Abstract
Different fibronectin (FN) isoforms are generated by the alternative splicing of the primary FN transcript. We previously demonstrated that the isoform containing the extra domain B sequence of fibronectin (B-FN), a complete type-III-homology repeat, is a marker of angiogenesis that accumulates around neovasculature only during angiogenic processes. We produced a single-chain human recombinant antibody (scFv), L19, which reacts specifically with B-FN and selectively targets tumor vasculature in vivo. We used this scFv and an antibody against a pan-endothelial marker (Factor VIII) in a double-staining procedure on specimens of low- and high-grade astrocytomas to determine the percentage of B-FN-positive vessels, (denominating the resulting value angiogenic index [AI]). Compared to vascular density and proliferative activity (evaluated using antibodies to Factor VIII and Ki67, respectively), AI correlated better with tumor grade (1.6 ± 2.6% and 92.0 ± 8.7% of B-FN-positive vessels in low- and high-grade astrocytomas, respectively) and was a more precise diagnostic tool than either of the two conventional methods. In fact, discriminating analysis using these three parameters showed that only AI accurately classified 100% of the cases studied, compared to 64% and 89% correctly diagnosed by vascular density and of proliferating cells, respectively.
Tumors cannot grow without a pronounced remodeling of the extracellular matrix (ECM) of the surrounding normal tissue in which the tumor grows. Remodeling of the ECM takes place through two main processes: proteolytic degradation of the normal tissue’s pre-existing ECM components, and neo-synthesis of new ECM components by both neoplastic and stromal cells. The interplay between these two processes results in the generation of a neoplastic ECM whose components may differ, qualitatively and quantitatively, from their nonneoplastic counterparts. Moreover, this provisional ECM also provides a permissive environment for tumor progression of which angiogenesis is a pivotal step. 1,2
One constituent of this provisional ECM is B-FN, the fibronectin (FN) isoform containing the extra domain B (ED-B), a complete type three repeat made up of 91 amino acids. 3 B-FN is, with very rare exceptions (eg, the female reproductive system where tissue remodeling and angiogenesis are recurrent physiological processes) virtually undetectable in normal adult tissues. 4 It is, by contrast, noticeably up-regulated in fetal and neoplastic tissues. 4 Because B-FN accumulates around neovasculature during angiogenic processes but not around mature vessels it is an ideal marker of angiogenesis and its visualization by immunohistochemistry can yield information on the level of angiogenic activities taking place within a tissue. 5
We produced a human recombinant antibody, L19, that reacts specifically with the ED-B domain of FN, 6 and have recently demonstrated that radiolabeled L-19 is able to selectively and specifically target tumor vasculature not only in experimental animal models but also in cancer patients. 7-10 Moreover, L19 has recently been shown to dramatically enhance the therapeutic index of cytokines and other bioactive molecules when used to deliver these to tumor neo-vasculature. 11-15 The possibility of using L19 to target tumors in patients both for in vivo diagnostic and therapeutic purposes requires meticulous preliminary studies on the biodistribution of the antigen, ED-B, in various types of tumors. Here, using a double-staining procedure, we determined the percentage of ED-B-positive vessels (denominating the resulting value as the angiogenic index [AI]) in specimens of low- and high-grade astrocytomas. We then compared these results with the vascular density and the proliferative activity assessed within the same tissues using the Ki67 antibody. High-grade astrocytomas showed a strikingly greater AI than low-grade forms, and this was independent of the vascular density. This observation could underpin relevant in vivo diagnostic and therapeutic approaches for astrocytic tumors.
Materials and Methods
Normal and neoplastic tissues were obtained from samples taken during the course of therapeutic surgical procedures. The samples investigated included 29 low-grade (grade I to II) astrocytomas (25 diffuse astrocytomas and 4 pilocytic astrocytomas) and 28 high-grade (grade III to IV) astrocytomas (15 glioblastomas and 13 anaplastic astrocytomas). The grading of the examined tumors followed the World Health Organization classification. 16,17 Each sample was immediately frozen in liquid nitrogen. Six-μm-thick cryostat sections were stained with hematoxylin and eosin, and additional frozen sections were used for immunohistochemical staining after fixation in absolute cold acetone for 10 minutes. Immunohistochemistry studies and double-staining experiments were performed according to Castellani and colleagues. 5 To avoid false-negative results arising from the heterogeneous distribution of FN within the tissues, at least three nonconsecutive sections of the biopsy were analyzed.
