Abstract
Social recognition constitutes the basis of social life. In male mice and rats, social recognition is known to be governed by the neuropeptide oxytocin (OT) through its action on OT receptors (OTRs) in the medial amygdala. In female rats and mice, which have sociosexual behaviors controlling substantial investment in reproduction, an important role for OT in sociosexual behaviors has also been shown. However, the site in the female brain for OT action on social recognition is still unknown. Here we used a customized, controlled release system of biodegradable polymeric microparticles to deliver, in the medial amygdala of female mice, “locked nucleic acid” antisense (AS) oligonucleotides with sequences specific for the mRNA of the OTR gene. We found that single bilateral intraamygdala injections of OTR AS locked nucleic acid oligonucleotides several days before behavioral testing reduced social recognition. Thus, we showed that gene expression for OTR specifically in the amygdala is required for normal social recognition in female mice. Importantly, during the same experiment, we performed a detailed ethological analysis of mouse behavior revealing that OTR AS-treated mice underwent an initial increase in ambivalent risk-assessment behavior. Other behaviors were not affected, thus revealing specific roles for amygdala OTR in female social recognition potentially mediated by anxiety in a social context. Understanding the functional genomics of OT and OTR in social recognition should help elucidate the neurobiological bases of human disorders of social behavior (e.g., autism).
Keywords: antisense locked nucleic acid oligonucleotides, Social Interaction, Controlled release, poly(lactic-co-glycolic) acid
True individual recognition, evidenced by unique modifications in the way an animal behaves toward another animal on the basis of past experiences with that specific individual, is a prerequisite for a wide range of social behaviors, from affiliative to agonistic (1). The neurochemical mechanisms that are known to be involved in social recognition in rodents include the two neuropeptides, oxytocin (OT) and vasopressin (2). Vasopressin is more abundant in the male brain than in the female brain, and it seems to mediate social recognition only in male rats and mice, in a manner dependent on androgenic hormones (3). OT, instead, mediates social recognition in both male and female rodents (e.g., ref. 4), with mice deficient in the gene for OT [OT knockout (OTKO) mice] being specifically impaired in social recognition and discrimination (5–7). In males, this could be rescued by OT infusion in the medial amygdala (MA), whereas infusion of an OT antagonist inhibited social recognition in wild-type (OTWT) mice (8). Whether amygdala OT also underlies social recognition in female mice is unknown. Data from male mice cannot be extrapolated to females. This is particularly true for the OT system, which has been implicated in several sex-specific behaviors such as parturition and nursing, maternal cares, and bonds (9–12), as well as in other prosocial [e.g., mate bonds (12, 13)] and antisocial behaviors [e.g., aggression (14–19)]. Moreover, there has not been a direct demonstration that blocking the gene for OT receptors (OTRs) in the MA of either male or female wild-type mice inhibits social recognition.
In the present study, we used poly(d,l-lactic-co-glycolic acid) polymer (PLGA) microparticles (20–23) to deliver locked nucleic acid (LNA) antisense (AS) oligodeoxynucleotides (24–29) targeted to the mRNA of the mouse OTR to assess the role of MA OTR genomic function in social recognition, activity, and anxiety-like behavior in female mice. Behavioral specificity of the treatment was assessed through detailed ethological analysis. Slow delivery of the AS oligonucleotides after injection and recovery of the animal allows us to bypass any stress-related side effects of intracerebral treatment administration (30), which have limited previous AS studies.
Results
Physical Properties of Microparticles.
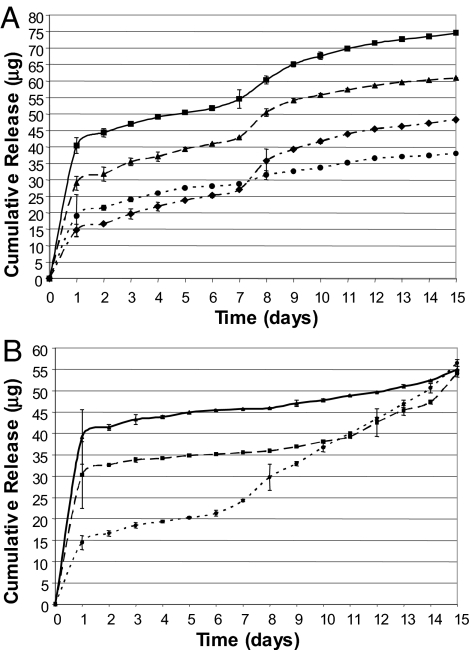
Scanning electron microscopy revealed that the surface of the microparticles was smooth and free of major defects (not shown). Particle sizes were fairly homogenous in the micrographs and correlated well with values obtained by using volume impedance (6 ± 1 μm). In addition, using different oligonucleotides in microparticle preparations resulted in no observable change in size and surface integrity. Encapsulation efficiency was almost identical for the formulations of microparticles with varying molecular weights of PLGA (502H, 50% 502H/50% 503H, and 503H) keeping oligonucleotide content fixed (i.e., a mixture of all four unmodified oligo sequences in all formulations; Fig. 1B). In the final preparation, encapsulation efficiency for formulations containing sequences 3 and 4 [scrambled (SCR)] were higher than preparations with sequences 1 and 2 (OTR AS; Fig. 1A).
