then incubated with Annexin V-FITS (5 μl per well) at room temperature for 15 min. Cells were washed with PBS and fixed in 2% paraformaldehyde for 10 min. The relative amount of apoptotic cells was evaluated after counting of fluorescent cells using an Axioplan 2 microscope (Carl Zeiss Microimaging Inc., NY).
Real-time PCR
Total RNA was isolated using Trizol reagent according to the manufacturer’s protocol (Invitrogen, CA). For cDNA synthesis, 1 μg of total RNA was reverse transcribed using SuperScript II first strand synthesis system (Invitrogen, CA) with a random primer. Primers 5′-CACCCAACATGTTTACAATCAACAATGAGAC-3′ (forward) and 5′-CTGCAGCAACAGTAAGGACAAACATCCTATT-3′ (reverse) were used for amplification of N-cadherin sequence [22]. Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) primers 5′-GCCTCGTCTCATAGACAAGATGGT-3′ (forward) and 5′-GAAGGCAGCCCTGGTAACC-3′ (reverse) were used for the normalization [23]. Real-time PCR was performed using the Applied Biosystems 7900HT real time thermocycler sequence detection system (Applied Biosystems, CA) and the SYBR green PCR master kit under conditions recommended by the manufacturer (Applied Biosystems, UK).
Luciferase reporter assay
HEK293 cells were kindly provided by Dr. Marvin Gershengorn (NIDDK, NIH). They were cultivated in DMEM supplemented with 10% fetal bovine serum, L-glutamine, and penicillin-streptomycin in a 5% CO2–containing atmosphere at 37° C. Cells were plated on the 12-well plates at the density of 2×105cells/well and transfected 24 hours after plating using FuGENE 6 (Roche Applied Science, Indianapolis, IN). Cell were transfected with 0.3 μg of Super8XTOPFlash luciferase construct containing 8 copies of TCF/LEF consensus sequence, 0.03 μg of pRL-SV40 (Promega, WI), and 0.6 μg of indicated DNAs. Super8XFOPFlash luciferase construct containing mutated consensus sequence was used as a negative control. Super8XTOPFlash and Super8XFOPFlash constructs were kindly provided by Dr. Randall Moon (HHMI, University of Washington School of Medicine, Seattle). Three independent transfection reactions were performed for each combination. Dual luciferase assay was performed 24 hours after transfection using Dual Luciferase Reporter®Assay 1000 System (Promega). All experiments were repeated at least twice.
Confocal microscopy
PC12 cells were plated on Permanox Lab-Tek Chamber Slides (Nulge Nunc International, Naperville, IL) coated with polylysine and transfected as described [24]. Cells were fixed in 4% paraformaldehyde prepared in phosphate buffered saline (PBS, pH 7.4) for 10 min, washed several times in PBS, and permeabilized with 0.1% Triton X-100 prepared in PBS for 5 min. For immunofluorescence, cells were first incubated in PBS with 2% bovine serum albumin (BSA) for 30 min and then incubated with different antibodies diluted in the same solution as follows: the monoclonal mouse anti-Flag antibody (dilution 1:2000), N-cadherin (dilution 1:50), β-catenin (dilution 1:50). After repetitive washing in PBS, the signals were visualized using rhodamine (TRITC) anti-mouse antibody (Jackson Immuno Research, West Grove, PA) in 1:100 dilution and with Alexa 488 anti-rabbit antibody (Molecular Probes) in 1:200 dilution or Alexa 488 anti-mouse antibody (Molecular Probes) at a dilution of 1:200 and Cy-3 anti-rabbit antibody (Jackson ImmunoResearch), diluted 1:100 in PBS with 2% BSA. F-actin fibers were stained with Rhodamin-phalloidin (Molecular Probe, Eugene, OR) in final concentration 5 u/ml for 30 min at room temperature. Images were collected using a Leica SP2 confocal microscope (Leica Microsystems, Exton, PA). Multiple fields (at least 10), containing several cells, were examined for each sample and typical images were collected.
siRNA experiments
N-cadherin and control siRNA oligonucleotides were synthesized by Dharmacon Research Inc. (Lafayette, CO) and used at a final concentration of 100nM. OptA(H) cells were transfected using siLentFect (Bio-Rad, Hercules, CA) Lipid Reagent for siRNA and incubated overnight in normal growth media. The normal growth media was replaced by differentiation media on the next morning. After incubation of cells in this media for two days, percent of single cells was counted and cell lysates were prepared for western blotting analysis as described above.
Results
Characterization of PC12 cell lines expressing optimedin
Since optimedin and olfactomedin 1 are both expressed in neural cells, we hypothesized that optimedin, similar to olfactomedin 1, may promote neurogenesis. To examine possible effects of optimedin on neurogenesis, we selected the PC12 rat pheochromocytoma cell line, a widely used model to study neuronal differentiation in vitro. PC12 cells can differentiate into neuron-like cells with neurite networks after treatment with nerve growth factor (NGF). The optimedin gene is not expressed in PC12 cells growing in the absence or presence of NGF, as judged by RT PCR analysis (not shown). We produced several PC12 cell lines stably transfected with optimedin under the control of the tetracycline-inducible promoter. Since appropriate antibodies against optimedin were not available, both optimedin A and optimedin B were tagged with the Flag epitope. 18, 20, and 3 individual PC12 cell lines were isolated after transfection with optimedin A, optimedin B and vector constructs, respectively. PC12 cell transfected with the vector construct will be called control cells through the paper. Individual optimedin-transfected cell lines demonstrated different levels of optimedin expression after stimulation with DOX as estimated by western blot experiments using Flag antibodies (Fig. 1). Although addition of DOX stimulated optimedin expression about 3–5 times, optimedin expression was detected even in the absence of DOX in most cell lines analyzed, indicating the leakage of the tet-inducible promoter. One line of PC12 cells expressing moderate levels of optimedin A before induction with DOX and high levels of optimedin after induction was used in most experiments and will be called OptH through the paper. Other lines were used occasionally for comparison. One line, which will be called OptL, expressed significantly lower levels of optimedin after induction compared with OptH line (Fig. 1). The level of optimedin in OptL cell line was hardly detectable before induction with DOX. Lines expressing different levels of optimedin B were called OptBL and OptBH (Fig. 1).
