Abstract
Collagen crosslinks are important to the quality of bone and may be contributors to the age-related increase in bone fracture. This study was performed to investigate whether age and gender effects on collagen crosslinks are similar in osteonal and interstitial bone tissues. Forty human cadaveric femurs were collected and divided into two age groups: Middle aged (42–63 years of age) and Elderly (69–90 years of age) with ten males and ten females in each group (n = 10). Micro-cores of bone tissue from both secondary osteons (newly formed) and interstitial regions (biologically old) in the medial quadrant of the diaphysis were extracted using a custom-modified, computer numerical controlled machine. The bone specimens were then analyzed using high performance liquid chromatography to determine the effects of age and gender on the concentration of mature, enzymatic crosslinks (hydroxylysyl-pyridinoline – HP and lysylpyridinoline – LP) and a non-enzymatic crosslink (pentosidine – PE) at these two bony sites. The results indicate that age has a significant effect on the concentration of LP and PE, while gender has a significant effect on HP and LP. In addition, the concentration of the crosslinks in the secondary osteons is significantly different from that in the interstitial bone regions. These results suggest that the rate of non-enzymatic crosslinking may increase while the formation of maturate enzymatic crosslinks may decrease with age. Such changes could potentially reduce the inherent quality of the bone tissue in the elderly skeleton.
Keywords: Osteon, Collagen Crosslinks, Metabolism, Glycation, Bone Quality
INTRODUCTION
Age-related bone fractures are a serious concern in the health care of the elderly population due to high treatment costs and morbidity. So far, clinical methods for identifying fracture risk rely mainly on the measurement of bone density. However, there are a number of additional factors rather than a loss in bone density that dictate susceptibility to failure [1-4]. One possible factor affecting the mechanical integrity of bone is the pattern and quantity of collagen crosslinks [5-11]. There are two potential types of collagen crosslinks affected by age. One involves an enzymatic pathway in which reducible crosslinks initially form between the head and tail of neighboring collagen molecules and mature into stable, trivalent bonds [12]. The other involves a glycation-mediated pathway in which intermolecular bonds between neighboring lysine or arginine residues accumulate over time [12].
The disruption of enzymatic crosslinking (e.g., by inhibiting the enzyme lysyl oxidase or causing copper deficiency) affects the mechanical properties of bone [5, 6, 10, 7]. Also, the stability of bone collagen decreases with age as measured by biochemical [13, 14] (extractability) and thermal [15] (i.e., shrinkage temperature and contraction rate) techniques. Furthermore, this decrease has been associated with a loss in bone toughness [16]. Yet, a clear connection between the decline in collagen integrity and age-related changes in crosslink concentrations has not emerged in the literature. A study by Zioupos et al. [16] did not find a correlation between mature, enzymatic crosslink concentrations and shrinkage temperature (nor with age). In addition, Oxlund et al. [14] reported in their study that there was no association between immature, enzymatic crosslink concentrations and collagen stability (nor with age). However, less collagen stability and less immature crosslink concentrations were observed in osteoporotic bone than in age- and gender-matched bone that had no signs of osteoporosis [14, 17]. Moreover, the ratio of mature to immature crosslinks was found to be greater near bone forming surfaces in osteoporotic bone compared to normal tissues [18]. Thus, at least with disease an imbalance in enzymatic crosslinking can occur in bone remodeling like the osteoporotic imbalance between resorption and formation. On the other hand, enzymatic crosslinks in long-lived collagen do not accumulate with age presumably due to the limited hydroxylation of the lysine residue and the activity of lysyl hydroxylase. In fact, the formation of enzymatic crosslinks is rather stereospecific, and repeats of head-to-tail covalent bonds are necessary for proper mineralization [19].
The loss in bone toughness with age could be affected by non-enzymatic crosslinking. With aging, this glycation-mediated process (i.e., the Maillard reaction) increases the number of intermolecular crosslinks in a variety of collagenous tissues [20-22] including bone [23-25].
Furthermore, an age-related increase in pentosidine, which is a useful biomarker for the damage to proteins caused by non-enzymatic, glycation-induced (NEG) crosslinks [26], has been associated with a decrease in strength and toughness of demineralized cortical bone [24] and a decrease in the structural ductility of individual trabeculae [25]. At least two studies indicate that increases in intermolecular crosslinks make bone more brittle. Currey at al. [27] observed that formaldehyde fixation for three hours (which presumably increases the concentration of crosslinks in bone) decreased impact energy absorption of bone at high strain rate, while Vashishth et al. [28] demonstrated that ribose incubation of bone for 38 days decreased the ratio of the initial elastic modulus to the failure modulus. This decrease indicates a loss in the ability to generate microdamage, which is thought to be a toughening mechanism of bone.