Fig. 1.
Release of OTR specific oligonucleotide from controlled release microparticles. (A) Release of oligonucleotide from 502H PLGA microparticles containing sequences 1 and 2 (diamonds/dotted line, OTR AS; encapsulation efficiency, 56%), sequence 3 and 4 (triangles/dashed line, scrambled; encapsulation efficiency, 74%), locked sequences 1 and 2 (circles/dotted line, OTR AS; encapsulation efficiency, 47%), locked sequences 3 and 4 (squares/solid line, scrambled; encapsulation efficiency, 99%). (B) Resomer 502H (circles/dotted line), 50:50 502H/50% 503H (squares/dashed line), and 503H (triangles/solid line). All microparticles had an encapsulation efficiency of 60 ± 2%. Experiments were performed in triplicate, and standard deviation bars are shown for each time point.
PLGA Molecular Weight Selection.
All formulations demonstrated an initial burst phase, with this effect being more pronounced in the higher molecular weight polymer (Fig. 1B). The lowest molecular weight formulation (100% 502H) demonstrated the most linear release profile with the smallest burst phase and was therefore chosen for subsequent formulations. Final preparations (Fig. 1A) demonstrate a consistent profile of released material but with magnitudes proportional to encapsulation efficiency (Fig. 1A, legend).
Social Recognition Test.
Overall, the AS-treated mice showed only a minor habituation response to the repeatedly introduced stimulus mouse and failed to show a dishabituation response to the novel stimulus mouse. There was a significant effect of treatment × time (F8,88 = 10.885; P < 0.0001) for the duration of social investigation at the various tests (T1–T5). Unlike the blank- and the SCR-treated mice, the AS-treated mice did not show a normal habituation dishabituation response (Fig. 2A). As a result, at T3 and T4, the duration of social investigation of the AS-treated mice was significantly higher than that of the SCR (T3, P < 0.03; T4, P < 0.001) or blank-treated (T3, P < 0.03; T4, P < 0.0002) mice. Blank- and SCR-treated, but not AS-treated, mice showed a habituation curve such that at T3 and T4 they were investigating the intruder less than at T1 (blank, both: P <0.0001; SCR, both: P < 0.0005). At T5, the novel stimulus mouse induced an increased social investigation in the blank (T4 vs. T5, P < 0.0001) and SCR (P < 0.0001) groups, but not in the AS-treated mice, which experienced social investigation that continued to decline (T1 vs. T5, P < 0.002; T4 vs. T5, NS). As a result, at T5 social investigation performed by the AS-treated mice was significantly lower than in both control groups (vs. blank, P < 0.0003; vs. SCR, P < 0.01). Stretched approaches toward the stimulus mouse at T1 (Fig. 2B) were significantly higher in the AS-treated mice than in the SCR-treated mice (P < 0.04). The effect of the AS injection was specific to the social aspect of the test in that there were no effects of treatment on nonsocial investigation (6, 7). As well, the AS-treated mice showed normal overall activity, e.g., no effects of treatment on horizontal, vertical (Fig. 2 C and D) or other (digging) active behaviors. In addition, there were no effects of treatment on nonlocomotor behavior (sitting and self-grooming). The blank- and SCR-treated mice showed no behavioral differences.
Fig. 2.
Social recognition test. Behavior of female mice treated with OTR AS (●), scrambled (Δ), or blank (■) microparticles in the social recognition test. Vertical lines represent the SEM.
Dark–Light Test.
All of the parameters analyzed [time in area, entries in area, distance traveled, active time, vertical (rearing) time and vertical bouts] failed to show any significant effects of treatment (not shown). For each measurement, the analysis was run on both the overall behavior, as well as when performed in the dark and the light areas.
Nissl Stained Tissue.
There was no significant effect of treatment on total amygdala cell density (not shown).
Immunohistochemistry.