In situ hybridization was performed as previously described. 18 Both sense and anti-sense probes entirely covered the ED-B repeat, but only the anti-sense probe gave a hybridization signal. A probe to human endoglin was used to recognize vascular structures on serial sections. 19 Discriminating analysis was applied to compare the ability of the three tested parameters (percentage of the ED-B-positive vessels, vascular density, and number of cells in proliferation) to assign patients to the correct group, ie, either high-grade or low-grade astrocytoma. 20
The anti-human Von-Willebrand-factor monoclonal antibody (mAb) (DAKO-Factor VIII) and the mAb specific for proliferating cells, Ki-67, were both purchased from DAKO (Carpinteria, CA). The mAb M2 to the FLAG tag peptide was purchased from Kodak (New Haven, CT). The scFv L-19 to the domain ED-B of FN was purified as previously reported. 6
Results
Figure 1 ▶ shows that the B-FN isoform is detectable in vascular structures of glioblastoma sections, but not in sections of normal brain tissue or grade I pilocytic astrocytoma. Moreover, double-staining experiments using L19 and a mAb to factor VIII on glioblastoma sections containing both tumor and normal brain demonstrated that the vessels of the normal tissue surrounding the tumor are devoid of B-FN (Figure 2; A to D) ▶ . Using L-19 in high-grade astrocytoma we observed a positive reaction both in vascular structures showing endothelial cells in proliferation and in structures not showing endothelial cell proliferation. We also performed in situ hybridization experiments and found that vascular cells are responsible for the production of this FN isoform (Figure 2, E and F) ▶ .
Figure 1.

Serial sections of a glioblastoma (A and B), normal brain (C and D), and a pilocytic astrocytoma (E and F) stained with the scFv L-19 specific for B-FN (A, C, E) and with the anti-factor VIII mAb (B, D, F). Scale bars, 10 μm.
Figure 2.

Serial sections of a anaplastic astrocytoma showing neoplastic and normal tissue (A and B) stained with the anti-factor VIII mAb and with scFv L-19, respectively. Only the vessels within the tumors were stained by the scFv L-19. Two different magnifications (C and D) of a glioblastoma multiforme specimen showing neoplastic and normal tissue double stained with the scFv L-19 (brown) and with anti-factor VIII mAb (red). Two different magnifications (E and F) of an in situ hybridization experiment using human glioblastoma cryostat sections with the DIG-labeled cRNA ED-B repeat probe. A positive signal was visible in the endothelial cells. Scale bars, 10 μm.
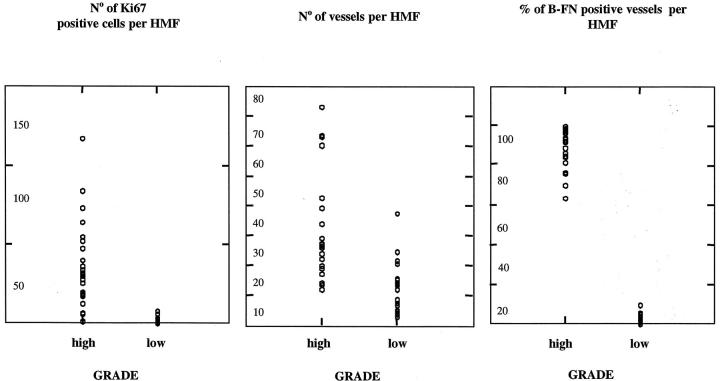
On the basis of this observation we quantitatively evaluated the percentage of B-FN-positive vessels in high-grade (28 cases) and low-grade (29 cases) astrocytoma specimens. Our results (Table 1) ▶ showed that 92 ± 8.7% of vessels were positive for B-FN in high-grade astrocytoma sections, whereas only 1.6 ± 2.6% of vessels were positive in low-grade astrocytoma sections. We also determined the vascular density and the number of proliferating cells per microscope field using the antibody Ki67. Discriminating analysis using these three parameters revealed that only the AI allowed the correct classification of 100% of the cases studied (Table 1) ▶ . Figure 3 ▶ shows the results obtained in each specimen of high- and low-grade astrocytoma. Although considerable overlapping of results for proliferating cells and vascular density was observed, no overlapping was seen when measuring the percentage of B-FN-positive vessels.
Table 1.
The Number of Ki67-Positive Cells, the Number of Vessels (Factor VIII-Positive), and the Percentage L-19-Positive Vessels Were Calculated in Five Different Areas of Each Specimen at ×250 Microscopy Magnification
| No. of cases | % of B-FN-positive vessels per HMF* | No. of vessels per HMF* | No. of Ki67-positive cells per HMF* | Survival of patients at 24 months | |
|---|---|---|---|---|---|
| Low grade | 29 | 1.6 ± 2.6 | 17.7 ± 11.7 | 1.0 ± 1.9 | 90% |
| High grade | 28 | 92.0 ± 8.7 | 40.7 ± 28.4 | 34.2 ± 26.8 | 12% |
| Discriminating analysis: % of cases correctly classified | 100 | 64 | 89 |
The average and standard deviation were calculated for each parameter. Discriminating analysis was applied to evaluate the percentage of cases correctly classified for each of the three parameters tested.
