Abstract
A breast carcinoma biopsy showed cytochemical evidence of epithelial mesenchymal transition and an α-smooth muscle actin-positive stromal reaction. To study the lineage, and the nature of the cells in the stromal reaction, we derived a novel cell line, HBFL-1, from the explanted biopsy. HBFL-1 cells are immortal and exhibit a shared non-random X-chromosome inactivation pattern with the epithelial tumor of origin. Yet they closely resemble normal, finite-life-span fibroblasts by morphology, lack of tumor formation in nude mice, marker expression profile, protein pattern using two-dimensional gel electrophoresis and the ability to undergo myofibroblast conversion. HBFL-1 interacts reciprocally with tumor cells in collagen gel to induce activation of MMP2, leading to tumor-like behavior of epithelial colonies. In vivo, HBFL-1 cells resembled normal-derived myofibroblasts and conferred a significant 3.5- to 7-fold increase in MCF-7 tumor size in nude mice. However, that they were indeed not normal fibroblasts was revealed by residual keratin expression and formation of epithelial microfoci in a reconstituted basement membrane and in nude mice. We conclude that breast cancer can generate its own nonmalignant stroma and that one function for this is that of a reciprocal interaction with epithelial tumor cells to facilitate tumor growth.
Epithelial mesenchymal transition (EMT) was originally described as a normal developmental process. 1,2 Later, it was adopted as an explanation for mesenchymal conversion in a number of cultured epithelial cells. 3,4 In cancer, EMT generally depicts a more aggressive behavior of the tumor cells. 5,6 In breast cancer, EMT has been estimated to occur in as much as 18% of tumors in vivo. 7-9 Under these conditions EMT is defined as the occurrence of a variable proportion of tumor cells that express mesenchymal markers such as vimentin, tenascin and stromelysin-3. 7,10 In its most elaborate form, EMT-derived cells of mixed epithelial-mesenchymal breast tumors may be difficult to distinguish from resident normal stromal cells. 11 These tumors which are also referred to as carcinosarcomas or metaplastic carcinomas in particular offer an excellent opportunity to study the nature and the consequence of this subset of EMT. 5,6 Such studies, however, have been hampered by lack of representative cell lines most likely due to low frequency of overtly metaplastic carcinomas but also to difficulties in culturing breast cancer cells in general. In the present study we succeeded in isolating a mesenchymal-like cell line from a metaplastic human breast carcinoma. Clonality analysis revealed that the cell line and the epithelial tumor cells of origin had a common ancestor. Even though the cells were immortal and severely aneuploid, and exhibited a rudimentary epithelial phenotype in terms of keratin expression and formation of microfoci in Matrigel and in vivo, they nevertheless behaved remarkably like normal resident fibroblasts. In particular they responded to transforming growth factor-β (TGF-β) by having α-smooth muscle actin (α-sm actin) induced and they were non-tumorigenic and reciprocally interactive with epithelial tumor cells in collagen gels and in co-inoculates in nude mice. The self-supply of a nonmalignant stroma that facilitates epithelial tumor growth may in part explain the poor prognosis of some breast cancers that show evidence of EMT.
Materials and Methods
Establishment of the HBFL-1 Cell Line
A breast carcinoma was obtained from the Department of Pathology, Rigshospitalet, from residual material. Histologically, the carcinoma was classified as metaplastic. Phenotypically normal tissue from the same patient was dissected from the mastectomy specimen at a site remote from the cancer. The use of such tissue material has been reviewed by the Regional Scientific-Ethical Committees for Copenhagen and Frederiksberg, Denmark and found consistent with Laws no. 503 of 24th June 1992 and no. 499 of 12th June 1996 (KF) 01–161/98. The tissue was collected in culture medium (Dulbecco’s modified Eagle’s medium-Ham’s F12 (DME-F12) supplemented with 20% fetal calf serum (FCS) and processed as previously described. 12 For zymography and TGF-β experiments, a subline (S2) was cultured in chemically defined medium alone (DME-F12). Clonal cultures for studies of multipotency were performed as previously described 13 using a subline (W2331) propagated in the presence of 20% FCS. One clonal subline (S4) was generated by limited dilution and isolation of the clonal cell islet by trypsinization under the microscope within a Perspex ring. The cells were split 1:3 once a week, and have been kept in culture for more than 3 years. They are referred to as human breast fibroblast-like-1 cells (HBFL-1).
Other Cell Lines
Purified primary breast fibroblasts were obtained from reduction mammoplasties or from within a normal appearing area of the HBFL-1 biopsy and cultured in the presence of 20% FCS as previously described to obtain experimentally generated myofibroblasts. 12 In some instances the cells were immortalized by transduction with a retroviral construct containing the HPV16 E6 and E7 oncogenes (ATCC) or the catalytic subunit of human telomerase hTERT ( 14 kindly provided by Dr. Judy Campisi, Lawrence Berkeley National Laboratory). For zymography MCF-7 subline 9 cells, kindly provided by Dr. Per Briand, 15 were cultured alone or recombined with HBFL-1 or primary breast fibroblasts in DME-F12. HMT3909S1, HMT3522T4-2 and MCF-7 (5% FCS) cells were cultured as previously described. 16-18 For induction of α-sm actin 20% fetal calf serum and/or 100 pg/ml TGF-β1 (Sigma Chemical Co., T-1654, Sigma-Aldrich, Vallensbeck Strand, Denmark) were added to the media for 6 days (up to 17 days for the MCF-7 cells), and the medium was changed every second day. For zymography (see below) HT-1080 cells were cultured in RPMI supplemented with 10% FCS. As controls for characterization of HBFL-1 cells we also used primary luminal epithelial cells and myoepithelial cells cultured as previously described. 19
Immunocytochemistry
The original routine formalin-fixed, paraffin-embedded biopsy was sectioned at a 4 μm thickness and stained for α-sm actin (1A4, Sigma), Vimentin (3B4 Boehringer Mannheim Roche, Hvidovre, Denmark), keratin CK7 (OV-TM, DAKO, Glostrup, Denmark) and Wide range keratins (MNF116, DAKO). The antibodies were visualized by streptavidin-biotin (DAKO 5004).