There was a significant effect of treatment on the density values for the MA (F2,22 = 96,140; P < 0.0001). Post hoc tests showed that mean OTR density in the AS-treated group was significantly lower than in both the SCR-treated (P < 0.0001) and the blank-treated (P < 0.0001) groups (Fig. 3A). Similarly, treatment was significant (F2,22 = 149.091; P < 0.0001) in the analysis run on the mean density values for the area immediately adjacent to the needle track (Fig. 3B). Again, OTR densities in the AS groups were lower than in the SCR-treated (P < 0.0001) and the blank-treated (P < 0.0001) groups. ANOVA analysis of the OTR density values from the septum (Fig. 3C) showed no significant effects. In all three areas, there were no differences between the blank and the SCR groups. Control slides with preimmunization antibody showed no observable binding (not shown). The overall staining patterns were consistent with results of receptor autoradiography studies (e.g., refs. 31 and 32). As expected, brain areas known to have high OTR binding in mice, such as amygdala, septum, paraventricular nucleus of the hypothalamus, or various cortical areas (e.g., entorhinal cortex), showed high immunohistochemistry staining, whereas areas that normally show low OTR binding (e.g., corpus striatum) had little or no immunohistochemistry staining.
Fig. 3.
OTR density. Mean density of OTR receptors in the MA (A), proximity of the needle tract (B), and septum (C) on mice treated with OTR AS (AS), scrambled (SCRAM), or blank (BLANK) microparticles. Vertical lines represent the SEM.
Discussion
Here we show (Fig. 2A) that wild-type female mice that received bilateral treatment with microparticles loaded with AS DNA targeted against the mRNA of the OTR gene in the MA were as impaired in individual recognition as their littermate OTKO mice (6, 7). Control mice that received either the SCR sequences or unloaded microparticles (blank) did not show any impairment in social recognition (Fig. 2A). The behavioral results were confirmed by immunohistochemical analysis (Figs. 3 and 4) showing a significant reduction in OTR expression in the AS-treated mice in the MA but not in a control, noninjected area (septum) known to express OTR (31, 32).
Fig. 4.
Amygdala OTR. Representative images of the MA of mice treated with blank microparticles (A) or microparticles containing scrambled (B) or OTR AS LNA (C) oligonucleotides.
Behavioral Results: Individual Recognition.
Gene expression for OTR in the amygdala is required for normal social recognition in female mice. The detailed ethological analysis showed that the AS-treated mice were not overall, impaired in other activities, including self-grooming, digging, nonsocial investigation, horizontal, and vertical activity (Fig. 2 C and D), nor were they affected in their locomotor and generalized anxiety responses in the dark–light test. The behavioral changes observed were, thus, specific for social behavior, rather than deriving from any generalized behavioral impairment. The present results show that the involvement of amygdala OTR in individual recognition in females is similar to that of males (8). This suggests that other modulatory systems, such as gonadal hormones (1), may underlie sex differences in mice sociosexual behaviors such as aggression, territoriality, reproduction, and parental cares (33).
As mice deposit their own odors onto other individuals (e.g., refs. 5 and 8), it has been proposed that results of studies on social recognition may reflect impairment in self-odor recognition (34), rather than the recognition of odor from another mouse. Our use (6, 7) of stimulus mice that were placed in a clean perforated cylinder prevented the experimental mice from using self-odors as the discriminatory cue. The present study, thus, supports the contention that the peptide hormone OT and OTR are involved in various social cognitive processes, such as individual recognition, social learning (35, 36), and the avoidance of parasitized conspecifics (37, 38). Whether these include the recognition of self-produced odors remains to be determined.
Behavioral Results: Risk Assessment and Anxiety/Fear.
OTR AS-treated mice showed an initial (T1) increase in the stretched approaches toward the stimulus mouse. This behavior, part of the risk assessment repertoire of the species, likely reflects the ambivalent motivational states of exploration (the approach component) and fear/anxiety (avoidance) (39). Stretched approaches have been introduced in social and nonsocial animal models of anxiety (e.g., refs. 40 and 41) and reliably respond to treatment with anxiolytic drugs (39, 42–44). Our OTR AS-treated mice, although still approaching the stimulus mouse at the same initial level as the control mice (Fig. 2A), did so in a more “cautious” manner (Fig. 2B). This suggests that the mice were exhibiting increased socially related anxiety, possibly elicited by decreased social recognition and not a generalized increased anxiety, as measured in the light–dark test. This is consistent with the differential effects of amygdalar administration of anxiolytic drugs on social and non social measures of anxiety (45).
Mechanisms of OT Control of Social Recognition.