*HMF; high magnification field.
Figure 3.
Left: Number of Ki-67-positive cells calculated in five different areas of each specimen of high- and low-grade astrocytomas using the mAb specific for proliferating cells. Middle: Number of vessels calculated in five different areas of each specimen of high- and low-grade astrocytomas using the mAb anti-factor VIII. Right: Percentage of B-FN-positive vessels calculated in five different areas of each specimen of high- and low-grade astrocytomas double stained using the scFv L-19 and the mAb anti-factor VIII. Although considerable overlapping of the results of high- and low-grade astrocytomas for proliferating cells and vascular density was observed, no overlapping was seen when measuring the percentage of B-FN-positive vessels.
A preliminary retrospective analysis considering whether the percentage of B-FN-positive vessels can be used to predict outcome among patients with tumor of the same grade yielded negative results.
Discussion
Expression of the ED-B domain of FN is regulated by alternative splicing, a process that is modulated by cytokines and extracellular pH. 3,21,22 The fact that this domain is 100% homologous in all mammalian species thus far tested offers both advantages and disadvantages. One major drawback initially lay in the impossibility to prepare mAbs that directly reacted with the ED-B domain because of immunotolerance. In earlier studies on ED-B expression in various normal and pathological tissues this problem was circumvented using the mAb BC-1. 4 BC-1 is able to react with an epitope present on all of the different FN isoforms, but it is cryptic when ED-B is omitted and is unmasked when the domain is present. 23 Thus, BC-1 is able to indirectly detect B-FN expression. BC-1 has been used extensively until now for studies on ED-B expression in normal and pathological tissues. 24-27 Nevertheless, because of all of the possible interactions of FN molecules in a tissue, we could not assume a priori that the epitope recognized by BC-1 could become unmasked even in the absence of the ED-B domain. To overcome these problems we used a phage display library to generate human antibodies able to react directly with the ED-B domain. 28 The dissociation constant of one of these antibodies, L19, was increased to 54 pmol through maturation procedures. 6
L19 has been extensively characterized in immunohistochemical experiments, confirming the earlier observation with the mAb BC-1 that ED-B is a marker of tissue remodeling and, in particular, of angiogenesis. 28 Furthermore, using radiolabeled L19 we demonstrated the selective cancer-targeting properties of the scFv in tumor-bearing mice as well as in patients. 7-10 Here, using such a recombinant antibody, we show that B-FN could be considered a clear-cut marker of differentiation between low-grade and high-grade astrocytoma, as well as between tumor and surrounding normal cerebral tissue. In fact, discriminating statistical analysis showed that the percentage of ED-B-positive vessels was the only parameter among those tested (ED-B-positive vessels, vascular density, and number of cells in proliferation) able to correctly assign 100% of the cases. Furthermore, L-19 clearly discriminates pilocytic astrocytoma (AI = 3.1 ± 2.0%) from grade IV astrocytoma (AI = 94.7 ± 5.1%), which can occasionally be confused because both may present necrosis, high vascular density, and mitoses.
We recently provided evidence that L19 radiolabeled with I123 and injected intravenously in cancer patients selectively accumulates in tumors, including glioblastoma. 7 This property could offer an appropriate diagnostic tool to differentiate high-grade from low-grade astrocytoma. Moreover, because it is known that a number of low-grade astrocytomas (B-FN-negative) may develop into anaplastic astrocytomas (B-FN-positive), the follow-up of low-grade astrocytoma by immunoscintigraphy using radiolabeled L19 would seem fitting. Such an approach would provide a viable alternative to the more invasive cerebral stereotactic biopsy, which entails a risk of morbidity as well as of mortality mainly because of hemorrhaging. 29 Beyond the intrinsic risks associated with stereotactic biopsies, and although these techniques can be used to obtain several biopsy specimens from different areas of a given lesion, it has been argued that small tissue specimens may not be consistently representative of the degree of malignancy. Indeed, it has been demonstrated that the grading of glial tumors obtained by stereotactic biopsy may lead to a significant underestimation of the degree of malignancy. Furthermore, a considerable variability in histological grading on small astrocytoma specimens has been reported, not only among different observers but, throughout time, between multiple readings by the same observer. 30 This is of particular relevance because the highest level of variability was observed between low-grade and anaplastic astrocytoma, in which the grading has profound implications on the adopted therapy and, consequently, on the patient’s outcome. 30 Immunohistochemical analysis of specimens could drastically reduce the level of uncertainty, because the AI, measured as the percentage of B-FN-positive vessels, is a much better indicator of malignancy than the commonly used parameters of vascular density and number of Ki67-positive cells. Moreover, the fact that the human antibody L19 specifically targets the vascular structures of high-grade astrocytoma paves the way for a noninvasive differential diagnosis between high- and low-grade astrocytoma by immunoscintigraphy. It also heralds a number of attractive treatment approaches, including the selective delivery of therapeutic substances to the tumor vasculature of high-grade astrocytoma, a disease that is still practically untreatable.