Fig. 1.

Expression of optimedin in stably transfected PC12 cell lines before and after induction with DOX. Control, OptL, OptH, OptBL and OptBH PC12 cells were grown in normal media without DOX or in the presence of 1μg/ml of DOX for two days. About 20 μg of cell lysates were separated by SDS–PAGE, transferred to nitrocellulose filter and stained with Flag monoclonal antibodies (1:4000 dilution). Peroxidase-conjugated secondary antibodies against mouse IgG were used in 1:5000 dilution.
In the absence of NGF and DOX, most of the control and optimedin-expressing cells had similar round morphology. We compared proliferation rate, apoptosis and adhesion to different extracellular matrix proteins of optimedin-expressing and control cells. OptH cells demonstrated higher rate of proliferation as compared to control and OptL cells (Fig. 2A). Higher proliferation rate of OptH cells compared to control cells was confirmed by cell labeling with bromo-deoxyuridine with subsequent cell sorting (not shown). At the same time, OptH cells demonstrated higher levels of apoptosis as compared to the control and OptL cells (Fig. 2B). OptH cells were more strongly attached to collagens I and collagen IV matrix as compared to control cells or OptL cells, while adhesion to other extracellular matrix molecules tested was similar for control and experimental cells (Fig. 2C). Since OptL cells expressed very low levels of optimedin and OptH expressed detectable levels of optimedin in the absence of DOX, these results demonstrated that expression of optimedin modifies properties of un-induced PC12 cells.
Fig. 2.

Comparison of some properties of control, OptH and OptL cells. (A) Growth rate comparison. 105 cells/well were plated onto collagen coated 12 wells plate and incubated in normal media without DOX and NGF. Cells were counted as described in Materials and Methods. (B) Relative amount of apoptotic cells two days after plating. Apoptotic cells were detected using Annexin V staining as described in Materials and Methods. (C) Cell adhesion to different extracellular matrix proteins. Cell adhesion was estimated using CytoMatrix Screening Kit (Chemicon, CA) as described in Materials and Methods.
Expression of optimedin stimulates aggregation of NGF-treated PC12 cells and inhibits neurite formation
It is well established that PC12 cells respond to NGF by extending neurites, thus acquiring the appearance of neurons. To study effects of optimedin on the NGF-induced differentiation of PC12 cells, control and optimedin-expressing cell lines were incubated with NGF in the presence or absence of DOX. About 50 % control cells produced neurites with a length longer than two cell bodies after incubation with 50 ng/ml of NGF for 10 days (Fig. 3A). Unlike control cells, OptH cells formed large aggregates with very few neurites and this happened both in the presence and absence of DOX (Fig. 3B). Separate experiments demonstrated that other OptH cells lines tested also aggregated in the presence of NGF (not shown). Similar to OptH cells, OptL cells also aggregated after stimulation with NFG (Fig. 3C). In all cases, the size of aggregates was larger on collagen-coated plates compared to polylysine-coated plates (not shown).
Fig. 3.

Aggregation of NGF-stimulated PC12 cells expressing optimedin. Control (A), OptH (B) and OptL (C) cells were incubated for 10 days in low serum in the presence of 50 ng/ml of NGF and 1μg/ml of DOX. In these conditions, only OptH and OptL cells formed large aggregates with a reduced amount of neurites. (D) Relative amount of cells producing neurites with a length longer than two cell bodies. Only single cells which were not parts of large aggregates were counted.
To test whether the formation of aggregates is critical for the inhibition of neurite growth, the neurite formation of the single cells was analyzed separately. Only about 5% of single OptH cells produced neurites with a length longer than two cell bodies, indicating that the formation of aggregates is not necessary for the inhibition of neurite outgrowth (Fig. 3D). Since optimedin is a secreted protein, we tested whether intracellular expression of optimedin is essential for cell aggregation or whether extracellular addition of optimedin alone may induce cell aggregation. Addition of conditional incubation media from NGF-induced DOX treated OptH cells to control cells did not induce aggregation of control cells (not shown), indicating that the presence of extracelluar optimedin is not sufficient for cell aggregation and that intracellular expression of optimedin may contribute to this process.
We next tested whether the aggregation of PC12 cells induced by optimedin was Ca2+-sensitive. The reduction of Ca2+ concentration to 0.03 mM from normal 1.8 mM did not significantly change properties of control cells (Fig. 4A,B) but completely inhibited aggregation of OptH (Fig. 4C) and OptL (not shown) cells observed in the presence of NGF and DOX. Calcium-dependence of cell aggregation indicates that cadherins, Ca2+-dependent cell-cell adhesion molecules, may be involved in this process.
Fig. 4.

Effect of Ca2+ concentration on aggregation of control and OptH cells. NGF-stimulated control (A,B) and OptH (C,D) cells were incubated in the presence of 1.8 mM (B,D) or 0.03 mM (A,C) Ca2+ for 2 days.