Avery and Bailey [29] have postulated that NEG crosslinking is an age-related process, at least in slowly metabolizing collagenous tissues. They reason that the slow turnover of mature collagen (i.e., slower than the rate of bone remodeling) allows for a continual accumulation of advanced glycation end-products (AGE) as glucose reacts with numerous side-chains in the triple helix of collagen, namely those associated with lysine and arginine. Then, the Maillard reaction continues through several steps (either oxidative or non-oxidative) eventually forming a stable, intermolecular crosslink. Previous research has shown that a fraction of bone tissue is not replaced by the remodeling process over a long time [30, 31]. However, bone remodeling activity or collagen turnover as measured by markers of bone resorption has been observed to increase with age in men [32, 33] and in women following menopause [34, 35]. This suggests that the amount of long-lived collagen in bone could be limited despite advanced aging of the skeleton.
There is the possibility that the bone remodeling process affects the quality of bone tissue. A recent study suggested that higher percentage of denatured collagen (i.e., less stability) exists in the osteoid of elderly donors compared with that in young ones [36]. In addition, irregular bone remodeling has been characterized in Paget's disease by histologically abnormal architecture of the bone matrix [37]. If age-related changes in collagen crosslinks were predominantly caused by abnormal bone remodeling, such changes would most likely be reflected in the newly formed bone tissues (i.e., secondary osteons). Thus, as an initial investigation into the role of bone remodeling in affecting the collagen crosslink profile with advancing age, we compared age- and gender-related differences in crosslinks between osteonal and interstitial bone tissue. To do so, pyridinoline and pentosidine concentration levels in micro-samples of osteonal tissue and interstitial tissue were measured from middle aged and elderly human cortical bone, taken from both male and female donors.
MATERIALS & METHODS
Collecting cross-sections of cortical bone
Forty fresh-frozen, human cadaveric femurs were obtained from several tissue banks (Musculoskeletal Transplant Foundation, Edison, NJ; The University of Texas Southwestern Medical Center at Dallas Willed Body Program, Dallas, TX; and National Disease Research Exchange, Philadelphia, PA) with the stipulation that donors had no known bone diseases. Bones from both male and female donors were equally divided into two age groups: middle aged and elderly. The middle-aged group included ages between 47 and 59 years for males (n = 10) and between 42 and 63 years for females (n = 10), while the elderly group included ages between 69 through 87 years for males (n = 10) and between 73 and 90 years for females (n = 10). Using a band saw and then a circular diamond saw (Isomet 2000, Buehler, Lake Bluff, IL), 3 mm thick cross-sections of the mid-diaphysis were obtained from each femur. The distal surface was lapped with successive grits of silicon carbide and polished with 0.05 micron Alumina suspension.
Collecting micro-cores of osteonal or interstitial tissue
To isolate bone specimens from the secondary osteonal and interstitial bone regions, a custom-modified, computer numerical controlled (CNC) machine system was used (Fig. 1). As shown in Figure 1A, a positioning system was attached to the spindle head of the CNC (ProLIGHT 1000, Light Machines, Manchester, NH). The positioning system consisted of a Nikon microscope (Measurescope UM-2, Nikon, Kanagawa, Japan) and a CCD camera (Spot Insight, Diagnostic Instruments, Inc., Sterling Heights, MI) connected to a computer. A micro-grain carbide blank (1/16th inch diameter) was made into an eccentric coring tool (Figure 1B) that produced cylindrical bone specimens with a nominal diameter of 200 – 250 μm (i.e., less than the average diameter of osteons and smaller than typical patches of interstitial tissue). With the cross-section of the femoral bone secured to the 2-axis moving stage of the CNC and positioned below the tool, coring was performed at a feed rate of 10 μm per revolution with a spindle speed of 1000 rev/min under continuous irrigation.
Figure 1.
A modified, computer numerical controlled milling machine (CNC) for isolating micro-specimens from osteonal and interstitial regions of human cortical bone (A). An eccentric mill bit is used to core out specimens from these small regions (B). The system identifies a region of interest with a microscope and then moves it under the eccentric mill bit for coring (C).
The following procedure was implemented for finding appropriate regions of osteonal or interstitial tissue. First, a cross section of polished femoral bone was positioned by the CNC stage such that the medial side was in view through the microscope objective. Second, at a magnification of 100x, either a secondary osteon (i.e., newly formed bone tissues as shown in Figure 2) or a region of interstitial bone (i.e., primary bone or remnants of early bone remodeling) was positioned within a preset ring of the eyepiece reticule. Finally, since the distance between the center of the microscope and that of the cutting tool was determined beforehand, the CNC was programmed to move the area of interest under the center of the cutting tool (positioning error < 5 μm).