Acknowledgments
We thank Mr. Thomas Wiley for manuscript revision and Drs. Vincenzo Fontana and Franco Merlo for statistical analysis.
Footnotes
Address reprint requests to Luciano Zardi, Laboratory of Cell Biology, Istituto Nazionale per la Ricerca sul Cancro, Largo Rosanna Benzi,10, 16132 Genoa, Italy. E-mail: luciano.zardi@tin.it.
Partially supported by the Associazione Italiana per la Ricerca sul Cancro (EU BIO4-CT97-2149 project “Neo-Vasculature Markers”), the Italian Health Ministry, the Bundesamt für Bilding und Wissenschaft, and Philogen Societǎ Responsabilità Limitata.
References
- 1.Van Den Hoff A: Stromal involvement in malignant growth. Adv Cancer Res 1988, 50:159-196 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1998, 86:353-564 [DOI] [PubMed] [Google Scholar]
- 3.Zardi L, Carnemolla B, Siri A, Petersen TE, Paolella G, Sebastio G, Baralle FE: Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J 1987, 6:2337-2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carnemolla B, Balza E, Siri A, Zardi L, Nicotra MR, Bigotti A, Natali PG: A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol 1989, 108:1139-1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellani P, Viale G, Dorcaratto A, Nicolò G, Kaczmarek J, Querzè G, Zardi L: The fibronectin isoform containing ED-B oncofetal domain: a marker of angiogenesis. Int J Cancer 1994, 59:612-618 [DOI] [PubMed] [Google Scholar]
- 6.Pini A, Viti F, Santucci A, Carnemolla B, Zardi L, Neri P, Neri D: Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J Biol Chem 1998, 273:21769-21776 [DOI] [PubMed] [Google Scholar]
- 7.Santimaria M, Moscatelli G, Viale GL, Giovannoni L, Neri G, Viti F, Leprini A, Borsi L, Castellani P, Zardi L, Neri D, Riva P: Immunoscintigraphic detection of the ED-B domain of fibronectin, a marker of angiogenesis, in patients with cancer. Clinical Cancer Res, in press [PubMed]
- 8.Neri D, Carnemolla B, Nissim A, Balza E, Leprini A, Querzè G, Pini A, Tarli L, Halin C, Neri P, Zardi L, Winter G: Targeting by affinity-matured recombinant antibody fragments of an angiogenesis associated fibronectin isoform. Nature Biotechnol 1997, 15:1271-1275 [DOI] [PubMed] [Google Scholar]
- 9.Tarli L, Balza E, Viti F, Borsi L, Castellani P, Berndorff D, Dinkelborg L, Neri D, Zardi L: A high-affinity human antibody that targets tumoral blood vessels. Blood 1999, 94:192-198 [PubMed] [Google Scholar]
- 10.Viti F, Tarli L, Giovannoni L, Zardi L, Neri D: Increased binding affinity and valence of recombinant antibody fragments lead to improved targeting of tumoral angiogenesis. Cancer Res 1999, 59:347-353 [PubMed] [Google Scholar]
- 11.Borsi L, Balza E, Bestagno M, Castellani P, Carnemolla B, Biro A, Leprini A, Sepulveda Y, Burrone O, Neri D, Zardi L: Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int J Cancer, in press. [DOI] [PubMed]
- 12.Birchler MB, Viti F, Zardi L, Spiess B, Neri D: Selective targeting and photocoagulation of ocular angiogenesis mediated by a phage-derived human antibody fragment. Nature Biotechnol 1999, 17:984-988 [DOI] [PubMed] [Google Scholar]
- 13.Nilsson F, Kosmehl H, Zardi L, Neri D: Targeted delivery of tissue factor to the ED-B domain of fibronectin, a marker of angiogenesis, mediates the infarction of solid tumors in mice. Cancer Res 2001, 61:711-716 [PubMed] [Google Scholar]
- 14.Carnemolla B, Borsi L, Balza E, Castellani P, Meazza R, Berndt A, Ferrini S, Kosmehl H, Neri D, Zardi L: Enhancement of the antitumor properties of interleukin-2 by its targeted delivery the blood vessels extracellular matrix. Blood 2002, 99:1659-1665 [DOI] [PubMed] [Google Scholar]