Cell cultures were stained for immunofluorescence as previously described, and double stainings were performed with isotype specific antibodies. 20,21 Immunoperoxidase cytochemistry and quantification of immunostainings were performed as previously described. 13,19 The following primary antibodies were used: cytokeratin K14 (C-8791, Sigma), EDA-fibronectin (DH1; Locus Genex, Helsinki, Finland), K17 (M-7046, DAKO), Ki-67 (M722, DAKO), P53 (M7001, DAKO), uPA (kindly provided by Dr. Boye Schnack Nielsen, The Finsen Laboratory, Copenhagen, Denmark), K18 (F3006, Monosan, Am Uden, The Netherlands), K19 (M772, DAKO), pankeratins (which stained also myoepithelial cells; LP34, M717, DAKO, 22 ); vimentin (6400-301, Labsystems, Helsinki, Finland), BG3C8 (kindly provided by Dr. J. E. Celis; the Danish Cancer Society, Denmark 23 ), β4-integrin (MAB 1964, Chemicon International Inc., Temecula, CA), α1-integrin (VLA-1, T Cell Diagnostics, Cambridge, MA), αV-integrin (MAB 1953, Chemicon), P- and E-Cadherin (NCC-CAD-299 and HECD-1; Kindly provided by Dr. Atsushi Ochiai, Tokyo, Japan), desmoplakin I+II (clone DP1&2–2.15; Boehringer Mannheim, GmbH), maspin (M45620, Transduction Laboratories, Lexington), connexin 43 (03-6900, Zymed Laboratories Inc., South San Francisco, CA), smooth muscle myosin heavy chain (kindly provided by Dr. V. E. Koteliansky 21 ), laminin (M638, DAKO), sialomucin (MAM6, clone 115D8, kindly provided by Dr. J. Hilgers, The Netherlands), estrogen receptor (M7047, DAKO), Thy-1 (ASO-2, Dianova, Hamburg, Germany; 24 ), 1B10 (MC-48, Sigma), α-sm actin, (A-2547, Sigma), type IV collagen (PHM12, Silenus, Victoria, Australia), HGF-receptor (c-met; 05-237, Upstate Biotechnology, Lake Placid, NY), and CALLA (J5, Coulter Immunology, Miami, FL). Immunofluorescence was visualized using a Zeiss LSM 510 laser scanning microscope (Carl Zeiss, Jena, GmbH).
Karyotyping
Chromosome preparations were made from exponentially growing cultures treated for 1.5 hours with colcemid (Gibco BRL, Invitrogen, Tåstrúp, Denmark) at final concentrations of 0.1 μg/ml. Cells were further harvested, centrifuged, and treated with hypotonic solution as previously described. 25 The cell pellet was fixed in methanol:glacial acetic acid (3:1) dropped on cold slides and stained for Giemsa bands. Chromosome counts were done on well-spread metaphases.
Telomeric Repeat Amplification Protocol (TRAP) Assay
Experimentally generated myofibroblasts and HBFL-1 cells were grown to 70 to 80% confluence, trypsinized and counted. Cells (106) were washed twice in PBS, pelleted, and stored at −80°C. The telomerase activity was determined with the TRAP assay 26 using the TRAPeze Telomerase Detection Kit (Intergen, Oxford, UK, purchased from AHdiagnostics, Aarhus, Denmark) according to the manufacturer’s instructions.
Human Androgen Receptor (HUMARA) Clonality Assay
Laser pressure catapulting was performed as described using 500 to 1000 cells for lysis. 27 To isolate DNA, the lysate was phenol-chloroform extracted with 200 μl of 25:24:1 phenol:chloroform:isoamyl alcohol (Sigma, P2069), followed by ethanol precipitation in the presence of glycogen (Roche, Hvidovre, Denmark). DNA extraction from HBFL-1 and tumor and normal breast specimens was performed with the Qiagen, QIAamp Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
The HUMARA clonality assay was performed in the original formulation with a few modifications. 28 The extracted DNA was divided in two, and incubated over night at 37°C in a final volume of 20 μl with or without 10 U HpaII (Roche) according to the manufacturer’s recommendations. It was heat inactivated for 10 minutes at 95°C, and 5 μl was PCR-amplified in a 15 μl volume. HPLC-purified primers were as follows: sense: 5′tcc aga atc tgt tcc aga gcg tgc-3′; and FAM-labeled antisense: 5′FAM-gct gtg aag gtt gct gtt cct cat-3′ 28 (Applied Biosystems). The cycling conditions were 15 minutes at 95°C, followed by 28 to 32 cycles of 95°C (1 minute), 60°C (1 minute), 72°C (1 minute), and extension (7 minutes). The PCR product (3 μl), together with a 350 TAMRA internal size marker for assessment of allelic sizes, was electrophoresed on an ABI PRISM 310 Genetic Analyzer using the Performance Optimized Polymer-4 (Applied Biosystems, Perkin Elmer). Fluorescent peaks were identified using the Genescan software version 3.1 (Applied Biosystems). Clonality ratios were calculated according to Willman et al, 29 and a clonal ratio of less than 0.5 was considered indicative of a clonal composition. 30,31
Two-Dimensional Gel Electrophoresis
Cells were treated and submitted to two-dimensional gel electrophoresis as previously described. 32
Tumorigenic Potential
HBFL-1 cells between passages 21–38 were cultured in T-75 flasks for transplantation. Confluent cultures were trypsinized and resuspended in DME-F12. Approximately 107 cells were inoculated adjacent to the fourth mammary gland of athymic female mice. A total of six mice were inoculated and were observed weekly for tumor formation for more than 3 months. In another series of experiments, HBFL-1 cells were co-inoculated with MCF-7 cells (n = 6 mice). Finally, two series of experiments (a total of 18 mice) were conducted to see the possible effect of implantation of cells in laminin-rich gels. The gels consisted of 300 μl of 20% reconstituted basement membrane (Matrigel, Engelbreth-Holm-Swarm matrix, Collaborative Biomedical Products, purchased from Becton Dickinson, Catalog no. 40230A, lot no. 913156) and 80% Vitrogen (Collagen Corporation, Fremont, CA 33 ) containing either HBFL-1 cells, cloned HBFL-1 cells (S4), MCF-7 cells, HBFL-1+MCF-7 cells, primary experimentally generated myofibroblasts or primary experimentally generated myofibroblasts from reduction mammoplasties+MCF-7 cells. Each cell type was added to the gel at a concentration of 106 cells. The gels were implanted in triplicate subcutaneously in the right flank adjacent to the fourth mammary gland following 24 hours in culture. The tumors were observed weekly for 1 month and the final size determined by weight. Significance was assessed by Student’s t-test.
Response to Cytokines
HBFL-1 cells cultured in DME-F12 without serum were exposed to 100 pg/ml TGF-β1 (T-1654, Sigma) or vehicle (4 mmol/L HCl with 1 mg/ml BSA), and the level of induction of α-sm actin was analyzed after 6 days by immunoperoxidase cytochemistry. 34 The effect of FGF-2 (basic fibroblast growth factor, bFGF, Pepro Tech EC Ltd., London, UK) on this induction was tested by concurrent exposure to 1 ng/ml FGF-2. TGF-β1 experiments are described above (see other cell lines and biopsies used).
Reconstituted Basement Membrane Assay
HBFL-1 cells or experimentally generated myofibroblasts (105) were embedded as single cells in 300 μl reconstituted basement membrane (Matrigel) and allowed to gel for 30 minutes. at 37°C. The cultures were kept for 2 to 3 weeks before the gels were frozen and stored. Some gels were sectioned and stained with immunoperoxidase for laminin, keratin K19, β4-integrin, and type IV collagen.