Figure 2.

A pictorial representation to identify secondary osteons and interstitial bone regions in human cortical bone: The secondary osteons (hollow arrows) are newly formed bone tissues through bone remodeling process, whereas the interstitial bone regions (solid arrows) are the older tissues formed previously.
Coring was performed to a depth of 1.3 mm as described above. After removing the core from the collection site, it was examined using a transmitted light microscope at 50x and 100x (ML5000, Meiji Techno, San Jose, CA) to ensure that the core came from the desired regions. Since Haversian canals do not always run parallel with the anatomical axis of long bones, it is possible that specimens are not solely from osteonal or interstitial tissue. Therefore, rejection criteria was used in this study (Fig. 3). Cores obtained from a secondary osteon having an Haversian canal spanning less than half of its length were rejected. Likewise, if a core obtained from the interstitial region included a Haversian canal more than one fourth of its length, it was ejected. Specimens were viewed from several orientations, while applying the rejection criteria.
Figure 3.
An example of bone cores prepared from the secondary osteons using the CNC coring device. (A) the secondary osteon identified prior to coring; (B) the core (diameter = 0.2 mm) prepared from the region; and (C) the lateral view of the specimen (the Haversian canal spanning over the length is visible). A score was assigned to each image of a micro-core to confirm that the samples were mainly osteonal or mainly interstitial tissue.
Measuring the concentration of collagen crosslinks
Each femur provided two micro-cores for each type of bone tissue (i.e., 2 osteonal and 2 interstitial tissues per donor). After measuring their mass with a precision microbalance (UMX2, Mettler Toledo, Greifensee, Switzerland), the two micro-cores of the same tissue type were combined and hydrolyzed in 500 μL of 6 N HCl at 110 °C for at least 20 hours. The hydrolysate was split into two disposable culture tubes (at a nominal ratio of 65:35), and the acid removed using a SpeedVac centrifuge (SPD111V, Thermo Savant, Holbrook, NY) at a temperature of 60 °C and a vacuum of ∼5 torr, attached to a refrigerated vapor trap (RVT400, Thermo Savant, Holbrook, NY). The residue of the 65% sample was re-suspended with a known volume (100 μL) of ultra-pure water plus a dissolved internal standard (0.51 mM pyridoxine). Next, following a method described by Bank et al. [38], the concentration of hydroxylysyl-pyridinoline (HP), lysyl-pyridinoline (LP), and pentosidine (PE) was determined using high performance liquid chromatography (HPLC). Briefly, the re-suspended residue of collagen was filtered and diluted with buffer (0.05% heptafluorobutyric acid in 10% acetonitrile), and a 50 μL sample was injected into a HPLC system (System Gold 126 Solvent Module, Beckman Coulter, Fullerton, CA) fitted with a silica-based column (Waters Spherisorb ODS-2 5u, Alltech Associates, Inc., Deerfield, IL). Samples of the hydrolysates, one from each test group (eight in all), were sequentially injected using an auto-sampler (System Gold 508 Autosampler, Beckman Coulter, Fullerton, CA). A diluted calibration standard (50 μL) was always injected prior to the sequence of eight injections. With a programmable fluorescence detector (FP-2020plus, Jasco, Tokyo, Japan), chromatograms were recorded as crosslinks eluted off of the column as dictated by the chemistry of the mobile phases and the column (see Bank et al. [38] for details).
To calculate crosslink concentrations, the area under each crosslink peak was 1) multiplied by the ratio of known crosslink concentration to the area of the respective peaks on the appropriate chromatogram of the calibration standard, 2) adjusted by the ratio of the known concentration of the internal standard (accounting for dilution) to the calculated concentration (as determined by calibration standard), and 3) divided by the mass of the bone sample after accounting for dilutions and divisions. Each crosslink concentration was then normalized by its respective collagen concentration, as determined by a micro-hydroxyproline (Hyp) assay on the remaining 35% of the sample. For this latter analysis, we used the colorimetric technique that is described by Morales et al. [39]. As in the case of HPLC, samples of collagen from each group were sequentially assayed in batches along with standards of varying Hyp concentrations. The mole of Hyp per mass of bone sample (after dilution and divisions) was divided by 0.14 because about 14% of Type I collagen is Hydroxyproline.