Optimedin does not stimulate TCF/LEF signaling
Optimedin’s action on stimulated PC12 cells in some respects resembles action of Wnt proteins [25–28]. Similar to optimedin expression, expression of Wnt1 or Wnt3a cDNAs in PC12 cells inhibited NGF-induced differentiation PC12 cells and neurite growth. Similar to optimedin, expression of Wnt1 increased the rate of multiplication of PC12 cells [26] and increased calcium-dependent cellular adhesion. Therefore, we examined whether optimedin may activate the Wnt canonical signaling pathway using Wnt-responsive TOPFlash luciferase reporter and HEK293 cells which are often used to study Wnt signaling. Control experiments demonstrated that Wnt3a produced about 20 fold stimulation of the TOPFlash luciferase activity over vector in transient transfection experiments (Fig. 5). Optimedin produced only 1.4 fold stimulation of the luciferase activity with the TOPFlash reporter. Co-transfection of optimedin together with Wnt3a did not produce higher TOPFlash luciferase activity as compared with Wnt3a transfection alone. On the basis of these results we concluded that optimedin action does not include TCF/LEF signaling.
Fig. 5.

Optimedin does not enhance the canonical Wnt signaling pathway. HEK293 cells were co-transfected with 0.3 μg Super8XTOPFlash-luceferase reporter, 0.03 μg of pRL-SV40, and 0.6 μg of indicated constructs. Luciferase activity was measured 24 h after transfection. Luciferase activity is shown relatively to the activity of Super8XTOPFlash-luceferase reporter co-transfected with 0.6 μg of vector pFLAG-CMV-2 (Sigma), optimedin A and Wnt3a.
Elevation of N-cadherin, α-catenin and β-catenin levels in NGF-treated optimedin-expressing cells
Gene expression analysis using the Affymetrix rat microarray system demonstrated that N-cadherin was the only cadherin among classical cadherins that changed its expression in OptH cells as compared with control cells (H.-S. L. and S.I.T., in preparation). Real-time PCR results showed that the level of N-cadherin mRNA expression was 8.5 times higher in differentiating OptH cells as compared with control cells at 0.03 mM and 1.8 mM Ca2+ concentrations after 2 days of incubation in the presence of NGF and DOX. Western blotting experiment were conducted to estimate changes in cadherin protein levels in control, OptH and OptL cells in the presence of normal and reduced Ca2+ concentrations. Two different OptH lines were analyzed to be sure that the observed effects are not line-specific. The levels of E-cadherin did not change dramatically in control, two different OptH and OptL lines at both Ca2+ concentrations (Fig. 6). The levels of N-cadherin were low in control cells at normal and low Ca2+ concentrations. The levels of N-cadherin were significantly increased in both OptH and OptL cells in the presence of normal Ca2+ concentration as compared with those in control cells after two days of incubation in the presence of NGF and DOX. OptH cells expressed higher levels of N-cadherin as compared to OptL cells. The levels of N-cadherin in OptH and OptL cells were dramatically reduced when Ca2+ concentration was reduced to 0.03 mM (Fig. 6).
Fig. 6.

The effects of optimedin and Ca2+ concenration on the levels of N-cadherin, E-cadherin, β-catenin and α-catenin. Control, two independent lines of OptH cells and OptL cells were grown in normal media with NGF and DOX for 2 days in the presence of 1.8 mM or 0.03 mM Ca2+. About 20 μg of cell lysates were separated by SDS–PAGE, transferred to nitrocellulose filters and stained with corresponding antibodies as described in Materials and Methods. (1, 2) Control cells in low and normal Ca2+, respectively; (3, 4) OptH cells in low and normal Ca2+; (5, 6); another OptH line in low and normal Ca2+; (7, 8) OptL cells in low and normal Ca2+.
N- and E-cadherins belong to type I cadherins that are organized in a core complex. In this complex, the cytoplasmic domain of cadherin binds β-catenin. The N-terminal region of β-catenin binds α-catenin, while α-catenin binds directly to actin and to several actin-binding proteins. The integrity of this core complex is essential for the formation and maintenance of stable adhesions. Changes in the level of N-cadherin may induce changes in α- and β-catenin. Therefore, we analyzed α- and β-catenins in control and optimedin-expressing cells at different Ca2+ concentrations. The levels of both α- and β-catenins were higher in OptH cells at normal Ca2+ concentration as compared with low Ca2+ concentration. At low Ca2+ concentration, the levels of α- and β-catenins in OptH cells were similar to those in control cells at normal Ca2+ concentration. Although the levels of β-catenin were higher at normal Ca2+ as compared with low Ca2+ concentration in OptL cells, this difference was not as big as in the case of OptH cells. The levels of α-catenin were similar at both Ca2+ concentrations in OptL cells.
Intracellular distribution of N-cadherin and β-catenin was also different in differentiating control and OptH cells. In control cells, N–cadherin and β-catenin were distributed between cellular membrane and cytoplasm (Fig. 7). In OptH cells that formed aggregates, N-cadherin and β-catenin showed preferential punctuated membrane staining. The punctuated staining probably reflects clustering of N–cadherin and β-catenin in adherens junctions. Adherens junctions may stabilize tight junctions [29]. So, we tested the levels of occludin, which is a component of tight junctions. After two days in culture in the presence of NGF, the levels of occludin were elevated in OptH cells as compared to control cells (Fig. 8).
Fig. 7.