Tumor Environment Assay
Collagen gels (Cellon S. A., Strassen, Luxemburg) were prepared at a concentration of 2 mg/ml at a volume of 2 ml per well in 6-well dishes (Nunc) as previously described. 17,21 2.2 × 105 HBFL-1 cells or primary breast fibroblasts and 5 × 105 MCF-7S9 cells, either separately or together. For substrate gels (MMP2, see below) medium was collected and frozen for later use. At the end of the experiments, gels were snap frozen in −80°C N-hexane, mounted, and sectioned for histological examination. 17
Zymography
Zymography was performed using 7.5% SDS-PAGE (BioRad) containing 0.1% gelatin as substrate for gelatinase activity. The serum-free supernatants from the tumor environment assay were concentrated using Centriplus concentrator, 10 kd cut-off (Amicon, Bedford, MA), and the protein concentration of each sample was determined spectrometrically (Beckman), adjusted to 1000 μg/ml. MMP bands were developed by incubation for 24 hours in 50 mmol/L Tris-HCl, 0.2 mol/L NaC1, 5 mmol/L CaCl2, pH 7.4.
Reverse Transcription-PCR
Total RNA from HBFL-1 cells cultured in DME-F12 with or without 1 ng/ml FGF-2 (see above), was isolated with the TRIZOL reagent (Life Technologies, Roskilde, Denmark) according to the manufacturer’s instructions. RNA (2 μg) was DNase-treated (DNase I Amp Grade, Gibco BRL) and served as a template for first strand cDNA synthesis with an oligo dT primer (Superscript Preamplification System, Gibco BRL). One μl of cDNA was subsequently PCR-amplified using Expand High Fidelity PCR System (Roche, Hvidovre, Denmark) and synthetic oligonucleotide primers for urokinase plasminogen activator (uPA) 5′-AAAATGCTGTGTGCTGCTGAC-3′ and 5′-CCCTGCCCTGAAGTCGTTAGTG-3′, product size 704 bp, 35 stromelysin-3 (STR-3) 5′-CCATGGCAGTTGGTGCAGGAGCAG and 5′-CTGCAGTCATCTGGGCTGAGACTC, product size 401 bp, and β-actin 5′-CACTGGCATCGTGATGGACTC-3′ and 5′-TCTGCATCCTGTCGGCAATGC-3′, product size 500 bp (TAG Copenhagen, Copenhagen, Denmark). PCR conditions for uPA were 94°C, 58°C, and 72°C for 1 minute each, 26 cycles for uPA and 94°C, 64°C and for β-actin, 72°C for 1 minute each, 18 cycles. Amplifications without reverse transcription were included as controls.
Results
HBFL-1 Cells Resemble Normal Fibroblasts and Myofibroblasts
A new cell line, HBFL-1, was established permanently in culture from a breast carcinoma. Initial morphological characterization showed that under serum-free conditions, the cells resembled non-activated primary fibroblasts (Figure 1A, a and b) ▶ . 17 In the presence of serum, they took up an appearance very similar to that of activated primary fibroblasts (Figure 1A, c and d) ▶ . 34 Two-dimensional gel electrophoresis of total proteins and staining for a panel of breast cell markers revealed a protein fingerprint which clearly resembled that of resident fibroblasts, the most common precursor of myofibroblasts (Figure 1A, e and f ▶ , and Table 1 ▶ ). 21 For lists of markers see also Refs. 21, 32, 36, 37 .
Figure 1.
HBFL-1 cells resemble normal fibroblasts and myofibroblasts. A: Morphology and protein pattern. Phase contrast morphology of HBFL-1 cells (a, c) and normal breast fibroblasts (b, d) cultured either under serum-free conditions (a, b) or in the presence of 20% fetal calf serum (c, d). HBFL-1 cells retain the ability to exhibit both the fibroblastic and the activated phenotype of myofibroblasts, a fundamental feature of normal fibroblasts. e, f: Two-dimensional gel electrophoresis of total cell extracts of [35S]methionine-labeled HBFL-1 cells and normal resident fibroblasts. Note the striking resemblance in the protein expression profiles. Scale bar, 50 μm. B: Induction of uPA after FGF-2 addition. RT-PCR of RNA extracted from HBFL-1 cells stimulated with FGF-2 (lane 1) and unstimulated cells (lane 2). Lanes 3 and 4: β-actin in the same RNA. There is a clear induction of uPA in HBFL-1 cells by FGF-2. C: Reciprocal interaction with MCF-7 cells in a tumor environment assay. Substrate gels (top row) with media conditioned by HBFL-1 cells alone (lane 1), primary fibroblasts (lane 2), HBFL-1 cells plus MCF-7 cells (lane 3), primary fibroblasts plus MCF-7 cells (lane 4), MCF-7 cells alone (lane 5), and HT-1080 cells as a positive control (lane 6). MCF-7 cells are negative and HBFL-1 cells alone produce some MMP2; however, the two cells together produce appreciably larger amounts of 62-kd MMP especially. A similar pattern was observed using primary breast fibroblasts. Bottom row: Phase contrast micrographs of HBFL-1 cells alone, the combined culture, and MCF-7 cells alone. The interaction between the two cell types allows the spread of MCF-7 cells inside the collagen gel. Scale bar, 100 μm. D: Tumor-like histology in the tumor environment assay. Cryostat sections of MCF-7 cells (left column) and MCF-7 cells plus HBFL-1 cells stained with immunoperoxidase (right column) for MMP2 (a, b), EDA-Fn (c, d), α-sm actin (e, f), and Ki-67 (g, h). With the exception of Ki-67, all markers are found in peritumoral HBLF-1 cells in co-cultures (arrows). Ki-67 is strongly induced in MCF-7 cells. Scale bar, 50 μm.
Table 1.
Characterization of HBFL-1 Cells Within the Context of Breast Differentiation
| Marker | HBFL-1 | Primary fibroblasts | Luminal epithelial cells | Myoepithelial cells |
|---|---|---|---|---|
| Myoepithelial markers | ||||
| K14 | − | − | −* | + |
| K17 | − | − | −* | + |
| BG3C8 | − | − | −* | + |
| β4-Integrin | − | − | −* | + |
| P-cadherin | − | − | − | + |
| Maspin | − | − | − | + |
| Connexin 43 | − | − | − | + |
| HC-myosin | − | − | − | + |
| Laminin | − | − | − | + |
| Luminal epithelial markers | ||||
| MAM6 | ±† | − | + | −* |
| K18 | ± | − | + | −* |
| K19 | − | − | + | − |
| ER | − | − | + | − |
| Pankeratins | ± | − | + | + |
| Fibroblastic markers | ||||
| Thy-1 | + | + | − | + |
| Vimentin | + | + | −* | + |
| 1B10 | + | + | − | − |
| α-sm actin | ± | ± | − | + |
| Type IV collagen | + | −* | −* | + |
| α1-integrin | ± | ± | − | + |
| HGFr | + | + | ND | ND |
| CALLA | +* | +* | − | + |
*Negative in situ but with a varying degree of induction in culture.