We have proposed (1, 6) a “micronet” involving the four genes coding for the two estrogen receptors (α and β), OT, and OTR as the basis of social recognition in the CNS. In this model, estrogens control OT production in the paraventricular nucleus of the hypothalamus through estrogen receptor β and the expression of the OTR gene in the MA through estrogen receptor α (1, 6). The chemical communication of rodents relies on sensory input from both the main and accessory olfactory systems, which converge in the MA (46, 47).
The present results are in full agreement with the predictions derived from our four-gene micronet model as the core of individual recognition in the brain (6) and add to evidence from various behavioral genetic and molecular studies (reviewed in ref. 1). The current results, in which selective block of OTRs in the MA blocked individual recognition in wild-type female mice, are consistent with results from males showing that infusion of OT in the MA reinstated individual recognition in OTKO males, whereas infusion of OT antagonists blocked individual recognition in WT mice (8). Thus, the essential role of the OT system in the regulation of individual recognition is confirmed in both male and female mice.
Note on the Delivery System.
Our slow delivery of locked nucleic DNA AS in the mouse brain proved highly effective, allowing for behavioral testing several days after injection. We favor PLGA microparticles over other systems (e.g., refs. 48 and 49) because of their high biocompatibility with brain tissue (50). We observed neither changes in amygdala cell density nor obvious signs of toxicity, in full agreement with electron microscopy studies showing preservation of neuronal structures in contact with PLGA microparticles (20–23, 50). The lack of toxicity effects also confirms the high biocompatibility of the LNA oligonucleotides (27, 29).
The reduced density of OTR in the MA and at the site of injection demonstrated the efficacy and specificity of the PLGA-delivered AS DNA. The lack of treatment effects on OTR binding in the septum, a site of high OTR density (31, 32) but far from the amygdala, confirms the specificity of our AS DNA injections and consequent behavioral effects. This innovative way of intracerebral delivery thus provides an important tool for the targeted manipulation of gene expression in specific brain areas in behavioral neuroscience investigations.
Materials and Methods
Materials.
PLGA RG502H (Mr ≈ 10,000) and RG503H (Mr ≈ 18,000) Resomers were purchased from Boehringer Ingelheim (Ingelheim, Germany). All oligonucleotides were obtained by custom synthesis from Proligo (Boulder, CO).
Preparation of Microparticles.
We used biodegradable polymeric structures that can incorporate and release chemicals in a degradation dependent manner (e.g., refs. 51 and 52). Oligonucleotide-containing microparticles were prepared by the following modification of the double emulsion technique described in (53). Lyophilized oligonucleotide (1 mg) was dissolved in 100 μl of 1 mM EDTA and 300 mM d(+)-Lactose. This solution was then emulsified in 4 ml of CH2Cl2 containing varying proportions of PLGA Resomers (100% Resomer 502, 50% Resomer 502 and 50% Resomer 503, 100% Resomer 503; 200 mg total) by using a probe sonicator (Sonics and Materials Inc., Danbury, CT). The resulting emulsion was then added to a solution of poly(vinyl alcohol) [50 ml, 5% (wt/wt)] and NaCl (0.1 M) and homogenized at 5,000 rpm in a Silverson L4R homogenizer (Silverson, East Longmeadow, MA). After 30 s, the water-oil-water mixture was added to 100 ml of 1% (wt/wt) poly(vinyl alcohol) solution and allowed to stir for 3 h at room temperature and 1 h at 4°C. Microparticles were centrifuged and washed four times (relative centrifugal force, <150 × g) to remove poly(vinyl alcohol), then lyophilized for 48 h. Yields were 50–75% by weight of a white, fluffy powder.
Characterization of Microparticles.
Encapsulation efficiency of the oligonucleotide microparticles was determined by dissolution in CH2Cl2 and extraction into 1× TAE buffer (pH 8.0) over a 2-h period. Oligo concentration was determined by using UV absorption (260 nm) with a SpectraMax Plus plate reading spectrophotometer (Molecular Devices, Sunnyvale, CA) normalized to a 1-cm path length cuvette and compared with a standard curve. Microparticle size distributions were measured via volume displacement impedance with a Multisizer 3 (Beckman Coulter, Miami, FL) by using a 30-μm orifice tube. Surface morphology of microspheres was imaged by scanning electron microscopy by using an AMR 1000 Microscope (Amray, Medford, MA).
Determination of Oligonucleotides Release.
Triplicate, 10-mg oligonucleotide-containing microparticle samples were suspended in 1.5 ml of artificial cerebrospinal fluid, composed of 149 mM NaCl, 10 mM d-glucose, 10 mM Hepes, 18 mM sucrose, 3.5 mM KCl, 2.5 mM CaCl2, and 1 mM MgCl2 with an osmolality of 328 mosmol/kg, by using a vapor pressure osmometer and rotated at 37°C. At predetermined time points, samples were centrifuged, supernatants were saved by freezing, and concentrations were determined as described above. Pellets were resuspended and the process repeated.