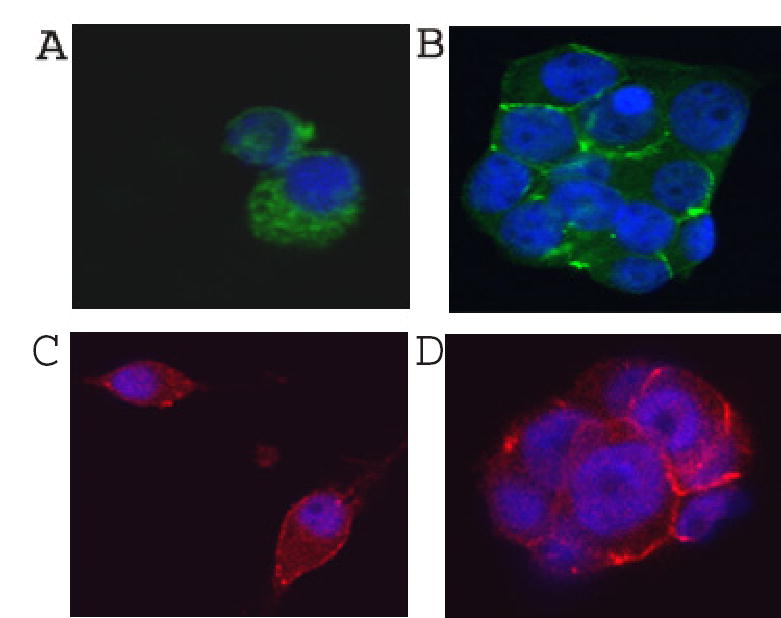
Subsellular localization of N-cadherin and β-catenin in control and OptH cells. Control (A, C) and OptH (B, D) cells were grown for two days in the presence of NGF and DOX. Cells were stained with antibodies against N-cadherin (A, B) or β-catenin (C, D). Nuclei were stained with DAPI. Typical images are shown.
Fig. 8.

Occludin levels in control (3) and OptH (1, 2) cells. Two different OptH cell lines and control cells were grown in the presence of NGF and DOX for two days as described in Materials and Methods. About 20 μg of cell lysates were separated by SDS–PAGE, transferred to nitrocellulose filter and stained with anti-occludin polyclonal antibodies (1:50 dilution). Peroxidase-conjugated secondary antibodies against rabbit IgG were used in 1:5000 dilution.
Possible reorganization and stabilization of both adherens and tight junctions would be expected to lead to the reorganization of the intracellular cytoskeletal filaments. Indeed, the organization of actin cytoskeleton was different in control and OptH cells as judged by staining with rhodamine-labeled phalloidin. Control cells showed large amount of neurite-like spikes stained with phalloidin at both low and normal Ca2+ concentrations (Fig. 9A, B). OptH cells did not produce significant amount of such neurite-like spikes and phalloidin staining was the most intense in the area of cell-cell contacts probably reflecting the formation of stress fibers at normal Ca2+ concentration (Fig. 9D). At low Ca2+ concentration, OptH cells also produced neurite-like spikes stained with phalloidin although in smaller amounts as compared with control cells (Fig. 9C). On the basis of the results described in this section we suggested that N-cadherin might be involved in the aggregation of differentiating OptH cells.
Fig. 9.
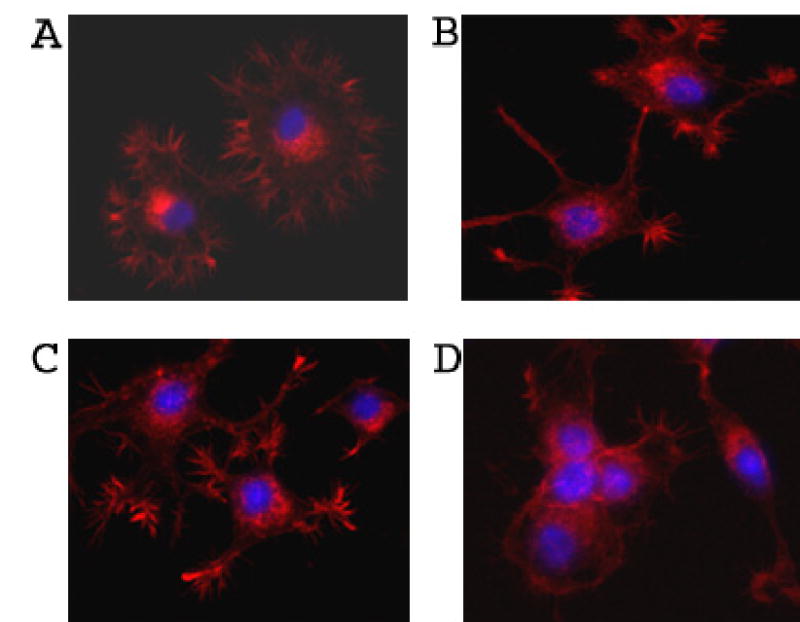
Localization of actin fibers in control and OptH cells. Control (A, B) and OptH (C, D) cells were incubated for 2 days with NGF and DOX in the presence of 1.8 mM (B, D) or 0.03 mM (A, C) Ca2+. Cells were stained with Rhodamin-phalloidin as described Material and Methods.