- 15.Halin C, Rondini S, Nilsson F, Berndt A, Kosmehl H, Zardi L, Neri D: Enhancement of the anti-tumor activity of interleukin-12 by targeted delivery to neo-vasculature. Nat Biotechnol 2002, 20:1-7 [DOI] [PubMed] [Google Scholar]
- 16. Kleihues P Cavenee WK eds. Pathology and Genetics of Tumours of the Nervous System. WHO Classification. 2000. IARC Press, Lyon
- 17.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK: The WHO classification of tumours of the nervous system. J Neuropathol Exp Neurol 2002, 3:215-225 [DOI] [PubMed] [Google Scholar]
- 18.Carnemolla B, Castellani P, Ponassi M, Borsi L, Urbini S, Nicolò G, Dorcaratto A, Viale G, Winter G, Neri D, Zardi L: Identification of a glioblastoma-associated tenascin-C isoform by a high affinity recombinant antibody. Am J Pathol 1999, 154:1345-1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balza E, Castellani P, Zijlstra A, Neri D, Zardi L, Siri A: Lack of specificity of endoglin expression for tumor blood vessels. Int J Cancer 2001, 94:579-585 [DOI] [PubMed] [Google Scholar]
- 20. Kleinbaum DG Kupper LL eds. Applied Regression Analysis and Other Multivariable Methods. 1978:pp 414-446 Duxbury Press, Boston
- 21.Borsi L, Castellani P, Risso AM, Leprini A, Zardi L: Transforming growth factor β regulates the splicing pattern of fibronectin messenger RNA precursor. FEBS Lett 1990, 261:175-178 [DOI] [PubMed] [Google Scholar]
- 22.Borsi L, Balza E, Gaggero B, Allemanni G, Zardi L: The alternative splicing pattern of the tenascin-C pre-mRNA is controlled by the extracellular pH. J Biol Chem 1995, 270:6243-6245 [DOI] [PubMed] [Google Scholar]
- 23.Carnemolla B, Leprini A, Allemanni G, Saginati M, Zardi L: The inclusion of the type-III repeat ED-B in the fibronectin molecules generates conformational modifications that unmask a cryptic sequence. J Biol Chem 1992, 267:24689-24692 [PubMed] [Google Scholar]
- 24.Kaczmarek J, Castellani P, Nicolò G, Spina B, Allemanni G, Zardi L: Distribution of oncofetal fibronectin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int J Cancer 1994, 58:11-16 [DOI] [PubMed] [Google Scholar]
- 25.Hauptmann S, Zardi L, Siri A, Carnemolla B, Borsi L, Castellucci M, Klosterhalfen B, Hartung P, Weis J, Stocker G, Haubeck H, Kirkpatrick CJ: Extracellular matrix proteins in colorectal carcinomas. Expression of tenascin and fibronectin isoform. Lab Invest 1995, 73:172-182 [PubMed] [Google Scholar]
- 26.Kosmehl H, Berndt A, Katenkamp D: Molecular variants of fibronectin and laminin: structure, physiological occurrence and histopathological aspects. Virchows Arch 1996, 429:311-322 [DOI] [PubMed] [Google Scholar]
- 27.Karelina TV, Eisen AZ: Interstitial collagenase and the ED-B oncofetal domain of fibronectin are markers of angiogenesis in human skin tumors. Cancer Detect Prev 1998, 22:438-444 [DOI] [PubMed] [Google Scholar]
- 28.Carnemolla B, Neri D, Castellani P, Leprini A, Neri G, Pini A, Winter G, Zardi L: Phage antibodies with pan-species recognition of the oncofoetal angiogenesis marker fibronectin ED-B domain. Int J Cancer 1996, 68:397-405 [DOI] [PubMed] [Google Scholar]
- 29.Melvin F, Witham TF, Flickinger JC, Kondziolka D, Lunsdorf D: Comprehensive assessment of hemorrhage risks and outcomes after stereotactic biopsies. J Neurosurg 2001, 94:545-551 [DOI] [PubMed] [Google Scholar]
- 30.Mittler MA, Beverly CW, Stopa EG: Observer reliability in histological grading of astrocytoma stereotactic biopsies. J Neurosurg 1996, 85:1091-1094 [DOI] [PubMed] [Google Scholar]