Statistical Analysis
A general linear model (GLM) of each measured property (HP, LP, and PE) initially included all main effects (gender, age group, and tissue type) plus all interaction effects. Tissue type was treated as a repeated measure (i.e., osteon and interstitial are correlated). Then, the model was adjusted by successively removing non-significant interaction effects to converge on a model with a p-value < 0.05 (MIXED Procedure, SAS/STAT User's Guide version 9, 2004, SAS Institute Inc., Cary, NC). For each crosslink, the final model did not include any interactions (i.e., none were significant), but for collagen content, the interaction between age group and tissue type was included. Since osteonal and interstitial samples were collected from the same donor, the error terms for these tissue type were correlated in the model (i.e., treated as a repeated measure). p-values for each main effect are given in Table 1. Two different post hoc analyses were performed to answer two distinct questions: 1) are there significant differences in osteonal or interstitial crosslinks between female and male, between middle and old, and among the combinations? and 2) are crosslinks greater in one type of tissue within each of the group pairings? For the first question, we analyzed the crosslink data from osteonal tissue separately from interstitial tissue and employed the Bootstrap method (MULTTEST Procedure), which provides an adjusted p-value and improves the power of the hypothesis tests through re-sampling techniques. The hypothesis tests were one sided, and the number of tests (influencing the adjusted p-value) depended on whether gender and age group were significant in the GLM (Table 2). For the second question, we employed a paired t-test that adjusted the p-value according to the Hochberg technique (TTEST Procedure). These hypothesis tests were also one sided. For each gender and age group pairing, the null hypotheses were: Interstitial HP > Osteonal HP, Interstitial LP > Osteonal LP, and Interstitial PE < Osteonal PE.
Table 1.
p-values obtained from general linear models with tissue type (osteonal or interstitial) being a repeated measure.
| Collagen | HP | LP | PE | |
|---|---|---|---|---|
| Age | 0.1944 | 0.2106 | 0.0074 | 0.0004 |
| Gender | 0.0809 | 0.0026 | 0.0014 | 0.1885 |
| Tissue type | 0.2454 | <0.0001 | <0.0001 | 0.0757 |
| Age*Tissue type | 0.0581 | NIa | NI | NI |
interactions were not included if removing them improved the model.
Table 2.
In post hoc analyses of osteonal and interstitial crosslinks, the family of null hypothesis tests only involved comparisons in which Gender or Age was significant at p-value < 0.05 in the general linear model.
| HP | LP | PE |
|---|---|---|
| Female-Middle > Male-Middle | Female-Middle > Male-Middle | Female-Middle < Female-Old |
| Female-Old > Male-Old | Female-Old > Male-Old | Male-Middle < Male-Old |
| Female-Middle > Female-Old | ||
| Male-Middle > Male-Old |
RESULTS
With tissue type included as a repeated measure, the general linear models indicated that all factors (i.e., age, gender, and tissue type) had significant effects on LP, gender and tissue type had significant effects on HP, and only age had a significant effect on PE (Table 1). However, it appeared that PE concentration in osteonal tissue tends to be higher than that in interstitial tissues since the p-value was 0.0757. The total collagen content seemed to be independent of the three factors, although an interaction between age and tissue type seemed to exist (Table 1).
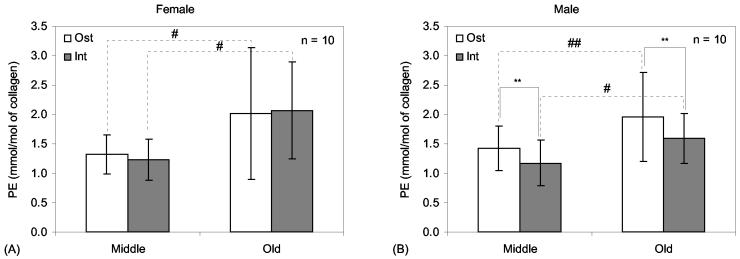
In post hoc comparisons between the gender groups, female bone tissues had significantly greater HP concentration than male bone tissues (Osteonal: p-value = 0.0156 and Interstitial: p-value = 0.0026). Post hoc comparisons between the gender groups within each age group found no significant difference in HP (p-value > 0.1) for both tissue types (Fig. 4). LP, another mature, enzymatic crosslink, was also greater in female than in male bone tissues (Osteonal: p-value = 0.0149 and Interstitial: p-value = 0.0019). Also, LP concentration was greater in the middle aged bone than in the elderly bone (Osteonal: p-value = 0.0288 and Interstitial: p-value = 0.0085). In post hoc comparisons of the four group pairings (Table 2), osteonal LP concentration appeared greater in female than in male bone, especially for middle aged bone (Middle: p-value = 0.0207 and Old: p-value = 0.0755). Interestingly, osteonal LP concentration decreased with age only in female bone, whereas interstitial LP concentration decreased with age in male bone alone (Fig. 5). In post hoc comparisons between the age groups, PE significantly increased with age in both osteonal (p-value = 0.0031) and interstitial tissue (p-value = 0.0002). For both osteonal and interstitial tissue, there is an age-related increase in PE for both female and male bone (Fig. 6).
Figure 4.