Animals.
All of the 49 adult (3–4 months) female experimental mice were the offspring of a first generation of breeding between wild-type mice of the strain of OTKO mice used in previous studies (6). Original breeding pairs (mixed background of 129/Sv and Black Swiss) were obtained from Washington University School of Medicine (54). After weaning, the mice were housed in same-sex groups of four to five per group. All mice were kept in polyethylene cages (26 × 16 × 12 cm) provided with Beta Chip bedding (Northeastern Products Corp., Warrensburg, NY), under a 12-h:12-h light/dark cycle (lights off at 11:00 a.m.) at 20 ± 2°C. Food (Purina Rat Chow, St. Louis, MO) and tap water were available ad libitum. The stimulus mice were 10 ovariectomized group-housed Swiss–Webster mice. Four to 5 days before testing, experimental mice were transferred from group to individual housing to permit establishment of a home cage territory. Behavioral tests were performed during the active dark phase, and the order of testing was randomized. This research was approved by the Institutional Animal Care and Use Committee of the Rockefeller University.
Oligonucleotide Sequences Design.
Sequence 1 (5′-CCC-tcc-atg-acc-AAC-3′) was an AS oligonucleotide spanning the translation start codon on the OTR mRNA sequence (55). Sequence 2 (5′-GAC-cgc-gac-cct-GAG-3′) was AS to a tract upstream of the start codon. Sequence 3 (5′-ACC-gca-cat-tcc-ACC-3′) was composed of the same 15 bases as sequence 1 in scrambled order. Similarly, sequence 4 (5′-GCG-cgc-cat-acg-AGC-3′) was the scrambled control for sequence 2. All four sequences were blasted in GenBank and had no homology for any mouse mRNA other than the OTR for sequences 1 and 2. The capitalized letters in the sequences (three at each end) represent the nucleic acids that were replaced with LNA, a synthetic nucleic acid that contains a 2′-O, 4′-C methylene bridge, which restricts the flexibility of the ribofuranose ring and locks the structure into a rigid bicyclic formation. This locked structure was shown to confer enhanced specificity (27, 29) and stability to the oligonucleotides, while being nontoxic (27).
Oligonucleotides Administration.
Each mouse was deeply anesthetized with 8 mg/kg pentobarbital (Nembutal, 0.5 ml/kg i.p.), placed in a stereotaxic instrument (Kopf Instruments, Tujunga, CA) with blunt-end 45° ear bars. The skin was cut along the midline, and bilateral holes were drilled in the skull with a 0.5-mm drill bit (Bovie Aaron, St. Petersburg, FL) 1.5 mm back from, and 2.55 mm lateral of, bregma (56). A 10-μl Hamilton syringe (Reno, NV) with a 26-gauge needle mounted on the stereotaxic instrument was lowered 5.5 mm below the pial surface, and 1.0 μl of blank (microparticles not loaded with DNA), AS (10 μg/ml; a mixture of sequences 1 and 2), or SCR (10 μg/ml; sequences 3 and 4) oligonucleotide-loaded microparticles were infused in the MA at a rate of 0.334 nl/min with a mircoinjector (Micro4; World Precision Instruments, Sarasota, FL) controlled minipump (UltraMicroPump II, World Precision Instruments). After the infusion, the needle was left in place for 1 min and was then raised slowly (1 mm/min) to avoid back flux of the infused solution. Bilateral injections were given in succession with the left–right order being randomized. The wound was then sutured with one surgical clip. After treatment, all mice were housed singly and left undisturbed.
Social Recognition Test.
Seven days after oligonucleotide administration, each mouse was exposed, in its home cage, four times (T1–T4) to an ovariectomized female mouse held inside a Plexiglas perforated cylinder. On a fifth exposure (T5) a novel ovariectomized mouse was introduced. Intertest intervals were 15 min. All tests lasted 5 min and were videotaped for subsequent analysis with specific software (The Observer Video Analysis; Noldus, Wageningen, The Netherlands). Behaviors collected (6, 7) included measures of social interest (active sniffing of the holes of the cylinder), nonsocial investigation, risk assessment, nonlocomotor behaviors (sit, groom), vertical and horizontal activity, and other active behaviors (digging). After testing, a vaginal smear was taken from each female. Mice in each phase of the estrus cycle were found in each group of treatment.
Dark–Light Test.
Eight days after oligonucleotide administration, each mouse was individually placed in a clear polycarbonate 43.2-cm square activity box divided by a black insert into dark and light rectangular compartments of equal sizes (MED Associates, St. Albans, VT). Mice were released in a corner of the dark area, and their activity was tracked by a set of three photo beam arrays for 10 min in a brightly lit room.