†Some cells are positive and some are negative as an indication of a biphasic culture.
We next determined how closely the tumor-derived myofibroblasts functionally resembled experimentally generated myofibroblasts derived from normal stroma. First, a hallmark of normal breast fibroblasts is their ability to convert to myofibroblasts on TGF-β1 stimulation, and the ability of bFGF (FGF-2) to inhibit this conversion. 34 HBFL-1 cells mimicked this behavior in a serum-free medium: the baseline level of α-sm actin positive cells was 14 ± 8%. TGF-β1 induced this level to 45 ± 8%, and FGF-2 reduced it even below the baseline to 8 ± 2%. Second, others have produced evidence that the addition of FGF-2 to the typical peritumoral myofibroblast induces uPA. 38 In this respect also the cell line conformed with the definition of myofibroblasts both at the mRNA (Figure 1B) ▶ and protein levels of uPA (not shown). Third, as assessed by RT-PCR analysis, HBFL-1 produced stromelysin-3 as do typical myofibroblasts 39 (data not shown). Fourth, the tumor-derived myofibroblasts were non-tumorigenic (0/6 mice) when inoculated under standard conditions.
Finally, as another evidence of the typical myofibroblast behavior, we analyzed the interaction of HBFL-1 cells with cancer cells in a tumor environment assay. 17,21,40 Cross-talk between the two cell types was clearly taking place, because tumor cells (MCF-7) could induce both inactive and active isoforms of MMP2 in HBFL-1 cells (Figure 1C) ▶ . The targeting of MMP2 activity to HBFL-1 cells was confirmed immunocytochemically (Figure 1D, a and b) ▶ . Similarly, peritumoral myofibroblasts expressed EDA-fibronectin and α-sm actin (Figure 1D, c–f ▶ ). Reciprocally, the fibroblasts allowed tumor cells to spread in the collagen gel (Figure 1C) ▶ and induced strong and widespread staining of Ki-67 in the tumor cells (Figure 1D, g and h) ▶ .
To ascertain a functional significance of HBFL-1 also in vivo, inoculates were made subcutaneously in the region of the fourth mammary gland of nude mice in the presence of Matrigel 33 with or without MCF-7 cells. Both HBFL-1 and experimentally generated myofibroblasts significantly augmented tumor formation by MCF-7 cells (3.5-fold, P < 0.01 in one experiment, Figure 2 ▶ , and 7.0 in another experiment, not shown) as evaluated by weight of the dissected tumors. Staining of tumors with human specific antibodies revealed a histological appearance of HBFL-1 cells in the peritumoral stroma very similar to that seen with experimentally generated myofibroblasts (Figure 2) ▶ . Double staining with human specific vimentin and α-sm actin revealed that the inoculated HBFL-1 cells and experimentally generated myofibroblasts resembled typical myofibroblasts also in vivo (Figure 2) ▶ . It is concluded that tumor-derived myofibroblasts facilitate epithelial tumor growth in vivo.
Figure 2.
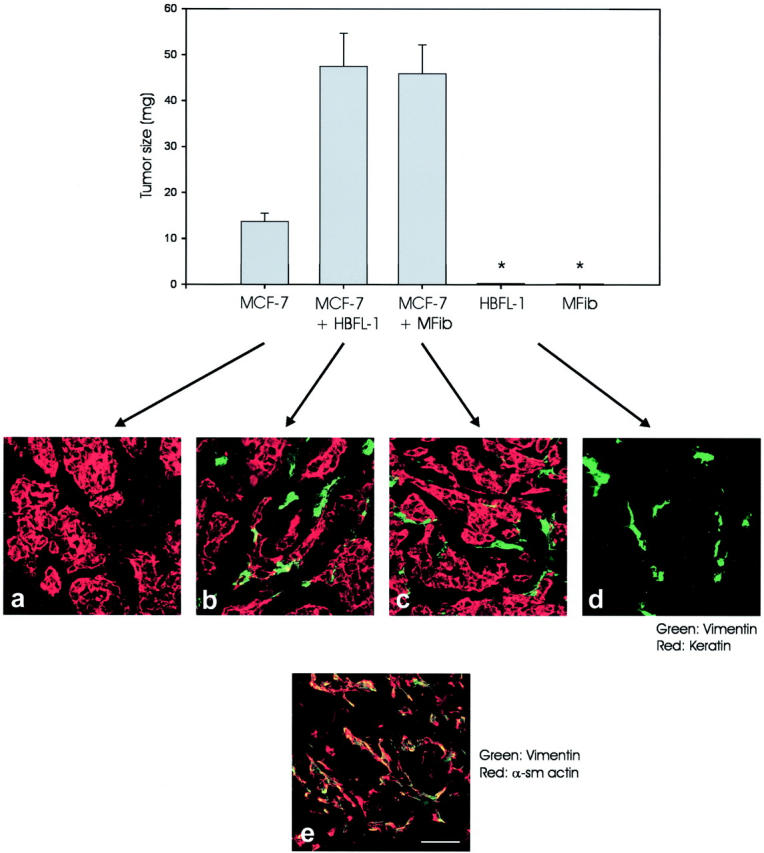
HBFL-1 cells provide a nonmalignant stroma in vivo. Top row: MCF-7 cells, MCF-7 cells plus HBFL-1 cells, MCF-7 cells plus experimentally generated myofibroblasts (MFib), HBFL-1 cells, or MFibs in laminin-rich gels in nude mice. HBFL-1 cells or MFibs by themselves did not form a solid tumor but consisted mainly of stromal cells in residual gel material. Therefore the solid tumor weight was set to zero (asterisk). Note that tumor cells co-embedded with either HBFL-1 or primary myofibroblasts (P < 0.01) increased tumor size significantly. Middle row: Cryostat sections of tumors double-stained with human-specific antibodies against keratin and vimentin revealed that HBFL-1 cells and primary myofibroblasts were identically positioned in the peritumoral stroma. HBFL-1 cells alone mostly resemble stromal fibroblasts (right). Bottom row: Double-staining for α-sm actin and vimentin confirms a myofibroblastic phenotype of the human-derived stromal cells. Scale bar, 50 μm.
Evidence for a Neoplastic Origin of HBFL-1
That HBFL-1 cells were in fact from a neoplastic origin, and were thus derived by EMT, was suggested by the following observations.