Comparison of hydroxylysyl-pyridinoline levels (mean and standard deviation) between different tissue types and age groups for female bone (A) and male bone (B). Connecting lines indicate differences: *adjusted p-value < 0.05 as determined by paired t-tests comparing osteonal to interstitial bone tissue.
Figure 5.
Comparison of lysyl-pyridinoline levels (mean and standard deviation) between different tissue types and age groups for female bone (A) and male bone (B). Connecting lines indicate differences: *adjusted p-value < 0.05 as determined by paired t-tests comparing osteonal to interstitial bone tissue. ##adjusted p-value < 0.1 as determined by separate bootstrap analyses of osteonal and interstitial crosslink data.
Figure 6.
Comparisons of pentosidine levels (mean and standard deviation) between different tissue types and age groups for female bone (A) and male bone (B). Connecting lines indicate differences: **adjusted p-value <0.1 as determined in paired t-tests comparing osteonal to interstitial bone tissue. #adjusted p-value < 0.05 and ##adjusted p-value < 0.1 as determined by separate bootstrap analyses of osteonal and interstitial crosslink data.
In a separate post hoc analysis, paired t-tests (giving Hochberg's adjusted p-values) also found that HP and LP concentrations were significantly greater in interstitial bone tissue than in osteon tissue. This difference in tissue type was significant for all four combinations of gender and age group (Fig. 4 and 5). Also, PE concentration was greater in osteonal bone than in interstitial tissues for male bone (Fig. 6), but did not show significant differences for female donors.
DISCUSSION
Mature collagen crosslinks (enzymatic and non-enzymatic) were quantified in two tissue types – osteonal and interstitial – in order to assess whether crosslinking in biologically younger tissue changes with age. The results of this study indicated that there exist significant age-related differences in the profile of collagen crosslinks within secondary osteons and interstitial bone regions. Since the activity level of bone remodeling results in different tissue age of osteons and in turn affects the level of maturation in the enzymatic crosslinks and the accumulation of nonenzymatic crosslinks, we cannot deduce that the decrease in LP with age is due to changes in newly formed tissue or an increase in remodeling activity with age. However, the similar and even slightly greater increase in osteonal PE compared to interstitial PE with increasing age suggests that bone remodeling may be a possible pathway for age-related changes in the collagen network of bone. Rates of crosslinking may vary with age in which enzymatic activity decreases or the maturation process decrease while non-enzymatic activity increases.
An interpretation of age-related effects on the enzymatic pyridinium crosslinks depends on the current understanding of their mechanism of formation and their relationship to the bone remodeling process. The divalent bond of reducible crosslinks (keto-imine) matures over time via lysyl oxidase into a trivalent bond forming the stable HP (present in most collagenous tissues) or the stable LP (present in only mineralized tissues [40]) [41]. In non-mineralized tissues like cartilage, such maturation requires a few weeks [42], but mineralization slows the process [43] or hinders the process [44]. Thus, the concentration of HP and LP in bone tissue could serve as an indicator of the age of the tissue (akin to the degree of mineralization). Furthermore, the higher concentration of mature crosslinks (HP and LP) in the interstitial tissue (Fig. 4 and 5) suggests that there exists a difference in the tissue age between the two tissue types.
Since bone remodeling serves to replace old tissue with new tissue, the stable crosslinks in the old tissue would be replaced by the reducible crosslinks in the newly formed tissue (which in time will mature). In fact, reducible crosslinks are always present in the skeleton of any age because remodeling is continual throughout life [43] and not all crosslinks mature due to mineralization and stereospecificity of enzymatic crosslinking [19]. A lower concentration of mature crosslinks is expected in secondary osteons compared with interstitial bone tissues, which is not affected by the bone remodeling process [43]. Indeed, we observed that both HP and LP were significantly less in secondary osteons than in interstitial bone tissues, thereby supporting the speculation that remodeling activity affects the concentration of mature, enzymatic crosslinks.
Besides age, gender was also found to affect the pyridinium crosslinks. To the best of our knowledge, the present study is the only one to have found greater HP and LP in female than in male bone. This finding appears at odds with the viewpoint that HP and LP indicate the level of remodeling activity. Given that the age range of the middle aged group in our study corresponded with the typical period of menopause, the remodeling activity in middle-aged females would be conceivably higher than that in the age matched males. This usually indicates that female bone tissues would have lower concentrations of HP and LP since on average higher remodeling activity would equate to more newly formed osteons. Yet, osteonal HP and LP of middle-aged bone was greater for females than males (Fig 4A and 5A). Perhaps the rate of maturation of enzymatic crosslinks is greater in female bone than in male. This requires further exploration.