Localization of the Site of Injections and of Signs of Cytotoxicity.
Approximately 2 h after the dark–light test, mice were killed by cervical dislocation and their brains were removed, quickly frozen with dry ice, and stored at −80°C. Coronal sections (12 μm) cut at −20°C were mounted on coated microscope slides (Superfrost Plus; Erie Scientific, Portsmouth, NH) and stored at −80°C. One of five sets of slides, with anatomically adjacent sections, was used for Nissl staining, and the location of needle tracts and microparticles were determined with a Zeiss Axioskop microscope (model 20, Gottingen, Germany). Only mice with bilateral MA injections were kept (final samples size: blank, n = 7; SCR, n = 7; AS, n = 11). Cell density was assessed from MA digital bilateral images obtained with a SPOT diagnostic camera (model 150) (Zeiss, Harrison, NJ) mounted on the microscope. Densitometry was conducted by using ImageJ freeware (National Institutes of Health) after calibration with a 0.5-increment density step wedge (exponential curve). The same density threshold, above background, was set for all images. The relative density of four circular areas (diameter 100 pixels) was measured from each image, and an average value per mouse was calculated.
Immunohistochemistry.
We obtained from Fred van Leeuwen (Nederland Instituut voor Hersenonderzoek, Amsterdam, The Netherlands) a polyclonal rabbit anti-mouse OTR antibody (57) that had been successfully used with mouse brains before (F. van Leeuwen, personal communication). We determined the optimal antibody concentration and immunohistochemistry paradigm through a dilution series run on control slides from strain-, age-, and sex-matched mice. One set of experimental slides was then processed: slides were fixed (10 min) in 0.1 M PBS (pH = 7.6) with 4% paraformaldehyde at 4°C; blocked with 0.5% hydrogen peroxide (15 min) in methanol; rinsed in PBS; coated (1 h) in 10% normal goat serum (Vector Laboratories, Burlingame, CA) in PBS with Triton X-100 (PBS/T; with 0.3% Triton X-100; Sigma, St. Louis, MO); and then incubated (120 h) in 1:3,000 primary antibody in 10% normal goat serum–PBS/T at 4°C. Control slides were incubated in preimmunization antibody. The slides were then rinsed in PBS/T, incubated (1 h) in secondary biotinylated goat anti-rabbit antibody (1:500 in PBS/T, Vector Laboratories), followed by 1 h in 1:1,000 avidin–biotin–horseradish peroxidase complex (Vectastain ABC, Elite Kit; Vector Laboratories) in PBS/T. The avidin–biotin–horseradish peroxidase complex was visualized with 0.025% cobalt chloride- and 0.02% ammonium nickel (II) sulfate-enhanced 0.5% DAB (3′,3′-diaminobenzidine tetrahydrochloride; Polysciences, Warrington, PA) in PBS activated with 0.05% H2O2. The blue–black chromogenic visualization was stopped with PBS. Slides were dehydrated in ethanol, left in xylenes for a minimum of 3 h, and cover slipped.
Digital images were taken (i) from the MA, (ii) at the exact location of the needle, and (iii) from the septum (56). The relative optic density of four circular areas (diameter, 100 pixels) was measured from each area, except the septum, where two measurements were taken. An average density value was then calculated per mouse/brain area.
Statistical Analyses.
Release of oligonucleotides from 502H microparticles.
SDs were calculated for each individual time point by using the three replicates and are shown with the error bars (Fig. 1 A and B).
Social recognition test.
Duration and frequency of each behavior were analyzed with ANOVA and multivariate ANOVA; with planned mean comparisons (SuperANOVA; Abacus Concepts, Berkeley, CA). Results obtained from the frequency of each behavior were in agreement with those of the respective durations and are not presented here.
Dark–light activity test.
Horizontal and vertical activity, place preferences (dark vs. lit compartment), and number of entries into each compartment were analyzed by using a one-way ANOVA (StatView, SAS Institute, Cary, NC).
Nissl-stained tissue and immunohistochemistry.
Cell and OTR density values were analyzed with a one-way ANOVA with treatment as the independent variable. Bonferroni–Dunn post hoc tests were performed (StatView, SAS Institute).
In all behavioral and cytological analyses, the factor of phase of estrous cycle never gave any statistical significance; therefore, it was removed from those statistical models.
In all analyses, P < 0.05 was considered statistically significant.