Inhibition of N-cadherin expression by siRNA inhibits aggregation of NGF-treated OptH cells
To prove that N-cadherin plays a critical role in the aggregation of differentiating OptH cells, expression of optimedin in these cells was inhibited by N-cadherin siRNA. Western blot analysis of protein extracts prepared from differentiating OptH cells transfected with control or N-cadherin siRNA demonstrated that N-cadherin siRNA induced dramatic reduction in the levels of N-cadherin (Fig. 10D). The reduction of N-cadherin level led to destabilization of β-catenin and reduction of its level without affecting the actin level (Fig. 10D). The reduction of N-cadherin level reduced the formation of cell aggregates by differentiating OptH cells (compare Fig. 10A, B and Fig. 3). The proportion of single cells was dramatically increased in N-cadherin siRNA transfected cultures of OptH cells as compared with those transfected with control siRNA (Fig. 10C). On the basis of these results we concluded that N-cadherin plays a central role in the aggregation of differentiating OptH cells.
Fig. 10.
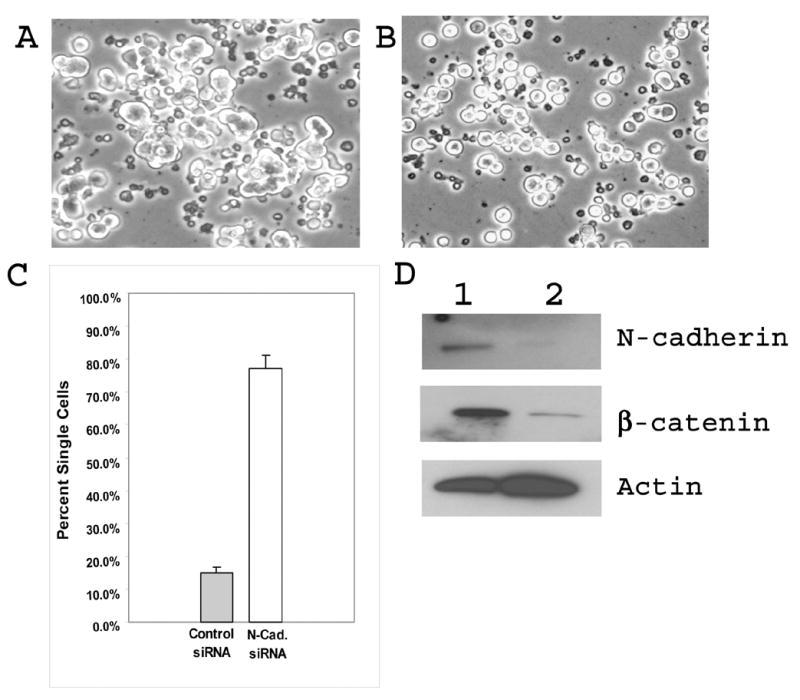
N-cadherin siRNA inhibits aggregate formation of differentiating OptH cells. Opt H cells were transfected with control (A) or N-cadherin (B) siRNA as described in Materials and Methods. NGF and DOX were added to cells 24 h after transfection. Relative amounts of single cells were counted 2 days after NGF addition (C). Changes in the levels of N-cadherin and β-catenin were evaluated after counting the cells (D). 1 – OptH cells transfected with control siRNA; 2 – OptH cells transfecetd with N-cadherin siRNA.
Discussion
Optimedin belongs to a family of olfactomedin domain-containing proteins that may be essential for normal development of different organs and tissues. The molecular mechanisms of action of this family of proteins are in most cases not clear. The results of microinjection of RNA encoding olfactomedin domain-containing proteins (noelin, tiarin, olfactomedin-like 3 or hnoel-iso) into the Xenopus embryos indicate that different olfactomedin-related proteins use multiple distinct response systems to mediate their signals in embryogenesis [30]. Recent data suggest that some olfactomedin-domain containing proteins may interact with membrane-bound proteins located at the cell surface. For example, gliomedin, a glial ligand for neurofascin and NrCAM, two axonal IgCAMs that are present at the nodes of Ranvier, interacts specifically with these two proteins but not with several related IgCAMs [10]. hGC-1, also known as GW112, OLFM4, and hOlfD, may interact with cell surface lectins and cadherin [31]. Amassin, an extracellular glycosylated protein found in the coelomic fluid of sea urchin, mediates the massive Ca2+-dependent intercellular adhesion of sea urchin coelomocytes through binding to a cell surface protein [5]. Myocilin, a secreted protein abundantly present in the tissues involved in the regulation of aqueous humor outflow [8;17;32;33], may interact with flotilin-1 [34]. Flotilin-1 is a key structural component and a marker of lipid rafts which play critical role in multiple biological processes and may serve as major assembly and sorting platforms for signal transduction complexes [35–37]. Our preliminary results indicate that optimedin, similar to other olfactomedin domain-containing proteins, may also interact with a protein located on the membrane of NGF-stimulated PC12 cells (H.-S. L. and S.I.T., unpublished observations). Our current experiments directed toward the identification of this protein.
The N-terminal parts of different olfactomedin domain-containing proteins appears to be essential for the formation of homodimers [8;38–40]. The olfactomedin domain is the most conserved part of the protein molecules and appears to be critical for interaction with other proteins. The olfactomedin domain of gliomedin, is essential for the interaction with neurofascin and NrCAM [10]. Olfactomedin domain of amassin is responsible for coelomocyte-binding activity [41]. Olfactomedin domain of optimedin is important for its interaction with myocilin [8], and olfactomedin domain of myocilin is critical for its interaction with flotilin-1 [34]. At present, we don’t know whether the olfactomedin domain of optimedin alone is able to stimulate aggregation of PC12 in the presence of NGF.