While the enzymatic crosslinks decreased, non-enzymatic collagen crosslinks increased significantly with age and without showing significant difference between the genders. There was a trend of less PE in interstitial tissues compared with secondary osteons (especially in male bone; Fig. 6), suggesting that rate of non-enzymatic crosslinking in osteons formed through the bone remodeling process increases with age. The present findings implicate that increases in advanced glycation end-products (AGEs) may not just be a result of aging tissue but a product of the bone remodeling process.
The mechanistic contribution of non-enzymatic crosslinks to bone quality is still unclear. The observation that patients with diabetes mellitus have a higher incidence of bone fracture than healthy individuals suggests glycation-mediated crosslinking compromises bone [46, 47]. Yet, a few ex vivo experiments that compared bone incubated in sugar (glucose or ribose) to control did not report significant differences between strength, stiffness, or toughness of mineralized bone [48, 28]. By incubating bone in ribose at successive time points, demineralizing it, and then measuring fluorescence and collagen stiffness, Vashishth et al. [28] demonstrated that mineral is not a barrier to glycation-mediated crosslinking and its stiffening effect on collagen. Nonetheless, this study did not investigate if this method of crosslinking caused concentrations comparable to in vivo levels nor did it analyze the nature of the crosslink (e.g., its type and location on the triple helix).
There are several limitations to the present study. One, it was impossible to ascertain the biological age of the tissues. Thus, osteonal (newly formed) and interstitial (existing) bone tissue can only provide a relative measure of tissue age. Two, although osteons are generally directed along the longitudinal axis of femurs, they do not necessarily run vertical to the cross section, as did the coring tool. Thus, bone specimens prepared from osteonal and interstitial bone regions are not necessarily pure. However, we used exclusion criteria to facilitate the retrieval of bone specimens that are predominately osteonal or interstitial tissue. As a check, images were taken of all samples, and three blind observes graded each image based on the presence of a Haversian canal (Fig. 3). The score for osteons was significantly less than the score for interstitial tissue in each group pairing. Three, there are number of other crosslinks that may be of equal to or more importance than pyridinolines and pentosidine. For example, the work of Knott et al. [9] observed a correlation between the pyrrole and bone strength, but not the pyridinolines, when comparing normal to osteoporotic avian bone. However, others have observed a correlation between the pyridinoline crosslinks and bone strength when testing the tibiae of boiler chicks of varying age [11]. Finally, the specimen size (Ø0.2 mm × 1.3 mm) was too small to be mechanically tested. Overcoming the technical challenges of mechanically testing micro-specimens may reveal a relationship between age-related changes in the collagen crosslink profile and increases in osteon stiffness and decreases in osteon toughness.
The accumulation of AGEs in aging bone may not solely be the result of long-term Maillard reactions at the existing tissue (interstitial), but may also occur in relatively newly formed tissues (secondary osteons) since interstitial PE was not found to be greater than that of osteonal tissues. This implicates that aging may accelerate the rate of non-enzymatic crosslinking in newly formed tissues. On the other hand, mature, enzymatic crosslinks appear to decrease with age, suggesting that aging may affect the collagen maturation process, cellular production of lysyl oxidase, or perhaps remodeling activity.
ACKNOWLEDGEMENT
This study was supported by a NIH/NIA grant (R01 AG022044). The authors are grateful to Dr. Ruud Bank for the collagen crosslink standards (i.e., HP, LP, and PE).