Acknowledgments
We thank Dr. Fred van Leeuwen (Nederland Instituut voor Hersenonderzoek, Amsterdam, The Netherlands) for providing the OTR-specific antibody, Dr. Martin Kavaliers for comments on the manuscript, and Dr. Lee Ming-Kow for consultation on some of the methodologies used. This work was funded by grants from the National Institute of Mental Health (to D.W.P.), National Institutes of Health (to R.L.), and Natural Sciences and Engineering Research Council of Canada (to E.C.).
Abbreviations
- AS
antisense
- LNA
locked nucleic acid
- MA
medial amygdala
- OT
oxytocin
- OTKO
oxytocin knockout
- OTR
oxytocin receptor
- OTWT
oxytocin wild type
- PBS/T
PBS with Triton X-100
- PLGA
poly(d,l-lactic-co-glycolic acid)
- SCR
scrambled.
Footnotes
The authors declare no conflict of interest.
References
- 1.Choleris E, Kavaliers M, Pfaff DW. J Neuroendocrinol. 2004;16:383–389. doi: 10.1111/j.0953-8194.2004.01178.x. [DOI] [PubMed] [Google Scholar]
- 2.Young LJ. Biol Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- 3.Bluthe RM, Gheusi G, Dantzer R. Psychoneuroendocrinology. 1993;18:323–335. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- 4.Engelmann M, Ebner K, Wotjak CT, Landgraf R. Behav Brain Res. 1998;90:89–94. doi: 10.1016/s0166-4328(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 6.Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. Proc Natl Acad Sci USA. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Genes Brain Behav. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson JN, Aldag JM, Insel TR, Young LJ. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendrick KM. Exp Physiol. 2000;85:111S–124S. doi: 10.1111/j.1469-445x.2000.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 11.Young LJ, Winslow JT, Wang Z, Gingrich B, Guo Q, Matzuk MM, Insel TR. Horm Behav. 1997;31:221–231. doi: 10.1006/hbeh.1997.1377. [DOI] [PubMed] [Google Scholar]
- 12.Cho MM, DeVries AC, Williams JR, Carter CS. Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 13.Insel TR, Hulihan TJ. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- 14.Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Physiol Behav. 1998;63:351–359. doi: 10.1016/s0031-9384(97)00434-4. [DOI] [PubMed] [Google Scholar]
- 15.Lubin DA, Elliott JC, Black MC, Johns JM. Behav Neurosci. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy MM. Horm Behav. 1990;24:365–375. doi: 10.1016/0018-506x(90)90015-p. [DOI] [PubMed] [Google Scholar]
- 18.Harmon AC, Moore TO, Huhman KL, Albers HE. Neuroscience. 2002;109:767–772. doi: 10.1016/s0306-4522(01)00523-1. [DOI] [PubMed] [Google Scholar]
- 19.Ragnauth AK, Devidze N, Moy V, Finley K, Goodwillie A, Kow LM, Muglia LJ, Pfaff DW. Genes Brain Behav. 2005;4:229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 20.Langer R. Science. 1990;249:1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 21.Benoit JP, Faisant N, Venier-Julienne MC, Menei P. J Control Release. 2000;65:285–296. doi: 10.1016/s0168-3659(99)00250-3. [DOI] [PubMed] [Google Scholar]
- 22.Bivas-Benita M, Romeijn S, Junginger HE, Borchard G. Eur J Pharm Biopharm. 2004;58:1–6. doi: 10.1016/j.ejpb.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freytag T, Dashevsky A, Tillman L, Hardee GE, Bodmeier R. J Control Release. 2000;69:197–207. doi: 10.1016/s0168-3659(00)00299-6. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy MM, Kleopoulos SP, Mobbs CV, Pfaff DW. Neuroendocrinology. 1994;59:432–440. doi: 10.1159/000126689. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy MM, Auger AP, Mong JA, Sickel MJ, Davis AM. Methods. 2000;22:239–248. doi: 10.1006/meth.2000.1075. [DOI] [PubMed] [Google Scholar]
- 26.Shi F, Hoekstra D. J Control Release. 2004;97:189–209. doi: 10.1016/j.jconrel.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Wahlestedt C, Salmi P, Good L, Kela J, Johnsson T, Hokfelt T, Broberger C, Porreca F, Lai J, Ren K, et al. Proc Natl Acad Sci USA. 2000;97:5633–5638. doi: 10.1073/pnas.97.10.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jepsen JS, Wengel J. Curr Opin Drug Discov Devel. 2004;7:188–194. [PubMed] [Google Scholar]