Optimedin, as well as several other olfactomedin domain-containing proteins, is a secreted protein. Experiments with Noelin-1 in Xenopus demonstrated that while non-secreted, endoplasmic reticulum located forms of Noelin-1 induced some neuronal markers to nearly the same extent as the secreted forms, the secreted forms of Noelin-1 have added activities that non-secreted form did not have [16]. Our experiment demonstrated that secreted form of optimedin alone is not sufficient to induce aggregation of NGF-stimulated PC12 cells which do not express optimedin endogenously. Optimedin in our system is expressed under the control of a tetracycline- inducible promoter. However, this promoter “leaked” and optimedin was detected in OptH cells even without addition of DOX. Although the level of optimedin was very low in OptL cells before induction (see Fig. 1), there were changes in the gene expression pattern in both OptH and OptL cells even before addition of DOX as judged by array hybridization experiments (H.-S. L. and S.I.T., manuscript in preparation). It is possible that expression of optimedin at low concentration “primes” these cells for subsequent aggregation after NGF addition. Expression of optimedin changed the properties of OptH cells even without DOX stimulation. In particular, OptH cells became more attached to collagen I and collagen IV extracellular matrix as compared with control or OptL cells (Fig. 2C). It is interesting to note that purified photomedin-1 and photomedin-2, two related olfactomedin domain-containing proteins that are expressed in the retina, were preferentially bound to chondrion sulphate-E and heparin among other extracellular matrix components tested [42].
Mutations in the olfactomedin domain may prevent secretion of olfactomedin domain-containing proteins and, in the case of myocilin, lead to glaucoma. Mutations in the olfactomedin domain of myocilin may also prevent interaction with other proteins as was demonstrated for interaction of myocilin with flotilin-1 [34]. Some changes in the olfactomedin domain of optimedin, corresponding to the glaucoma-leading mutations in myocilin, prevent secretion of optimedin [43]. It is not known whether a non-secreted fraction of wild-type optimedin or non-secreted mutated optimedin is able to induce PC12 cells aggregation alone without participation of a secreted form. It should be pointed out that olfactomedin domain by itself is not required for secretion since Noelin-4, an isoform of olfactomedin 1 without the olfactomedin domain, is efficiently secreted and may have functions different from the functions of olfactomedin domain-containing isoform, Noelin-1 [40]. Optimedin, similar to some other secreted glycoproteins, may interact with cellular receptor(s) and stimulates or inhibits cellular signaling pathways. Altough optimedin’s action on stimulated PC12 cells in some respects resembles action of Wnt proteins [25–28], optimedin does not stimulate the canonical Wnt pathway through TCF/LEF signaling (Fig. 5).
The key observation of this work is that expression of optimedin induces aggregation of NGF-stimulated PC12 cells and that up-regulation of N-cadherin is essential for this aggregation. Inhibition of N-cadherin by N-cadherin siRNA expression suppressed cell aggregation. Up-regulation of N-cadherin occurred at both mRNA and protein levels under physiological levels of Ca2+. A number of transcription and growth factors are implicated in the regulation of N-cadherin gene expression [44]. At present, we don’t understand how optimedin expression may up-regulate expression of the N-cadherin gene or increase stability of N-cadherin mRNA. β-catenin, being a key component of Wnt and other growth factor signaling pathways as well as a component of the cadherin complex, might be a protein providing a connection between signaling and cell adhesion [45]. N-cadherin may sequester β-catenin at the cellular membrane and thus restrict its availability for signaling to the nucleus. Indeed, although the level of β-catenin was higher in NGF-stimulated OptH cells as compared with control cells, β-catenin was located mainly on the membrane and did not show increased accumulation in the nucleus (Fig. 6). It reasonable to assume that optimedin, similar to some other olfactomedin domain-containing proteins (amassin, gliomedin) may stimulate the formation of protein complexes on the cell membrane. In particular, optimedin may stimulate the formation of adherent and tight junctions on the cell surface.
In vivo, the optimedin gene is expressed in the ganglion and inner nuclear layers of the retina, epithelial cells of the iris and ciliary body [8]. In the developing mouse, the optimedin gene is expressed in the trigeminal ganglia, pituitary gland, ventral telencephalon and thalamus, and spinal cord [46]. Optimedin, by modulating cytoskeleton organization, cell-cell adhesion and migration, may play an important role in differentiation of these structures.