Footnotes
Sources of funding: NIH/NIA grant (1 R01 AG022044-01)
REFERENCES
- 1.Parfitt AM. Bone remodeling and bone loss: understanding the pathophysiology of osteoporosis. Clin Obstet Gynecol. 1987;30:789–811. doi: 10.1097/00003081-198712000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Mosekilde L. Vertebral structure and strength in vivo and in vitro. Calcif Tissue Int. 1993;53:S121–5. doi: 10.1007/BF01673420. discussion S125-6. [DOI] [PubMed] [Google Scholar]
- 3.van der Meulen MC, Jepsen KJ, Mikic B. Understanding bone strength: size isn't everything. Bone. 2001;29:101–4. doi: 10.1016/s8756-3282(01)00491-4. [DOI] [PubMed] [Google Scholar]
- 4.Jepsen KJ. The aging cortex: to crack or not to crack. Osteoporos Int. 2003;14(Suppl 5):57–66. doi: 10.1007/s00198-003-1475-3. [DOI] [PubMed] [Google Scholar]
- 5.Opsahl W, Zeronian H, Ellison M, Lewis D, Rucker RB, Riggins RS. Role of copper in collagen cross-linking and its influence on selected mechanical properties of chick bone and tendon. J Nutr. 1982;112:708–16. doi: 10.1093/jn/112.4.708. [DOI] [PubMed] [Google Scholar]
- 6.Jonas J, Burns J, Abel EW, Cresswell MJ, Strain JJ, Paterson CR. Impaired mechanical strength of bone in experimental copper deficiency. Ann Nutr Metab. 1993;37:245–52. doi: 10.1159/000177774. [DOI] [PubMed] [Google Scholar]
- 7.Oxlund H, Barckman M, Ortoft G, Andreassen TT. Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone. 1995;17:365S–371S. doi: 10.1016/8756-3282(95)00328-b. [DOI] [PubMed] [Google Scholar]
- 8.Banse X, Sims TJ, Bailey AJ. Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross-links. J Bone Miner Res. 2002;17:1621–8. doi: 10.1359/jbmr.2002.17.9.1621. [DOI] [PubMed] [Google Scholar]
- 9.Knott L, Whitehead CC, Fleming RH, Bailey AJ. Biochemical changes in the collagenous matrix of osteoporotic avian bone. Biochem J. 1995;310(Pt 3):1045–51. doi: 10.1042/bj3101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lees S, Eyre DR, Barnard SM. BAPN dose dependence of mature crosslinking in bone matrix collagen of rabbit compact bone: corresponding variation of sonic velocity and equatorial diffraction spacing. Connect Tissue Res. 1990;24:95–105. doi: 10.3109/03008209009152426. [DOI] [PubMed] [Google Scholar]
- 11.Rath NC, Balog JM, Huff WE, Huff GR, Kulkarni GB, Tierce JF. Comparative differences in the composition and biomechanical properties of tibiae of seven- and seventy-two-week-old male and female broiler breeder chickens. Poult Sci. 1999;78:1232–9. doi: 10.1093/ps/78.8.1232. [DOI] [PubMed] [Google Scholar]
- 12.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–7. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 13.Oxlund H. Alterations in the stability of collagen from human trabecular bone with respect to age. In: J. JS CC, BJ R, editors. Osteoporosis. Osteopress; Copenhagen: 1987. 1987. [Google Scholar]
- 14.Oxlund H, Mosekilde L, Ortoft G. Reduced concentration of collagen reducible cross links in human trabecular bone with respect to age and osteoporosis. Bone. 1996;19:479–84. doi: 10.1016/s8756-3282(96)00283-9. [DOI] [PubMed] [Google Scholar]
- 15.Danielsen CC, Mosekilde L, Bollersleve J, Mosekilde L. Thermal stability of cortical bone collagen in relation to age in normal individuals and in individuals with osteoprosis. Bone. 1994;19:91–96. doi: 10.1016/8756-3282(94)90897-4. [DOI] [PubMed] [Google Scholar]
- 16.Zioupos P, Currey JD, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res. 1999;45:108–16. doi: 10.1002/(sici)1097-4636(199905)45:2<108::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 17.Bailey AJ, Wotton SF, Sims TJ, Thompson PW. Biochemical changes in the collagen of human osteoporotic bone matrix. Connect Tissue Res. 1993;29:119–32. doi: 10.3109/03008209309014239. [DOI] [PubMed] [Google Scholar]
- 18.Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone fragility and collagen cross-links. J Bone Miner Res. 2004;19:2000–4. doi: 10.1359/JBMR.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamauchi M, Katz EP, Otsubo K, Teraoka K, Mechanic GL. Cross-linking and stereospecific structure of collagen in mineralized and nonmineralized skeletal tissues. Connect Tissue Res. 1989;21:159–67. doi: 10.3109/03008208909050006. discussion 168-9. [DOI] [PubMed] [Google Scholar]
- 20.Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989;264:21597–602. [PubMed] [Google Scholar]
- 21.Monnier VM, Sell DR, Nagaraj RH, Miyata S, Grandhee S, Odetti P, et al. Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes. 1992;41(Suppl 2):36–41. doi: 10.2337/diab.41.2.s36. [DOI] [PubMed] [Google Scholar]
- 22.Zieman S, Kass D. Advanced glycation end product cross-linking: pathophysiologic role and therapeutic target in cardiovascular disease. Congest Heart Fail. 2004;10:144–9. doi: 10.1111/j.1527-5299.2004.03223.x. quiz 150-1. [DOI] [PubMed] [Google Scholar]