- 29.Jepsen JS, Sorensen MD, Wengel J. Oligonucleotides. 2004;14:130–146. doi: 10.1089/1545457041526317. [DOI] [PubMed] [Google Scholar]
- 30.Kim DH, Jung JS, Song DK, Suh HW, Huh SO, Kim YH. J Pharmacol Toxicol Methods. 1998;39:71–73. doi: 10.1016/s1056-8719(97)00105-6. [DOI] [PubMed] [Google Scholar]
- 31.Insel TR, Gingrich BS, Young LJ. Prog Brain Res. 2001;133:59–66. doi: 10.1016/s0079-6123(01)33005-4. [DOI] [PubMed] [Google Scholar]
- 32.Gould BR, Zingg HH. Neuroscience. 2003;122:155–167. doi: 10.1016/s0306-4522(03)00283-5. [DOI] [PubMed] [Google Scholar]
- 33.Latham N, Mason G. Appl Anim Behav Sci. 2004;86:261–289. [Google Scholar]
- 34.Insel TR, Fernald RD. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- 35.Choleris E, Kavaliers M. Pharmacol Biochem Behav. 1999;64:767–776. doi: 10.1016/s0091-3057(99)00141-0. [DOI] [PubMed] [Google Scholar]
- 36.Popik P, van Ree JM. Behav Neural Biol. 1993;59:63–68. doi: 10.1016/0163-1047(93)91173-k. [DOI] [PubMed] [Google Scholar]
- 37.Kavaliers M, Choleris E, Agmo A, Pfaff DW. Horm Behav. 2004;46:272–283. doi: 10.1016/j.yhbeh.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Kavaliers M, Choleris E, Agmo A, Muglia LJ, Ogawa S, Pfaff D. Anim Behav. 2005;70:693–702. [Google Scholar]
- 39.Blanchard RJ, Yudko EB, Rodgers RJ, Blanchard DC. Behav Brain Res. 1993;58:155–165. doi: 10.1016/0166-4328(93)90100-5. [DOI] [PubMed] [Google Scholar]
- 40.Lister RG. Pharmacol Ther. 1990;46:321–340. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- 41.Roy V, Chapillon P. Behav Brain Res. 2004;154:439–448. doi: 10.1016/j.bbr.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Choleris E, Thomas AW, Kavaliers M, Prato FS. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 43.Rodgers RJ, Cao BJ, Dalvi A, Holmes A. Braz J Med Biol Res. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- 44.Mikics E, Barsy B, Barsvari B, Haller J. Horm Behav. 2005;48:152–162. doi: 10.1016/j.yhbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez LE, Andrews N, File SE. Brain Res. 1996;732:145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- 46.Dulac C, Torello AT. Nat Rev Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- 47.Johnston RE. J Mammal. 2003;84:1141–1162. [Google Scholar]
- 48.Lysik MA, Wu-Pong S. J Pharm Sci. 2003;92:1559–1573. doi: 10.1002/jps.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoji Y, Nakashima H. Curr Pharm Des. 2004;10:785–796. doi: 10.2174/1381612043453009. [DOI] [PubMed] [Google Scholar]
- 50.Veziers J, Lesourd M, Jollivet C, Montero-Menei C, Benoit JP, Menei P. J Neurosurg. 2001;95:489–494. doi: 10.3171/jns.2001.95.3.0489. [DOI] [PubMed] [Google Scholar]
- 51.Chirila TV, Rakoczy PE, Garrett KL, Lou X, Constable IJ. Biomaterials. 2002;23:321–342. doi: 10.1016/S0142-9612(01)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Twaites BR, de Las Heras AC, Cunliffe D, Lavigne M, Pennadam S, Smith JR, Gorecki DC, Alexander C. J Control Release. 2004;97:551–566. doi: 10.1016/j.jconrel.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 53.Odonnelland PB, McGinty JW. Adv Drug Deliv Rev. 1997;28:25–42. doi: 10.1016/s0169-409x(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 54.Gross GA, Imamura T, Luedke C, Vogt SK, Olson LM, Nelson DM, Sadovsky Y, Muglia LJ. Proc Natl Acad Sci USA. 1998;95:11875–11879. doi: 10.1073/pnas.95.20.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kubota Y, Kimura T, Hashimoto K, Tokugawa Y, Nobunaga K, Azuma C, Saji F, Murata Y. Mol Cell Endocrinol. 1996;124:25–32. doi: 10.1016/s0303-7207(96)03923-8. [DOI] [PubMed] [Google Scholar]
- 56.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York: Academic; 2001. [Google Scholar]
- 57.Adan RA, Van Leeuwen FW, Sonnemans MA, Brouns M, Hoffman G, Verbalis JG, Burbach JP. Endocrinology. 1995;15:573–593. doi: 10.1210/endo.136.9.7649111. [DOI] [PubMed] [Google Scholar]