Acknowledgments
We thank Drs. R. Fariss and J.Y. Tsai for their help with confocal microscopy, Dr. R. Moon for providing Super8XTOPFlash and Super8XFOPFlash constructs, Dr. C. Bianco (NCI, NIH) for providing the Wnt3a expression construct, Dr. M. Gershengorn (NIDDK, NIH) for providing HEK293 cells, and Drs. Peggy Zelenka and Janine Davis for critical reading of the manuscript,
Abbreviations
- DOX
doxycyclin
- NGF
nerve growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Snyder DA, Rivers AM, Yokoe H, Menco BP, Anholt RR. Olfactomedin: purification, characterization, and localization of a novel olfactory glycoprotein. Biochemistry. 1991;30:9143–9153. doi: 10.1021/bi00102a004. [DOI] [PubMed] [Google Scholar]
- 2.Barembaum M, Moreno TA, LaBonne C, Sechrist J, Bronner-Fraser M. Noelin-1 is a secreted glycoprotein involved in generation of the neural crest. Nature Cell Biology. 2000;2:219–225. doi: 10.1038/35008643. [DOI] [PubMed] [Google Scholar]
- 3.Danielson PE, Forss-Petter S, Battenberg EL, deLecea L, Bloom FE, Sutcliffe JG. Four structurally distinct neuron-specific olfactomedin-related glycoproteins produced by differential promoter utilization and alternative mRNA splicing from a single gene. JNeurosciRes. 1994;38:468–478. doi: 10.1002/jnr.490380413. [DOI] [PubMed] [Google Scholar]
- 4.Graveel CR, Harkins-Perry SR, Acevedo LG, Farnham PJ. Identification and characterization of CRG-L2, a new marker for liver tumor development. Oncogene. 2003;22:1730–1736. doi: 10.1038/sj.onc.1206309. [DOI] [PubMed] [Google Scholar]
- 5.Hillier BJ, Vacquier VD. Amassin, an olfactomedin protein, mediates the massive intercellular adhesion of sea urchin coelomocytes. JCell Biol. 2003;160:597–604. doi: 10.1083/jcb.200210053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni NH, Karavanich CA, Atchley WR, Anholt RR. Characterization and differential expression of a human gene family of olfactomedin-related proteins. GenetRes. 2000;76:41–50. doi: 10.1017/s0016672300004584. [DOI] [PubMed] [Google Scholar]
- 7.Mukhopadhyay A, Talukdar S, Bhattacharjee A, Ray K. Bioinformatic approaches for identification and characterization of olfactomedin related genes with a potential role in pathogenesis of ocular disorders. MolVis. 2004;10:304–314. [PubMed] [Google Scholar]
- 8.Torrado M, Trivedi R, Zinovieva R, Karavanova I, Tomarev SI. Optimedin: a novel olfactomedin-related protein that interacts with myocilin. HumMolGenet. 2002;11:1291–1301. doi: 10.1093/hmg/11.11.1291. [DOI] [PubMed] [Google Scholar]
- 9.Tsuda H, Sasai N, Matsuo-Takasaki M, Sakuragi M, Murakami Y, Sasai Y. Dorsalization of the neural tube by Xenopus tiarin, a novel patterning factor secreted by the flanking nonneural head ectoderm. Neuron. 2002;33:515–528. doi: 10.1016/s0896-6273(02)00590-1. [DOI] [PubMed] [Google Scholar]
- 10.Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR, Jr, Peles E. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47:215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Davletov BA, Shamotienko OG, Lelianova VG, Grishin EV, Ushkaryov YA. Isolation and biochemical characterization of a Ca2+-independent alpha-latrotoxin-binding protein. JBiolChem. 1996;271:23239–23245. doi: 10.1074/jbc.271.38.23239. [DOI] [PubMed] [Google Scholar]
- 12.Krasnoperov VG, Bittner MA, Beavis R, Kuang Y, Salnikow KV, Chepurny OG, Little AR, Plotnikov AN, Wu D, Holz RW, Petrenko AG. alpha-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron. 1997;18:925–937. doi: 10.1016/s0896-6273(00)80332-3. [DOI] [PubMed] [Google Scholar]
- 13.Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, Totty NF, Ushkaryov YA. Alpha-latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J Biol Chem. 1997;272:21504–21508. doi: 10.1074/jbc.272.34.21504. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita H, Lelianova VG, Ushkaryov YA. The latrophilin family: multiply spliced G protein-coupled receptors with differential tissue distribution. Febs Letters. 1999;443:348–352. doi: 10.1016/s0014-5793(99)00005-8. [DOI] [PubMed] [Google Scholar]
- 15.Sudhof TC. alpha-Latrotoxin and its receptors: neurexins and CIRL/latrophilins. AnnuRevNeurosci. 2001;24:933–962. doi: 10.1146/annurev.neuro.24.1.933. [DOI] [PubMed] [Google Scholar]
- 16.Moreno TA, Bronner-Fraser M. The secreted glycoprotein Noelin-1 promotes neurogenesis in Xenopus. DevBiol. 2001;240:340–360. doi: 10.1006/dbio.2001.0472. [DOI] [PubMed] [Google Scholar]
- 17.Adam MF, Belmouden A, Binisti P, Brezin AP, Valtot F, Bevhetoille A, Dascotte J-C, Copin B, Gomez L, Chaventre A, Bach J-F, Garchon H-J. Recurrent mutations in a single exon encoding the evolutionary conserved olfactomedin-homology domain of TIGR in familial ope-angle glaucoma. HumMolGenet. 1997;6:2091–2097. doi: 10.1093/hmg/6.12.2091. [DOI] [PubMed] [Google Scholar]
- 18.Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Kawase Rait K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations [In Process Citation] Hum Mol Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 19.Gong G, Kosoko-Lasaki O, Haynatzki GR, Wilson MR. Genetic dissection of myocilin glaucoma. HumMolGenet. 2004;13(Spec1):R91–102. doi: 10.1093/hmg/ddh074. [DOI] [PubMed] [Google Scholar]