The cells were spontaneously immortal as evidenced by the presence of telomerase activity (Figure 3A) ▶ 41 and viability for more than 3 years (70+ passages) in culture. Also, the karyotype was highly aneuploid with a modal value of 87 already in passage 9 (not shown; earlier passages not done). Moreover, in the carcinoma of origin we found a frequent and intimate association of regular nests of epithelial cancer cells with elongated fibroblastoid cells which stained for keratins with CAM 5.2 (Figure 3B) ▶ . All fibroblastoid cells stained for vimentin and some stained for α-sm actin (not shown). Normal stroma in the periphery of the cancer biopsy was completely keratin-negative (not shown). The epithelial stromal relationship was further substantiated by the fact that HBFL-1 and laser pressure catapulted carcinoma cells from the tissue of origin were clonal with an identical non-random X-chromosome inactivation pattern (ratio less than 0.5) 30,31 as assessed with the HUMARA clonality assay (Figure 3C) ▶ . A rudimentary epithelial phenotype was revealed in the majority of cells also by double staining with antibodies against keratins and α-sm actin (Figure 4a) ▶ . Identical data were obtained in clonal cultures (not shown). Two-dimensional gel electrophoresis of cytoskeletal extracts confirmed a weak expression of simple keratins (Figure 4b) ▶ which was not seen in normal fibroblasts (not shown). To show a broader evidence that a combined induction of α-sm actin and loss of keratin could arise as a consequence of an EMT phenomenon was demonstrated by use of two additional basaloid breast carcinoma cell lines (HMT-3909S1 and HMT-3522T4). Under standard culture conditions these cell lines were vimentin- and keratin-positive and entirely α-sm actin-negative (not shown). In the presence of serum (HMT-3522T4)- or TGF-β (HMT3909S1) cells, they down-regulated keratin and induced α-sm actin (Figure 4, c and d) ▶ . Luminal epithelial MCF-7 cells failed to respond to both serum and TGF-β treatments (not shown). To exclude the possibility that the keratin expression was an artifact of immortalization induced in culture, we compared HBFL-1 cells with two breast fibroblast cell lines we established from primary breast fibroblasts after infection with a retroviral construct containing either E6/E7 or hTERT. While these cells had extended life spans, neither expressed keratins (not shown).
Figure 3.
Evidence for a neoplastic origin of HBFL-1. A: HBFL-1 cells are immortal. TRAP-assay of equal cell numbers of HBFL-1 cells and normal breast primary fibroblasts showed telomerase activity in HBFL-1 cells only. Lane 1: molecular weight marker; lane 2: primary fibroblasts; lanes 3 and 5: heat inactivated negative controls of experimentally generated primary myofibroblasts and HBFL-1; lane 4: HBFL-1; lane 6: positive control; lane 7: negative control without cell lysate; lane 8: positive control TSR8 control template (IC) 36-bp internal control. B: HBFL-1-like cells are found in the biopsy of origin. Epithelial-stromal transitions were evident by staining for keratin. At the epithelial-stromal junction glandular epithelial cells, E, were strongly stained and adjacent elongated stromal cells, S, were weakly stained. Scale bar, 50 μm. C: HBRL-1 cells and the epithelial carcinoma cells in the biopsy of origin are monoclonal. Areas of carcinoma cells were microdissected from the surrounding stroma and laser pressure catapulted and used for clonality analysis. Arrows indicate the photolysed separation area. Right: Clonal analysis with the HUMARA assay of tumor tissue, microdissected carcinoma cells from the tissue of origin, HBFL-1 cells and normal breast tissue (control). Whereas the normal tissue shows a slightly skewed X-chromosome inactivation (CR = 0.85), the longer allele is reduced on HpaII digestion in both tumor tissue (CR = 0.50), carcinoma cells (CR = 0.42) and HBFL-1 cells (CR = 0.33) indicating clonal populations. Scale bar, 50 μm.
Figure 4.

HBFL-1 cells exhibit a rudimentary epithelial phenotype and a plasticity reminiscent of EMT-derived breast cancer cell lines. HBFL-1 cells (a), serum-stimulated HMT-3522T4 (c) and TGF-β-stimulated HMT-3909S1 (d) were double-stained for an epithelial marker (keratins; red) and a myofibroblast marker (α-sm actin; green), or HBFL-1 cells were extracted with a cytoskeletal buffer and submitted to two-dimensional gel electrophoresis (b). All cell lines generate myofibroblast-like cells as determined by loss of keratins and gain of α-sm actin (green cells). The keratins expressed in HBFL-1 cells appear to be keratin K7, K8, and K18. Scale bars, 50 μm.
Finally, inside a reconstituted basement membrane (Matrigel), HBFL-1 differed from normal breast fibroblasts taken from the same patient by the ability to form cellular clusters. The cells further stained for the epithelial markers β4-integrin, type IV collagen (not shown) and keratin and laminin (Figure 5A, a ▶ inset). We found no staining for E-cadherin or desmoplakin. The clusters produced elongated cells which projected into the gel (Figure 5A, a) ▶ . Normal fibroblasts from the same specimen, either formed only elongated cells, or remained as rounded single cells (Figure 5A, b) ▶ , a morphology also observed in experimentally generated myofibroblasts 17 (data not shown). Also, when transplanted into nude mice in the absence of MCF-7 cells, HBFL-1 cells inside a laminin-rich gel did not form solid tumors per se but mostly resembled resident fibroblasts in an interstitial stroma (compare Figure 2d and 5B, b ▶ ▶ ). Nevertheless, evidence for a rudimentary neoplastic epithelial phenotype of HBFL-1 cells in vivo was provided by the occasional clonal appearance of microfoci of vimentin-negative epithelial cancer cells surrounded by keratin-negative fibroblast-like cells analogous to those obtained in Matrigel cultures (Figure 5B, a) ▶ . Experimentally generated (normal) myofibroblasts which showed no signs of epithelial plasticity in mice inoculates (n = 3 mice) served as controls (Figure 5B, b) ▶ .
Figure 5.
HBFL-1 cells generate microfoci of epithelial tumor cells in laminin-rich gels and in nude mice. A: Laminin-rich gels (Matrigel). HBFL-1 incubated for 3 weeks inside Matrigel (a) compared with experimentally generated myofibroblasts from normal breast from the same biopsy under identical conditions (b). HBFL-1 cells formed cellular clusters inside Matrigel and send projections into the gel (arrows). The clusters (blue nuclei) stained for human laminin (upper inset) and keratin K19 (lower inset). In contrast, normal breast fibroblasts (MFib) became either rounded single cells or formed elongated fibroblast-like cells (arrow). Scale bar, 100 μm. B: Nude mice. Sections double-stained with keratin and vimentin showed occasional vimentin-negative epithelial colonies surrounded by fibroblastic cells in cloned HBFL-1 cells (a). No keratin expression was recorded in transplants of experimentally generated myofibroblasts (MFib) in three different mice (b). Scale bar, 25 μm.