- 23.Tomasek JJ, Meyers SW, Basinger JB, Green DT, Shew RL. Diabetic and age-related enhancement of collagen-linked fluorescence in cortical bones of rats. Life Sci. 1994;55:855–61. doi: 10.1016/0024-3205(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, et al. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone. 2005;37:825–32. doi: 10.1016/j.bone.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991;266:11654–60. [PubMed] [Google Scholar]
- 27.Currey JD, Brear K, Zioupos P, Reilly GC. Effect of formaldehyde fixation on some mechanical properties of bovine bone. Biomaterials. 1995;16:1267–71. doi: 10.1016/0142-9612(95)98135-2. [DOI] [PubMed] [Google Scholar]
- 28.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 29.Avery NC, Bailey AJ. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. Scand J Med Sci Sports. 2005;15:231–40. doi: 10.1111/j.1600-0838.2005.00464.x. [DOI] [PubMed] [Google Scholar]
- 30.Simmons ED, Jr., Pritzker KP, Grynpas MD. Age-related changes in the human femoral cortex. J Orthop Res. 1991;9:155–67. doi: 10.1002/jor.1100090202. [DOI] [PubMed] [Google Scholar]
- 31.Boivin G, Meunier PJ. The degree of mineralization of bone tissue measured by computerized quantitative contact microradiography. Calcif Tissue Int. 2002;70:503–11. doi: 10.1007/s00223-001-2048-0. [DOI] [PubMed] [Google Scholar]
- 32.Fatayerji D, Eastell R. Age-related changes in bone turnover in men. J Bone Miner Res. 1999;14:1203–10. doi: 10.1359/jbmr.1999.14.7.1203. [DOI] [PubMed] [Google Scholar]
- 33.Clarke BL, Ebeling PR, Jones JD, Wahner HW, O'Fallon WM, Riggs BL, et al. Predictors of bone mineral density in aging healthy men varies by skeletal site. Calcif Tissue Int. 2002;70:137–45. doi: 10.1007/s00223-001-1072-4. [DOI] [PubMed] [Google Scholar]
- 34.Melton LJ, 3rd, Khosla S, Atkinson EJ, O'Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res. 1997;12:1083–91. doi: 10.1359/jbmr.1997.12.7.1083. [DOI] [PubMed] [Google Scholar]
- 35.Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res. 1997;12:498–508. doi: 10.1359/jbmr.1997.12.4.498. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Li X, Shen X, Agrawal CM. Age-related changes of noncalcified collagen in human cortical bone. Ann Biomed Eng. 2003;31:1365–71. doi: 10.1114/1.1623488. [DOI] [PubMed] [Google Scholar]
- 37.Ingram RT, Collazo-Clavell M, Tiegs R, Fitzpatrick LA. Paget's disease is associated with changes in the immunohistochemical distribution of noncollagenous matrix proteins in bone. J Clin Endocrinol Metab. 1996;81:1810–20. doi: 10.1210/jcem.81.5.8626840. [DOI] [PubMed] [Google Scholar]
- 38.Bank RA, Beekman B, Verzijl N, de Roos JA, Sakkee AN, TeKoppele JM. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J Chromatogr B Biomed Sci Appl. 1997;703:37–44. doi: 10.1016/s0378-4347(97)00391-5. [DOI] [PubMed] [Google Scholar]
- 39.Morales TI, Woessner JF, Howell DS, Marsh JM, LeMaire WJ. A microassay for the direct demonstration of collagenolytic activity in Graafian follicles of the rat. Biochim Biophys Acta. 1978;524:428–34. doi: 10.1016/0005-2744(78)90180-8. [DOI] [PubMed] [Google Scholar]
- 40.Eyre DR, Koob TJ, Van Ness KP. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984;137:380–8. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- 41.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 42.Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–48. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 43.Eyre DR, Dickson IR, Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem J. 1988;252:495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banes AJ, Yamauchi M, Mechanic GL. Nonmineralized and mineralized compartments of bone: the role of pyridinoline in nonmineralized collagen. Biochem Biophys Res Commun. 1983;113:975–81. doi: 10.1016/0006-291x(83)91094-x. [DOI] [PubMed] [Google Scholar]
- 45.Bailey AJ, Sims TJ, Ebbesen EN, Mansell JP, Thomsen JS, Mosekilde L. Age-related changes in the biochemical properties of human cancellous bone collagen: relationship to bone strength. Calcif Tissue Int. 1999;65:203–10. doi: 10.1007/s002239900683. [DOI] [PubMed] [Google Scholar]
- 46.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. Diabetes and risk of fracture: The Blue Mountains Eye Study. Diabetes Care. 2001;24:1198–203. doi: 10.2337/diacare.24.7.1198. [DOI] [PubMed] [Google Scholar]
- 47.Leidig-Bruckner G, Ziegler R. Diabetes mellitus a risk for osteoporosis? Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S493–514. doi: 10.1055/s-2001-18605. [DOI] [PubMed] [Google Scholar]
- 48.Reddy GK. Glucose-mediated in vitro glycation modulates biomechanical integrity of the soft tissues but not hard tissues. J Orthop Res. 2003;21:738–43. doi: 10.1016/S0736-0266(03)00006-8. [DOI] [PubMed] [Google Scholar]