- 20.Stone EM, Fingert JH, Alward WM, Nguyen TD, Polansky JR, Sunden SF, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma [see comments] Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 21.Kondo D, Yamamoto T, Yaoita E, Danielson PE, Kobayashi H, Ohshiro K, Funaki H, Koyama Y, Fujinaka H, Kawasaki K, Sutcliffe JG, Arakawa M, Kihara I. Localization of olfactomedin-related glycoprotein isoform (BMZ) in the golgi apparatus of glomerular podocytes in rat kidneys. JAmSocNephrol. 2000;11:803–813. doi: 10.1681/ASN.V115803. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q, Chen TJ, Letourneau PC, Costa LF, Schubert D. Modifier of cell adhesion regulates N-cadherin-mediated cell-cell adhesion and neurite outgrowth. JNeurosci. 2005;25:281–290. doi: 10.1523/JNEUROSCI.3692-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrickx J, Doggen K, Weinberg EO, Van Tongelen P, Fransen P, De Keulenaer GW. Molecular diversity of cardiac endothelial cells in vitro and in vivo. Physiol Genomics. 2004;19:198–206. doi: 10.1152/physiolgenomics.00143.2004. [DOI] [PubMed] [Google Scholar]
- 24.Mertts M, Garfield S, Tanemato K, Tomarev SI. Identification of the region in the N-terminal domain responsible for the cytoplasmic localization of Myoc/Tigr and its association with microtubules. Laboratory Investigation. 1999;79:1237–1245. [PubMed] [Google Scholar]
- 25.Bradley RS, Cowin P, Brown AM. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. JCell Biol. 1993;123:1857–1865. doi: 10.1083/jcb.123.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou AH, Zheng S, Itsukaichi T, Howard BD. Wnt-1 inhibits nerve growth factor-induced differentiation of PC12 cells by preventing the induction of some but not all late-response genes. Brain ResMolBrain Res. 2000;77:232–245. doi: 10.1016/s0169-328x(00)00058-9. [DOI] [PubMed] [Google Scholar]
- 27.Chou AH, Howard BD. Inhibition by Wnt-1 or Wnt-3a of nerve growth factor-induced differentiation of PC12 cells is reversed by bisindolylmaleimide-I but not by several other PKC inhibitors. Oncogene. 2002;21:6348–6355. doi: 10.1038/sj.onc.1205791. [DOI] [PubMed] [Google Scholar]
- 28.Shackleford GM, Willert K, Wang J, Varmus HE. The Wnt-1 proto-oncogene induces changes in morphology, gene expression, and growth factor responsiveness in PC12 cells. Neuron. 1993;11:865–875. doi: 10.1016/0896-6273(93)90116-9. [DOI] [PubMed] [Google Scholar]
- 29.Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. AdvDrug DelivRev. 2005;57:815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Sakuragi M, Sasai N, Ikeya M, Kawada M, Onai T, Katahira T, Nakamura H, Sasai Y. Functional analysis of chick ONT1 reveals distinguishable activities among olfactomedin-related signaling factors. MechDev. 2006;123:114–123. doi: 10.1016/j.mod.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Liu W, Chen L, Zhu J, Rodgers GP. The glycoprotein hGC-1 binds to cadherin and lectins. ExpCell Res. 2006 doi: 10.1016/j.yexcr.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Karali A, Russell P, Stefani FH, Tamm ER. Localization of myocilin/trabecular meshwork-inducible glucocorticoid response protein in the human eye. Investigative Ophthalmology & Visual Science. 2000;41:729–740. [PubMed] [Google Scholar]
- 33.Tomarev SI, Wistow G, Raymond V, Dubois S, Malyukova I. Gene Expression Profile of the Human Trabecular Meshwork. NEIBank Sequence Tag Analysis. InvestOphthalmolVisSci. 2003;44:2588–2596. doi: 10.1167/iovs.02-1099. [DOI] [PubMed] [Google Scholar]
- 34.Joe MK, Sohn S, Choi YR, Park H, Kee C. Identification of flotillin-1 as a protein interacting with myocilin: Implications for the pathogenesis of primary open-angle glaucoma. BiochemBiophysResCommun. 2005;336:1201–1206. doi: 10.1016/j.bbrc.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons K, Toomre D. Lipid rafts and signal transduction. NatRevMolCell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 37.Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM., Jr Lipid rafts in neuronal signaling and function. Trends Neurosci. 2002;25:412–417. doi: 10.1016/s0166-2236(02)02215-4. [DOI] [PubMed] [Google Scholar]
- 38.Ando K, Nagano T, Nakamura A, Konno D, Yagi H, Sato M. Expression and characterization of disulfide bond use of oligomerized A2-Pancortins: extracellular matrix constituents in the developing brain. Neuroscience. 2005;133:947–957. doi: 10.1016/j.neuroscience.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Fautsch MP, Johnson DH. Characterization of myocilin-myocilin interactions. Invest Ophthalmol Vis Sci. 2001;42:2324–2331. [PubMed] [Google Scholar]
- 40.Moreno TA, Bronner-Fraser M. Noelins modulate the timing of neuronal differentiation during development. DevBiol. 2005;288:434–447. doi: 10.1016/j.ydbio.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 41.Hillier BJ, Sundaresan V, Stout CD, Vacquier VD. Expression, purification, crystallization and preliminary X-ray analysis of the olfactomedin domain from the sea urchin cell-adhesion protein amassin. Acta Cryst. 2006;F62:16–19. doi: 10.1107/S1744309105038996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furutani Y, Manabe R, Tsutsui K, Yamada T, Sugimoto N, Fukuda S, Kawai J, Sugiura N, Kimata K, Hayashizaki Y, Sekiguchi K. Identification and characterization of photomedins: novel olfactomedin-domain-containing proteins with chondroitin sulphate-E-binding activity. BiochemJ. 2005;389:675–684. doi: 10.1042/BJ20050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malyukova I, Lee HS, Fariss RN, Tomarev SI. Mutated mouse and human myocilins have similar properties and do not block general secretory pathway. Invest Ophthalmol Vis Sci. 2006;47:206–212. doi: 10.1167/iovs.05-0220. [DOI] [PubMed] [Google Scholar]
- 44.Derycke LD, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. IntJDevBiol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- 45.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grinchuk O, Kozmik Z, Wu X, Tomarev S. The Optimedin gene is a downstream target of Pax6. JBiolChem. 2005;280:35228–35237. doi: 10.1074/jbc.M506195200. [DOI] [PubMed] [Google Scholar]