Discussion
The human breast cell line described here was derived from a metaplastic carcinoma, and provides a unique opportunity to investigate the consequences of an aspect of epithelial mesenchymal transition. The cell line is essentially mesenchymal and for all purposes behave very much like normal human breast myofibroblasts as we and others have characterized them extensively previously. 42,43 Here we show that while they are non-tumorigenic by themselves, in combination with epithelial breast cancer cells they sort out in the stromal compartment and facilitate tumor growth substantially. This, we believe, is a hitherto unappreciated function of EMT in breast tumors. We propose that this may be at least part of the explanation for the poor clinical outcome of breast cancers that show evidence of EMT.
A major conclusion from our study is that EMT-derived mesenchymal cells may differentiate into nonmalignant myofibroblast-like cells. Transdifferentiation or metaplasia is seen both in human breast cancer and in rodent mammary tumors. In breast tumors the most frequent transdifferentiation takes place within the epithelial lineages leading to squamous metaplasia. 44 However, in mixed mesenchymal breast tumors, reports on unequivocal mesenchymal lineages are numerous including chondroid and osteogenic differentiation. 45,46 That it really represents metaplasia (the divergent hypothesis) instead of a multiclonal tumor (the convergent hypothesis) has become increasingly clear based on the numerous studies of genetic markers in microdissected cells from within different compartments of the tissue. 46,47 The criteria in the present study used to categorize the cells as myofibroblastic were a fibroblastoid morphology, expression of the mesenchymal markers Thy-1 and vimentin, and most importantly the concomitant loss of keratins in some of the cells expressing α-sm actin. It is important though to emphasize that acquisition of α-sm actin in itself should not be classified as EMT. This is a frequent phenomenon in normal resident fibroblasts and only reflects an activation of the cells. 43 Thus, the myofibroblast-like definition depends on complete loss of keratins and would apply only to some of the cells. 22 It is, however, remarkable that the cells also respond like myofibroblasts to cytokines and sort out in the stroma like myofibroblasts inside collagen gels and in vivo. These criteria for myofibroblasts differentiation of EMT-derived cells are similar to other reports on EMT of kidney epithelial cells. 48,49
It could be questioned why the HBFL-1 are not classified as myoepithelial cells since Thy-1, vimentin, and α-sm actin are also markers for myoepithelial cells in vivo. 13,50-52 Indeed, the mesenchymal compartment of metaplastic carcinomas has been firmly believed to reflect myoepithelial differentiation primarily based on the expression of S-100. 53 However, since keratin expression is also a hallmark of myoepithelial cells and given the criteria listed above for the definition of myofibroblasts, we found it reasonable to categorize at least those cells completely lacking keratin expression as myofibroblasts. It is possible to postulate that those cells in HBFL-1 cultures that express keratins represent the myoepithelial lineage, a possibility raised in reports on the evolution of rat mammary mixed mesenchymal tumors. 54 It should however be pointed out that the cells are not expressing the myoepithelial keratin K14. It has often been stated that oncogenesis mirrors ontogenesis. 55 Thus it is intriguing that in the closely related sweat gland, normal myoepithelial cells down-regulate keratin 14 by cultivation on irradiated fibroblasts. 56 It is also worthwhile to recall that in mouse mammary gland, the basal cells on the tip of the developing gland ie, the cap cells, were reported to lack keratin expression. 57 Thus, the loss of keratins in EMT in breast cancer to acquire a myofibroblastic phenotype may in fact recapitulate a developmental state.
Since cap cells have also been assumed to represent progenitor cells in the mouse mammary gland, another explanation for the observed EMT in mixed mesenchymal tumors would be that the origin of those tumors is an epithelial stem cell. 6 This hypothesis has been used to describe the mixed phenotype of another related tumor type namely the lung carcinosarcomas. 58 The large group of EMT-derived breast cancers with primarily basaloid phenotype has also been referred to as bimodal. 59 HBFL-1 cells do not generate bona fide luminal epithelial cells or myoepithelial cells and as such should not be classified as stem or progenitor cells. However, in analogy with lung carcinosarcomas, they may well be downstream of an ancestral progenitor cell.
A most surprising final conclusion of our functional studies of HBFL-1 is that they are not intrinsically tumorigenic. Therefore, it is worthwhile to incorporate into the list of consequences of EMT, the indirect action of nonmalignant EMT-derived cells on associated tumor cells as a mechanism of facilitating tumor growth. The current paradigm would appear then to be that some EMT-derived cells encourage their neighbors to be more malignant rather than performing themselves.
Acknowledgments
We thank Tove Marianne Lund and Winnie Hansen for expert technical assistance, Keld Ottosen for printing the micrographs, and Professor Dabelsteen for kindly placing the laser pressure catapulting equipment at our disposal. Dr. Derek Radisky is acknowledged for critical reading of the manuscript. The Aasted Clinic, the Private Clinic, and the Søllerød Plastic Surgery Clinic are gratefully acknowledged for providing the biopsy material.
Footnotes
Address reprint requests to Ole William Petersen, Structural Cell Biology Unit, Department of Medical Anatomy, Section A, The Panum Institute, Blegdamsvej 3, DK-2200 Copenhagen N, Denmark. E-mail: o.w.petersen@mai.ku.dk.
Supported by The Icelandic Research Fund for Graduate Students, Dansk Kræftforskningsfond, The Dagmar Marshallsfond (to T.G.), The Danish Research Council, The Novo Nordisk Foundation, The Thaysen Foundation, Friis Fonden, The Meyer Foundation, The Danish Cancer Society, The Danish Research Council, The Danish Medical Association Research Fund (to O.W.P. and L.R.-J.), Weimanns Legat (to L.R.-J.) and The US National Cancer Institute (grant CA-64786-02 to M.J.B. and O.W.P.) and United States Department of Energy Office of Biological and Environmental Research (contract DE-AC03-76SF00098 to M.J.B.).
References
- 1.Hay ED: An overview of epithelio-mesenchymal transformation. Acta Anat 1995, 154:8-20 [DOI] [PubMed] [Google Scholar]
- 2.Boyer B, Valles AM, Thiery JP: Model systems of epithelium-mesenchyme transitions. Acta Anat 1996, 156:227-239 [DOI] [PubMed] [Google Scholar]
- 3.Stoker M, Gherardi E, Perryman M, Gray J: Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 1987, 327:239-242 [DOI] [PubMed] [Google Scholar]
- 4.Boyer B, Tucker GC, Vallés AM, Franke WW, Thiery JP: Rearrangement of desmosomal and cytoskeletal proteins during the transition from epithelial to fibroblastoid organization in cultured rat bladder carcinoma cells. J Cell Biol 1989, 109:1495-1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilles C, Thompson EW: The epithelial to mesenchymal transition and metastatic progression in carcinoma. Breast J 1996, 2:83-96 [Google Scholar]
- 6.Thiery JP: Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002, 2:442-454 [DOI] [PubMed] [Google Scholar]
- 7.Dandachi N, Hauser-Kronberger C, More E, Wiesener B, Hacker GW, Dietze O, Wirl G: Co-expression of tenascin C and vimentin in human breast cancer cells indicates phenotypic transdifferentiation during tumour progression: correlation with histopathological parameters, hormone receptors, and oncoproteins. J Pathol 2001, 193:181-189 [DOI] [PubMed] [Google Scholar]
- 8.Jones C, Nonni AV, Fulford L, Merrett S, Chaggar R, Eusebi V, Lakhani SR: CGH analysis of ductal carcinoma of the breast with basaloid/myoepithelial cell differentiation. Br J Cancer 2001, 85:422-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL: Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001, 98:10869-10874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad A, Hanby A, Dublin E, Poulsom R, Smith P, Barnes D, Rubens R, Anglard P, Hart I: Stromelysin 3: an independent prognostic factor for relapse-free survival in node-positive breast cancer and demonstration of novel breast carcinoma cell expression. Am J Pathol 1998, 152:721-728 [PMC free article] [PubMed] [Google Scholar]
- 11.Gobbi H, Simpson JF, Borowsky A, Jensen RA, Page DL: Metaplastic breast tumors with a dominant fibromatosis-like phenotype have a high risk of local recurrence. Cancer 1999, 85:2170-2182 [DOI] [PubMed] [Google Scholar]
- 12.Rønnov-Jessen L, van Deurs B, Celis JE, Petersen OW: Smooth muscle differentiation in cultured human breast gland stromal cells. Lab Invest 1990, 63:532-543 [PubMed] [Google Scholar]
- 13.Petersen OW, van Deurs B: Growth factor control of myoepithelial-cell differentiation in cultures of human mammary gland. Differentiation 1988, 39:197-215 [DOI] [PubMed] [Google Scholar]
- 14.Counter CM, Meyerson M, Eaton EN, Ellison LW, Caddle SD, Haber DA, Weinberg RA: Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene 1998, 16:1217-1222 [DOI] [PubMed] [Google Scholar]
- 15.Briand P, Lykkesfeldt A: Long-term cultivation of a human breast cancer cell line, MCF-7, in a chemically defined medium Effect of estradiol Anticancer Res 1986, 6:85-90 [PubMed] [Google Scholar]
- 16.Petersen OW, van Deurs B, Vang Nielsen K, Madsen MW, Laursen I, Balslev I, Briand P: Differential tumorigenicity of two autologous human breast carcinoma cell lines HMT-3909 S1 and HMT-3909 S8 established in serum-free medium. Cancer Res 1990, 50:1-14 [PubMed] [Google Scholar]
- 17.Rønnov-Jessen L, van Deurs B, Nielsen M, Petersen OW: Identification, paracrine generation and possible function of human breast carcinoma myofibroblasts in culture. In Vitro Cell Dev Biol 1992, 28A:273-283 [DOI] [PubMed] [Google Scholar]
- 18.Briand P, Nielsen KV, Madsen MW, Petersen OW: Trisomy 7p and malignant transformation of human breast epithelial cells following epidermal growth factor withdrawal. Cancer Res 1996, 56:2039-2044 [PubMed] [Google Scholar]
- 19.Petersen OW, van Deurs B: Preservation of defined phenotypic traits in short-time cultured human breast carcinoma derived epithelial cells. Cancer Res 1987, 47:856-866 [PubMed] [Google Scholar]
- 20.Rønnov-Jessen L, Celis JE, van Deurs B, Petersen OW: A fibroblast-associated antigen: characterization in fibroblasts and immunoreactivity in smooth muscle differentiated stromal cells. J Histochem Cytochem 1992, 40:475-486 [DOI] [PubMed] [Google Scholar]
- 21.Rønnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ: The origin of the myofibroblasts in breast cancer: recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest 1995, 95:859-873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen OW, van Deurs B: Distinction between vascular smooth muscle cells and myoepithelial cells in primary monolayer cultures of human breast tissue. In Vitro Cell Dev Biol 1989, 25:259-266 [DOI] [PubMed] [Google Scholar]
- 23.Pallesen G, Nielsen S, Celis JE: Characterization of a monoclonal antibody (BG3C8) that reacts with basal cells of stratified epithelia. Histopathol 1987, 11:591-601 [DOI] [PubMed] [Google Scholar]
- 24.Saalbach A, Kraft R, Herrmann K, Haustein UF, Anderegg U: The monoclonal antibody AS02 recognizes a protein on human fibroblasts being highly homologous to Thy-1. Arch Dermatol Res 1998, 290:360-366 [DOI] [PubMed] [Google Scholar]
- 25.Briand P, Petersen OW, van Deurs B: A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cell Dev Biol 1987, 23:181-188 [DOI] [PubMed] [Google Scholar]
- 26.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW: Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 265:2011-2015 [DOI] [PubMed] [Google Scholar]
- 27.Nielsen HL, Rønnov-Jessen L, Villadsen R, Petersen OW: Identification of EPSTI1, a novel gene induced by epithelial-stromal interaction in human breast cancer. Genomics 2002, 79:703-710 [DOI] [PubMed] [Google Scholar]
- 28.Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW: Methylation of HpaII and HhaI sites near the polymorphic repeat in the human androgen-receptor gene correlates with chromosomal inactivation. Am J Hum Genet 1992, 51:1229-1239 [PMC free article] [PubMed] [Google Scholar]
- 29.Willman CL, Busque L, Griffith BB, Favara BE, McClain KL, Duncan MH, Gilliland DG: Langerhans’-cell histiocytosis (histiocytosis-X) - a clonal proliferative disease. N Engl J Med 1994, 331:154-160 [DOI] [PubMed] [Google Scholar]
- 30.Li M, Cordon-Cardo C, Gerald WL, Rosai J: Desmoid fibromatosis is a clonal process. Hum Pathol 1996, 27:939-943 [DOI] [PubMed] [Google Scholar]
- 31.Middleton SB, Frayling IM, Philips RKS: Desmoids in familial adenomatous polyposis are monoclonal proliferations. Br J Cancer 2000, 82:827-832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Péchoux C, Gudjonsson T, Rønnov-Jessen L, Bissell MJ, Petersen OW: Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev Biol 1999, 206:88-99 [DOI] [PubMed] [Google Scholar]
- 33.Popnikolov N, Yang J, Liu A, Guzman R, Nandi S: Reconstituted normal human breast in nude mice: effect of host pregnancy environment and human chorionic gonadotropin on proliferation. J Endocrinol 2001, 168:487-496 [DOI] [PubMed] [Google Scholar]
- 34.Rønnov-Jessen L, Petersen OW: Induction of α-smooth muscle actin by transforming growth factor-β1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia Lab Invest 1993, 68:696-707 [PubMed] [Google Scholar]
- 35.Barchowsky A, Roussel RR, Krieser RJ, Mossman BT, Treadwell MD: Expression and activity of urokinase and its receptor in endothelial and pulmonary epithelial cells exposed to asbestos. Toxicol Appl Pharmacol 1998, 152:388-396 [DOI] [PubMed] [Google Scholar]
- 36.Celis JE, Dejgaard K, Madsen P, Leffers H, Gesser B, Honore B, Rasmussen HH, Olsen E, Lauridsen JB, Ratz G: The MRC-5 human embryonal lung fibroblast two-dimensional gel cellular protein database: quantitative identification of polypeptides whose relative abundance differs between quiescent, proliferating and SV40 transformed cells. Electrophoresis 1990, 11:1072-1113 [DOI] [PubMed] [Google Scholar]
- 37.Celis JE, Gesser B, Holm Rasmussen H, Madsen P, Leffers H, Dejgaard K, Honore B, Olsen E, Ratz G, Lauridsen JB, Basse B, Mouritzen S, Hellerup M, Andersen A, Walbum E, Celis A, Bauw G, Puype M, Van Damme J, Vandekerckhove J: Comprehensive two-dimensional gel protein databases offer a global approach to the analysis of human cells: the transformed amnion cells (AMA) master database and its link to genome DNA sequence data. Electrophoresis 1990, 11:989-1071 [DOI] [PubMed] [Google Scholar]
- 38.Sieuwerts AM, Klijn JGM, Henzen-Logmans SC, Foekens JA: Cytokine-regulated urokinase-type-plasminogen-activator (uPA) production by human breast fibroblasts in vitro. Breast Cancer Res Treat 1999, 55:9-20 [DOI] [PubMed] [Google Scholar]
- 39.Shao Z-M, Nguyen M, Barsky SH: Human breast carcinoma desmoplasia is PDGF initiated. Oncogene 2000, 19:4337-4345 [DOI] [PubMed] [Google Scholar]
- 40.Petersen OW, Rønnov-Jessen L, Bissell MJ: The microenvironment of the breast: three-dimensional models to study the roles of the stroma and the extracellular matrix in function and dysfunction. Breast J 1995, 1:22-35 [Google Scholar]
- 41.Garbe J, Wong M, Wigington D, Yaswen P, Stampfer MR: Viral oncogenes accelerate conversion to immortality of cultured conditionally immortal human mammary epithelial cells. Oncogene 1999, 18:2169-2180 [DOI] [PubMed] [Google Scholar]
- 42.Sappino A-P, Schürch W, Gabbiani G: Biology of disease. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations Lab Invest 1990, 63:144-161 [PubMed] [Google Scholar]
- 43.Rønnov-Jessen L, Petersen OW, Bissell MJ: Cellular changes involved in conversion of normal to malignant breast: the importance of the stromal reaction. Physiol Rev 1996, 76:69-125 [DOI] [PubMed] [Google Scholar]
- 44.Raju GC: The histological and immunohistochemical evidence of squamous metaplasia from the myoepithelial cells in the breast. Histopathology 1990, 17:272-275 [DOI] [PubMed] [Google Scholar]
- 45.Smith DM, Rongaus VA, Wehmann TW, Agarwal PJ, Classen GJ: Metaplastic breast carcinoma. J Am Osteopath Assoc 1996, 96:419-421 [PubMed] [Google Scholar]
- 46.Wang X, Mori I, Tang W, Yang Q, Nakamura M, Nakamura Y, Sato M, Sakurai T, Kennichi K: Metaplastic carcinoma of the breast: p53 analysis identified the same point mutation in the three histologic components. Mod Pathol 2001, 14:1183-1186 [DOI] [PubMed] [Google Scholar]
- 47.Teixeira MR, Qvist H, Bohler PJ, Pandis N, Heim S: Cytogenetic analysis shows that carcinosarcomas of the breast are of monoclonal origin. Genes, Chromosomes Cancer 1998, 22:145-151 [PubMed] [Google Scholar]
- 48.Fan J-M, Huang X-R, Ng Y-Y, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY: Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-β1-dependent mechanism in vitro. Am J Kidn Dis 2001, 37:820-831 [DOI] [PubMed] [Google Scholar]
- 49.Yang J, Liu Y: Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 2001, 159:1465-1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lennon VA, Unger M, Dulbecco R: Thy-1: a differentiation marker of potential mammary myoepithelial cells in vitro. Proc Natl Acad Sci USA 1978, 75:6093-6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mørk C, van Deurs B, Petersen OW: Regulation of vimentin expression in cultured human mammary epithelial cells. Differentiation 1990, 43:146-156 [DOI] [PubMed] [Google Scholar]
- 52.Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rønnov-Jessen L, Bissell MJ: The plasticity of human breast carcinoma cells is more than epithelial to mesenchymal conversion. Breast Cancer Res 2001, 3:213-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wargotz ES, Norris HJ: Metaplastic carcinomas of the breast. IV. Squamous cell carcinoma of ductal origin. Cancer 1990, 65:272-276 [DOI] [PubMed] [Google Scholar]
- 54.Bartsch C, Szadowska A, Karasek M, Bartsch H, Geppert M, Mecke D: Serial transplants of DMBA-induced mammary tumors in fischer rats as model system for human breast cancer: v. Myoepithelial-mesenchymal conversion during passaging as possible cause for modulation of pineal-tumor interaction. Exp Toxicol Pathol 2000, 52:93-101 [DOI] [PubMed] [Google Scholar]
- 55.Pierce GB, Speers WC: Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res 1988, 48:1996-2004 [PubMed] [Google Scholar]
- 56.Schon M, Benwood J, O’Connell-Willstaedt T, Rheinwald JG: Human sweat gland myoepithelial cells express a unique set of cytokeratins and reveal the potential for alternative epithelial and mesenchymal differentiation states in culture. J Cell Sci 1999, 112:1925-1936 [DOI] [PubMed] [Google Scholar]
- 57.Sapino A, Macri L, Gugliotta P, Pacchioni D, Liu Y-J, Medina D, Bussolati G: Immunophenotypic properties and estrogen dependency of budding cell structures in the developing mouse mammary gland. Differentiation 1993, 55:13-18 [DOI] [PubMed] [Google Scholar]
- 58.Dacic S, Finkelstein SD, Sasatomi E, Swalsky PA, Yousem SA: Molecular pathogenesis of pulmonary carcinosarcoma as determined by microdissection-based allelotyping. Am J Surg Pathol 2002, 26:510-516 [DOI] [PubMed] [Google Scholar]
- 59.Malzahn K, Mitze M, Thoenes M, Moll R: Biological and prognostic significance of stratified epithelial cytokeratins in infiltrating ductal breast carcinomas. Virchows Arch 1998, 433:119-129 [DOI] [PubMed] [Google Scholar]





